An amorphous wrapped nanorod LiV3O8 electrode with enhanced performance for lithium ion batteries†
Qian
Shi
ab,
Jiangwen
Liu
a,
Renzong
Hu
a,
Meiqin
Zeng
a,
Minjiang
Dai
b and
Min
Zhu
*a
aSchool of Materials Science and Engineering, South China University of Technology, Guangzhou, PR China. E-mail: memzhu@scut.edu.cn; Fax: +86-20-87111317; Tel: +86-20-87113924
bGuangzhou Research Institute of Non-ferrous Metals, Guangzhou, PR China
First published on 12th July 2012
Abstract
To achieve a good combination of high capacity, cyclability and rate capability in an electrode is still a great challenge for lithium-ion batteries, especially those used for electric vehicles. The present work has developed a simple and effective strategy to solve this problem in the layer structured LiV3O8 positive electrode. This has the highest theoretical capacity of the currently available positive electrodes but it has not yet been achieved in practice along with high rate capability. In our approach, an amorphous wrapped [100] orientated nanorod structure has been fabricated in a LiV3O8 thin film by adjusting the oxygen partial pressure in the deposition process using radio frequency (RF) magnetron sputtering. With this structure, a record combination of high capacity and superior high-rate capability, namely 388 mA h g−1 at C/5 and 102 mA h g−1 at 40 C, has been achieved along with stable cycle life. The result revealed that the orientated nanorods provide additional ionic transport channels and their amorphous wrapping layer can withstand the anisotropy of the surface during the intercalation of Li ions.
Introduction
Considerable effort has been made to develop high power lithium-ion batteries (LIBs) to satisfy the increasing demands for power sources in electronic technology and, in particular, the electric vehicles necessary for a low carbon society.1 Although the current LIBs, using LiCoO2, LiFePO4 and LiMn2O4 as positive electrodes, exhibit superior performance in terms of energy density, rate capability and cycling life in comparison with other rechargeable batteries, such as the Ni–MH battery, their electrochemical performances have hardly been improved, due to the limited theoretical capacity for these electrode materials.2 Therefore, significant research has been carried out to develop novel positive electrode materials, in particular new nanostructured materials, such as Li2FeSiO4 and Li3V2(PO4)3.3,4As an attractive positive electrode material, LiV3O8 has a high theoretical capacity of 372 mA h g−1, which is approximately 3 times higher than that of LiCoO2. However, its high capacity and long cycle lifetime can barely be achieved with a conventional microstructure.5,6 The initial capacity and cyclability of this electrode material have been substantially improved by manipulating the crystal sizes, morphologies and growth texture.7,8 For example, Patey et al. have shown that nanoparticulate LiV3O8 (50 nm) delivers a capacity of 315 mA h g−1 in the potential range 2.0–4.0 V.9 In addition, one dimensional and two dimensional nanoscale LiV3O8 electrodes also deliver a high capacity with excellent cycle performance.10,11 Although these nanostructured LiV3O8 electrodes exhibit high capacity and good cyclability, their rate capability has not been significantly improved. To the best of our knowledge, the best rate capability of 82 mA h g−1 at 20 C was obtained for the LiV3O8–carbon composite electrode.12 The poor rate capability is attributed to its intrinsic limitations. The existence of repulsive interactions between pre-existing and inserted Li ions leads to sluggish kinetics of the insertion of Li ions.
In general, nanostructures favor both Li storage and kinetics due to the large surface area and short transport pathway, consequently enhancing the capacity and rate performance of lithium-ion batteries.13–15 Tremendous progress has been made in improving the rate capability through nanoscaling or by consideration of three-dimensional structures.16 For instance, a high rate performance of 100 mA h g−1 at 50 C has been obtained for hollow sphere LiFePO4.17 These hollow particles provide easy accessibility of Li ions to each primary particle. Okubo et al. have clearly demonstrated that the size of the crystallites of LiCoO2 plays a significant role in the reactivity of this material with Li ions.18 Similarly, Arrebola et al. have improved the kinetic properties of the LiNi0.5Mn1.5O4 electrode by controlling its crystallinity using a special synthetic procedure.19 These highly crystalline nanoparticles exhibit a very low level of imperfections and microstrain, so providing a high capacity of 100 mA h g−1, at a high rate of 15 C.
For the LiV3O8 electrode, as mentioned above, the improvement of sluggish kinetics by simply utilizing the compound material or nanosize effect is still far from optimal, and therefore other improvement strategies should be considered. Several researchers have proven that the grain orientation and state of the particle surface play important roles in the diffusion of Li ions and the rate capability of LiCoO2 and LiFePO4.20,21 Based on the above, we have speculated that the [100] orientated and nanoscale LiV3O8 electrodes should deliver high electrochemical performance because the most appropriate Li ion diffusion pathway is parallel to the (100) plane. However, intercalation compounds such as layered oxides are highly anisotropic materials, which might block the flow of Li ions.22 To overcome such challenges, we have proposed and realized a simple strategy to obtain a structure consisting of amorphous wrapped orientated LiV3O8 nanorods, which greatly enhances their rate capability.
Experimental
The preparation of LiV3O8 powder and targets was in accordance with our previous study.23 The nanostructured LiV3O8 films were deposited on a stainless steel (SS) substrate by radio frequency (RF) magnetron sputtering at a sputtering power of 130 W for 4 h. The distance between target and substrate was set to 5 cm. The film was grown in an atmosphere of Ar and O2 with constant flow of 10 sccm and 5 sccm, respectively. The total working pressure in the deposition chamber was set at 1.0 Pa. Before deposition, the SS substrate was cleaned with dilute hydrochloric acid, acetone and ethanol in an ultrasonic bath.The phase and microstructure of the as-deposited films were characterized by X-ray diffraction (XRD) using a Philips X’pert MPD diffractometer equipped with Cu-Kα radiation, scanning electron microscopy (SEM) using a Nova NanoSEM 430 and transmission electron microscopy (TEM) using a JEOL 2100 operating at 200 kV. The specimens for TEM observation were thinned by ion milling using a Gatan model 691 precision ion polishing system.
Charge–discharge tests and cyclic voltammetry (CV) of the LiV3O8 thin films were carried out using a two-electrode system. CR2016-type coin cells were assembled in an argon filled glove box using lithium foil as the anode, the as-deposited LiV3O8 thin films as the cathode and LiPF6 (1 M)/ethylene carbonate (EC) + diethyl carbonate (DEC) + ethyl methyl carbonate (EMC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume) as the electrolyte; these were separated by a polyethylene membrane. For determination of the C-rate, 1 C corresponds to a current allowing a full discharge in 1 h, and here 1 C = 50 μA cm−2. For rate-capacity performance, the charge rate was increased every 10 cycles and varied from C/5 to 50 C. The cycle test was performed in a battery test system using an Arbin BT-2000. CV tests were scanned between 1.5 and 3.8 V at a sweep rate of 0.5 mV s−1 using an AutoLab Electrochemical System from ECO Chemie.
1 by volume) as the electrolyte; these were separated by a polyethylene membrane. For determination of the C-rate, 1 C corresponds to a current allowing a full discharge in 1 h, and here 1 C = 50 μA cm−2. For rate-capacity performance, the charge rate was increased every 10 cycles and varied from C/5 to 50 C. The cycle test was performed in a battery test system using an Arbin BT-2000. CV tests were scanned between 1.5 and 3.8 V at a sweep rate of 0.5 mV s−1 using an AutoLab Electrochemical System from ECO Chemie.
Results and discussion
The structure of the as-deposited LiV3O8 thin film was examined by X-ray diffraction and is shown in Fig. 1a. The XRD patterns of the target and substrate are also given in the figure for reference. The target has the typical monoclinic P21/m (JCPDS No.72-1193) structure of LiV3O8. Except for the diffraction peak of the stainless steel substrate, the as-deposited thin film exhibits only one relatively broad diffraction peak, which is indexed as the (100) reflection of LiV3O8. The presence of only the (100) reflection indicates that the LiV3O8 film has a preferential orientation and most of its grains are oriented with the (100) plane parallel to the substrate. The broadening of the peak reveals the fine crystalline grain size of the LiV3O8 film. The crystal structure of LiV3O8, as shown in Fig. 1b, consists of VO5 trigonal bipyramids and VO6 octahedral layers on the (100) plane. Li cations lie in the interlayer space, coordinating with six oxygen atoms to build LiO6 octahedral chains, which are separated from each other by a V3O8− layer. According to the crystal structure schematic diagram in Fig. 1c, the inserted Li ions can easily diffuse along the [010] orientation and occupy octahedral and tetrahedral sites between the V3O8 layers in the (100) plane to form the Li1+xV3O8 (x = 1∼4) phase, as illustrated.24,25 This structural feature of LiV3O8 indicates that the [100] orientation strongly affects the insertion of Li ions. This will be discussed in detail below.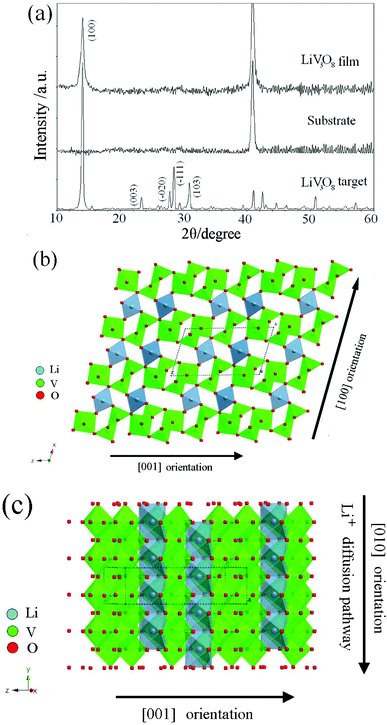 | ||
| Fig. 1 (a) X-ray diffraction patterns of the substrate, LiV3O8 target and film; (b) LiV3O8 crystal structure with polyhedrons along the b axis and (c) the lithium diffusion pathway along the a axis. | ||
The morphology of the as-deposited LiV3O8 film prepared under an oxygen partial pressure of Ar/O2 = 2 is shown in Fig. 2a. The film is composed of uniform nanoparticles which are approximately 50 nm in size and close-packed with each other. However, some rod-like particles were observed on the surface of the film with a length of 150 nm (ESI Fig. S1†). The XRD results show that most particles must grow along the [100] orientation, which should be perpendicular to the substrate. Hence, the particle size obtained from Fig. 2a should be associated with the short direction of the rod-like particles. We therefore deduced that the film is composed of LiV3O8 nanorods 150 nm long, aligned in the [100] direction and 50 nm wide in the [010] or [001] directions. It is worth noting that these nanorods do not show distinct boundaries between each other, indicating the presence of heteropic compounds in the interspaces between nanorods. To confirm this, a TEM analysis was performed and the typical structure is shown in Fig. 2b and c, in which two distinct phases are clearly observed. One is the nanorod phase and the relevant selected area electron diffraction (SAED) pattern proves that it belongs to monoclinic LiV3O8. The other is an amorphous phase, which can be identified by the enlarged TEM image in Fig. 2c. TEM observation clearly shows that the nanorods are wrapped and interconnected by the amorphous layer, forming the compact network architecture. The structure of this as-deposited LiV3O8 film is clearly different from the nanoparticles previously reported by others.8 Hence we define it as the amorphous wrapped orientated nanorod (AWON). Furthermore, it was noted that this amorphous wrapping is in a metastable state during the TEM observation, and is easily crystallized after continuous exposure to the electron beam. The transformation of amorphous wrapping, as indicated by the arrow in Fig. 2c, was recorded by high resolution TEM imaging and is given in the ESI Fig. S2.† The fringe spacing of the crystallized wrapping can be indexed as the (020) plane of LiV3O8, indicating that the amorphous wrapping also belongs to LiV3O8.
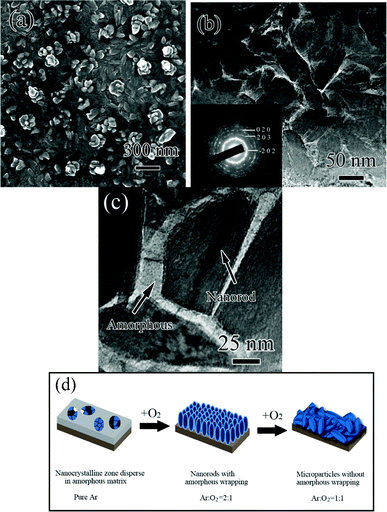 | ||
| Fig. 2 (a) SEM and (b, c) TEM images of the nanostructured LiV3O8 thin film and (d) the scheme describing the production of the special LiV3O8 thin film morphology with increase of oxygen partial pressure. The inset in (b) shows the corresponding SAED pattern of the film. | ||
The morphology and microstructure of the orientated nanorod LiV3O8 with amorphous wrapping clearly differs from that of the mixed amorphous–nanocrystalline microstructure film reported previously,23 in which amorphous LiV3O8 is the major constituent. The deposition process of those two films is completely the same except for the oxygen partial pressure. After altering the sputtering atmosphere from pure Ar to Ar/O2 in the ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, LiV3O8 film exhibits a nanorod nature and is orientated along the [100] direction. This experiment indicates that the crystallinity of the LiV3O8 film was enhanced with increasing oxygen partial pressure (ESI Fig. S3†). Oxygen therefore plays an important role in the crystal growth mechanism of this thin film. This should probably be attributed to the smaller momentum transfer of oxygen in comparison with argon during ionic bombardment, which leads to a low deposition rate during the sputtering process.26 With the decreasing proportion of Ar, the sputtering particles deliver a longer mean free path and improve the mobility of atoms in the substrate, which ultimately increases the film crystallinity. The sputtering particles may exist in the atomic state or as molecular groups in an atmosphere of oxygen when a transition metal is contained in the target.27 As a result, the driving force for nucleation of crystals is probably reduced after the atom or molecular group is deposited on the substrate, yielding an increased crystallinity for the film.
1, LiV3O8 film exhibits a nanorod nature and is orientated along the [100] direction. This experiment indicates that the crystallinity of the LiV3O8 film was enhanced with increasing oxygen partial pressure (ESI Fig. S3†). Oxygen therefore plays an important role in the crystal growth mechanism of this thin film. This should probably be attributed to the smaller momentum transfer of oxygen in comparison with argon during ionic bombardment, which leads to a low deposition rate during the sputtering process.26 With the decreasing proportion of Ar, the sputtering particles deliver a longer mean free path and improve the mobility of atoms in the substrate, which ultimately increases the film crystallinity. The sputtering particles may exist in the atomic state or as molecular groups in an atmosphere of oxygen when a transition metal is contained in the target.27 As a result, the driving force for nucleation of crystals is probably reduced after the atom or molecular group is deposited on the substrate, yielding an increased crystallinity for the film.
The growth mechanism for thin films as a function of oxygen partial pressure is schematically illustrated in Fig. 2d. The amorphous constituent is first formed, accompanied by a small amount of the nanocrystalline zone scattered in it. After increasing the oxygen partial pressure, nanocrystalline zones grow as nanorods and the amorphous constituent is left as a wrapping layer. When the Ar/O2 mixture ratio is altered to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the LiV3O8 film exhibits the typical crystalline structure without the existence of an amorphous phase (ESI Fig. S4†). Excellent studies have actually proved that the addition of oxygen in a sputtering atmosphere would dramatically change the crystallinity and orientation of the thin films.28,29 Thus, it is reasonable that the microstructure consisting of crystallized particles with an amorphous wrapping can be simply prepared by adjusting the Ar/O2 ratio.
1, the LiV3O8 film exhibits the typical crystalline structure without the existence of an amorphous phase (ESI Fig. S4†). Excellent studies have actually proved that the addition of oxygen in a sputtering atmosphere would dramatically change the crystallinity and orientation of the thin films.28,29 Thus, it is reasonable that the microstructure consisting of crystallized particles with an amorphous wrapping can be simply prepared by adjusting the Ar/O2 ratio.
The sharp (100) peak indicates that the dimensions of the crystallites normal to the (100) plane are relatively large, which means that the orientated nanorods can provide more entrances for Li insertion into the interstitial site, as illustrated in Fig. 1b. Therefore, improved electrochemical performance for the LiV3O8 film with this amorphous wrapped orientated nanorod structure would be expected. Fig. 3 gives the electrochemical performance of the as-deposited LiV3O8 thin film. The cyclic voltammogram (Fig. 3a) exhibits two reduction peaks, at 2.56 V and 2.81 V, and oxidation peaks at 2.52 V and 2.78 V, corresponding to the extraction and insertion of Li ions in monoclinic LiV3O8, respectively. The sharp peaks at 2.81 and 2.52 V indicate the depth of the phase transformation during the charge and discharge processes. The curves obtained for five cycles remain identical to each other, indicating a stable and good reversible insertion/extraction reaction. This is similar to the typical charge–discharge process of the nanostructured LiV3O8 electrode.6Fig. 3b shows the cycling performance of the present LiV3O8 film at a constant current density of C/5. The film delivers an initial specific discharge capacity of 388 mA h g−1, which is equivalent to 4.0 mol Li per mol of LiV3O8. After 40 cycles, it still reaches 350 mA h g−1, 87.5% of the initial value and a coulombic efficiency of 100%. This capacity is far superior to that of the so-called advanced positive electrodes, such as V2O5·nH2O and LiMnO2, for which capacities of 300 mA h g−1 and 200 mA h g−1 were obtained, respectively.30,31
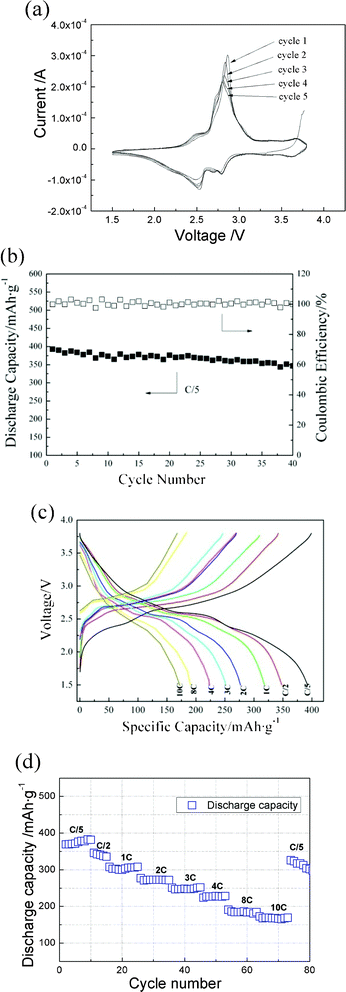 | ||
| Fig. 3 (a) Cyclic voltammograms of nanostructured LiV3O8 thin film recorded in the first 5 cycles; (b) capacity versus cycle number at a C-rate of C/5; (c) voltage vs. discharge capacity curves obtained at different charge rates and (d) cycling performance of the nanostructured LiV3O8 thin film at various discharge rates. | ||
In order to fully estimate the electrochemical performance of the LiV3O8 film electrode, the charge–discharge curves at increasing rates from C/5 to 10 C were determined, with the results shown in Fig. 3c. The voltage profiles always retain the same extraction/insertion plateau at 2.5 V, which means that serious polarization does not occur. The discharge capacity is 302 mA h g−1 at 1 C and remains at 167 mA h g−1 even at 10 C. The rate capability of the present electrode is much higher than that of the LiV3O8 electrode consisting of single crystal nanorods, being 80 mA h g−1 at a rate of 8 C.8 The cycle performance as a function of discharge capacity at various C-rates is shown in Fig. 3d. The film electrode was cycled at charge rates that increased stepwise from C/5 to 10 C. The capacity is fairly stable at each C-rate, demonstrating the excellent cycle reversibility and stability. After 70 cycles, a capacity of 325 mA h g−1 was obtained when the C-rate was returned to C/5. This result indicates the excellent rate capability and cycle performance of the LiV3O8 film with the structure consisting of amorphous wrapped orientated nanorods.
It is believed that the nanosized material favors a shorter Li-ion diffusion length and provides more active sites for electrochemical reactions. A few reports have been published recently on high capacity, due to the favorable transport properties of nanostructured LiV3O8 itself. However, the poor rate capability remains a problem.32 The present film exhibits not only a very high capacity, but also an excellent rate capability, as shown in Fig. 4a. The specific capacity of the present film electrode at 20 C is 134 mA h g−1, which is much higher than the value 65 mA h g−1 for the LiV3O8–carbon composite electrode obtained at the same C-rate.12 To the best of our knowledge, the present film with the amorphous wrapped oriented nanorod structure displays the best high rate capability of the LiV3O8 electrodes that have been reported up to now. At the high rate of 40 C, our LiV3O8 film can be fully charged and discharged in the short period of 45 s and still maintain a high capacity of 102 mA h g−1. Moreover, as an important cycle-life-related property for commercial batteries, the LiV3O8 film can withstand fast-charge–slow-discharge processes, confirming the excellent rate capability (ESI Fig. S5†).
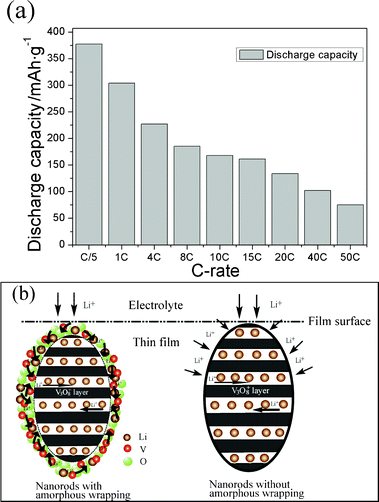 | ||
| Fig. 4 (a) Discharge capacity of the nanostructured LiV3O8 film at successively increasing C-rates and (b) illustration of the diffusion pathway for Li ions in LiV3O8 nanorods with and without amorphous wrapping. | ||
From the structure analysis, the high-power discharge performances and outstanding rate capability of the present LiV3O8 film should arise from (i) the nano-scale particles with [100] orientation and (ii) the amorphous LiV3O8 wrapping layer on the surface of each particle. From consideration of the LiV3O8 crystal structure in Fig. 1b, we understand that Li ions can easily move into the bulk of the crystal along the (100) plane during the charge and discharge processes. Hence, LiV3O8 nanorods with a (100) preferred orientation can provide more entrances and shorter diffusion pathways for insertion of Li ions, which facilitate high capacity and excellent rate capability. However, the lithiation planes are perpendicular to the Li ion transport pathways when the (100) planes are parallel to the substrate for the film electrode, which blocks the flow of Li ions, as shown in Fig. 4b. Li ions therefore have to move through the close-packed oxygen layer, which leads to poor rate properties for a film electrode. In our research, such a diffusion limitation can be overcome by the amorphous wrapping layer.
As demonstrated, the limitation factor for the rate capability, when particles are considered in nano dimensions, is the delivery of Li ions to the surface rather than bulk diffusion.21 After introducing the amorphous wrapping layer, the migration of Li ions proceeds firstly in a three-dimensional manner along the outside of the nanorods and then across the interface towards the (100) plane. This amorphous wrapping layer can avoid the close-packed oxygen layer and remove the anisotropy of the nanorods, which therefore improves the sluggish kinetics of Li ion insertion, as shown schematically in Fig. 4b. Moreover, the amorphous layer is not an inert phase but the same as the bulk material, as indicated by the TEM measurements, so that it can provide Li ions in various sites and be convenient for delivery of Li ions to the (100) planes where it can be inserted.
In our previous work, we have reported that the diffusion coefficient of the mixed amorphous and nanostructured LiV3O8 film is lower than that of the crystalline LiV3O8 electrodes, which probably induces the poor rate capability.33 The excellent rate performance of the present electrode can be attributed to the unique properties of this particular amorphous wrapping layer. Firstly, an amorphous wrapping layer with characteristic loose packing of atoms might possibly permit more freedom for diffusion due to the ability to withstand more structural deformation, which therefore facilitates charge transfer reactions at the interface between the electrode and the electrolyte.34,35 This is based on the fact that the migration of inserted Li ions from site to site is less hindered by impurities or the repulsion effects of other Li ions already accommodated in the available sites.36 Secondly, the amorphous wrapping layer can accommodate the excess of Li ions, which cannot transfer immediately to the (100) plane, and therefore avoids any surface polarization phenomenon. Finally, unlike the bulk amorphous material, the amorphous wrapping layer has a thickness of about 15 nm, a small proportion of the whole electrode, and therefore releases the negative influence of slow diffusion velocity in an amorphous material. As a result, the amorphous wrapping layer soaks and stores Li ions like a sponge, thus dramatically decreasing the surface polarization phenomena for a film electrode. In conclusion, the amorphous wrapping structure removes the polarization of the film electrode and the anisotropy of the nanorods, giving efficient Li diffusion.
The improved kinetic behavior of an electrode incorporating amorphous wrapped nanorods should become evident through its lower activation energies in comparison with the mixed microstructure LiV3O8 film reported previously.23 The activation energies of LiV3O8 incorporating amorphous wrapped nanorods at 2.8 V and 2.4 V are 73.3 and 65.7 kJ mol−1, respectively (ESI Fig. S6†). By comparison, the activation energies of the mixed microstructure LiV3O8 electrode are 89.5 and 83.2 kJ mol−1, respectively. This result further demonstrates that the amorphous wrapped nanorod structure leads to improved kinetics by creation of a shorter diffusion pathway for Li insertion.
Conclusions
In summary, the present work has developed a new strategy to achieve an excellent combination of capacity, rate capability and cyclability in the positive electrode of a lithium-ion battery. This was realized in LiV3O8 by fabricating a film electrode with an amorphous wrapped [100] orientated nanorod structure created by using RF magnetron sputtering with a mixture of Ar/O2 in the appropriate ratio. The LiV3O8 film electrode with this structure exhibits the highest known discharge capacity and high-rate capability, delivering a capacity of 388 mA h g−1 at C/5 and 102 mA h g−1 at 40 C. There is no doubt that both the [100] orientated nanorods and amorphous wrapping layer play crucial roles in the electrochemical performance of the LiV3O8 film. The former contributes to the high capacity by providing a greater number of insertion entrances and the latter results in an excellent rate capability by overcoming the polarization of the film electrode and anisotropy of the nanorod structure. The present studies illustrate that LiV3O8 with the amorphous wrapped orientated nanorod structure is a most promising cathode material for the lithium-ion battery.Acknowledgements
This work was supported by NSFC under projects No. 50971060, and the Fundamental Research Funds for the Central Universities under project No. 2012ZM0001.References
- V. Etacheri, R. Marom, R. Elazari, G. Salitra and D. Aurbach, Energy Environ. Sci., 2011, 4, 3243–3262 CAS.
- M. S. Whittingham, Chem. Rev., 2004, 104, 4271–4301 CrossRef CAS.
- Z. L. Gong and Y. Yang, Energy Environ. Sci., 2011, 4, 3223–3242 CAS.
- K. Nagamine, T. Honma and T. Komatsu, J. Power Sources, 2011, 196, 9618–9624 CrossRef CAS.
- Y. Feng, F. Hou and Y. L. Li, J. Power Sources, 2009, 192, 708–713 CrossRef CAS.
- H. L. Zhang, J. R. Neilson and D. E. Morse, J. Phys. Chem. C, 2010, 114, 19550–19555 CAS.
- S. Jouanneau, A. Vervaera, S. Lascaud and D. Guyomard, Solid State Ionics, 2006, 177, 311–315 CrossRef CAS.
- N. Tran, K. G. Bramnik, H. Hibst, J. Prölß, N. Mronga, M. Holzapfel, W. Scheifele and P. Novák, J. Electrochem. Soc., 2008, 155, A384–A389 CrossRef CAS.
- T. J. Patey, S. H. Ng, R. Büchel, N. Tran, F. Krumeich, J. Wang, H. K. Liu and P. Novák, Electrochem. Solid-State Lett., 2008, 11, A46–A50 CrossRef CAS.
- H. W. Liu, H. M. Yang and T. Huang, Mater. Sci. Eng., B, 2007, 143, 60–63 CrossRef CAS.
- X. L. Li, P. P. Li, M. Luo, X. Y. Chen and J. J. Chen, J. Solid State Electrochem., 2010, 14, 1325–1332 CrossRef CAS.
- N. H. Idris, M. M. Rahman, J. Z. Wang, Z. X. Chen and H. K. Liu, Compos. Sci. Technol., 2011, 71, 343–349 CrossRef CAS.
- P. Balaya, Energy Environ. Sci., 2008, 1, 645–654 CAS.
- L. F. Nazar, G. Goward, F. Leroux, M. Duncan, H. Huang, T. Kerr and J. Gaubicher, Int. J. Inorg. Mater., 2001, 3, 191–200 CrossRef CAS.
- Y. G. Wang, H. Q. Li, P. He, E. Hosono and H. S. Zhou, Nanoscale, 2010, 2, 1294–1305 RSC.
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930–2946 CrossRef CAS.
- M. H. Lee, J. Y. Kim and Hyun-Kon Song, Chem. Commun., 2010, 46, 6795–6797 RSC.
- M. Okubo, E. Hosono, J. Kim, M. Enomoto, N. Kojima, T. Kudo, H. S. Zhou and I. Honma, J. Am. Chem. Soc., 2007, 129, 7444–7452 CrossRef CAS.
- J. C. Arrebola, A. Caballero, M. Cruz, L. Hernán, J. Morales and E. R. Castellón, Adv. Funct. Mater., 2006, 16, 1904–1912 CrossRef CAS.
- J. B. Bates, N. J. Dudney, B. J. Neudecker, F. X. Hart, H. P. Jun and S. A. Hackney, J. Electrochem. Soc., 2000, 147, 59–70 CrossRef CAS.
- B. Kang and G. Ceder, Nature, 2009, 458, 190–193 CrossRef CAS.
- Y. S. Meng and M. E. Arroyo-de Dompablo, Energy Environ. Sci., 2009, 2, 589–609 CAS.
- Q. Shi, R. Z. Hu, L. Z. Ouyang, M. Q. Zeng and M. Zhu, Electrochem. Commun., 2009, 11, 2169–2172 CrossRef CAS.
- S. Jouanneau, A. Verbaere and D. Guyomard, J. Solid State Chem., 2005, 178, 22–27 CrossRef CAS.
- R. Benedek, M. M. Thackeray and L. H. Yang, J. Power Sources, 1999, 81-82, 487–490 CrossRef CAS.
- J. H. Kim, J. H. Lee, Y. W. Heo, J. J. Kim and J. O. Park, J. Electroceram., 2009, 23, 169–174 CrossRef CAS.
- V. Vancoppenolle, P. Y. Jouan, M. Wautelet, J. P. Dauchot and M. Hecq, Surf. Coat. Technol., 1999, 116–119, 933–937 CrossRef CAS.
- A. Giesa, B. Pecquenard, A. Benayad, H. Martinez, D. Gonbeau, H. Fuess and A. Levasseur, Solid State Ionics, 2005, 176, 1627–1634 CrossRef.
- L. H. Yang, G. S. Wang, C. L. Mao, Y. Y. Zhang, R. H. Liang, C. Soyer, D. Rémiens and X. L. Dong, J. Cryst. Growth, 2009, 311, 4241–4246 CrossRef CAS.
- Y. Wang, K. Takahashi, K. Lee and G. Cao, Adv. Funct. Mater., 2006, 16, 1133–1144 CrossRef CAS.
- X. L. Xiao, L. Wang, D. S. Wang, X. M. He, Q. Peng and Yadong Li, Nano Res., 2009, 2, 923–930 CrossRef CAS.
- A. Q. Pan, J. Liu, J. G. Zhang, G. Z. Cao, W. Xu, Z. M. Nie, X. Jie, D. Choi, B. W. Arey, Ch. M. Wang and H. Q. Liang, J. Mater. Chem., 2011, 21, 1153–1161 RSC.
- Q. Shi, R. Z. Hu, M. Q. Zeng, M. J. Dai and M. Zhu, Electrochim. Acta, 2011, 56, 9329–9336 CrossRef CAS.
- D. W. Liu and G. Z. Cao, Energy Environ. Sci., 2010, 3, 1218–1237 CAS.
- A. Kayyar, H. J. Qian and J. Luo, Appl. Phys. Lett., 2009, 95, 221905–221908 CrossRef.
- R. Tossici, R. Marassi, M. Berrettoni, S. Stizza and G. Pistoia, Solid State Ionics, 1992, 57, 227–234 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra20769a/ |
| This journal is © The Royal Society of Chemistry 2012 |
