Nano-micelles based on a rosin derivative as potent sorbents and sinking agents with high absorption capabilities for the removal of metal ions†
Wei-Xin
Tan‡
a,
Zuan-Tao
Lin‡
a,
Huai-Tian
Bu
b,
Yanan
Tian
c and
Gang-Biao
Jiang
*a
aDepartment of Pharmaceutical Engineering, South China Agricultural University, Guangzhou, 510642, China. E-mail: jgb3h@163.com; Tel: +86 20 85280293; Fax: +86 20 85280292
bSINTEF Materials and Chemistry, PO Box 124 Blindern, N-0314 Oslo, Norway
cDepartment of Veterinary Physiology and Pharmacology, Mail Stop 4466, Texas A&M University, College Station, TX 77843, USA
First published on 12th July 2012
Abstract
In this paper, a novel stable nano-micelle based on a rosin derivative was prepared via the conjugation of tetraethylenepentamine (TEPA) with maleopimaric acid anhydride (MPA). The TEPA-MPA micelle depicts potent absorption and sinking abilities for metal ions. Its structure was characterized by FT-IR. The size of micelle measured by dynamic lighter scattering (DLS) was 96.5 nm, and its morphology observed by transmission electron microscopy (TEM) is approximately regular spherical. Besides, the low critical micelle concentration (CMC) value of micelles (6.575 × 10−4 mg mL−1) determined by fluorometric measurement indicates that the micelle has great self-assembly capability and excellent stability in aqueous solution. The adsorption mechanisms of Cu(II), Ni(II), Cd(II) and Cr(III) by TEPA-MPA were investigated by FT-IR and XPS, which suggests that both the carboxyl and amino groups are involved in metal adsorption, and that Cr(III) is the most firmly chelated ion by TEPA-MPA. Moreover, the effects of initial concentration and pH value of metal ions solution on adsorption were investigated to ascertain the adsorbing capacity. The results indicate that the adsorption of metal ions by TEPA-MPA is not pH dependent, and TEPA-MPA has a higher affinity for Ni(II) than that for Cu(II) and Cd(II). The absorption experiment data reveal that TEPA-MPA has outstanding metal absorption capacities (293.74–1655.08 mg g−1, metal ions/TEPA-MPA).
Introduction
Recently, there have been arising attentions on effluent which contains metal contaminants due to serious pollution caused by metals. Since metals are persistent and bioaccumulative, it has become a global environmental problem.1 Approaches to recycling heavy metal ions from effluent are widely investigated. Diverse methods including exchange, electroosmosis, and membrane separation, are employed to remove metal ions.2–6 However, many of them have limitations including high operating cost and energy requirements. Hence, as an effective method for metal ion removal, adsorption has received increasing attention.7Although many low-cost adsorbents, such as chitosan, clays, and marine algae, have been studied for their utility, their adsorption capacities are low and seldom satisfied in practice.8–11 Therefore, it is crucial and desired to develop highly efficient sorbents with excellent adsorption capacities.
Adsorbents made from nano-scale materials can be dispersed uniformly into effluents. With minute size and large surface area, the permeation ability and amount of functional groups can both be enhanced, which increase the tendency and opportunity of adhesion of pollution molecules, resulting in great adsorption abilities.12 In view of their unprecedented properties, several pioneering studies using nanoparticles to remove pollution substances were reported. For instance, Liu et al. utilized humic acid coated Fe3O4 magnetic nanoparticles to absorb metal ions, yet their maximum adsorption capacities only ranged from 46.3 to 97.7 mg g−1.13 Among nano-materials, stable nano-micelles which possess both hydrophobic and hydrophilic groups, and can self-aggregate to form shell–core structures in water, are one of the most vital materials for a wide range of pharmaceutical and biomedical applications.14
However, there are only a few reports using nano-micelles as adsorbents, and most of them focused on organic pollution adsorption.15–18 For example, poly(2-cinnamoylethyl methacrylate)-block-poly(acrylic acid) nanospheres, reported by Henselwood et al., were used to remove perylene from water.15 Porras-Rodriguez et al. reported using Al3+ coated lauryl sulfate micelles to remove 2, 4-diclorophenoxyacetic acid.16 The common limitation of using nano-micelles as adsorbents is the rareness of nano-micelles. We believe that utilization of micelles may be an efficient strategy for metal adsorption due to the high density of ligand dispersed on the micelle surfaces, which are capable of binding with metal ions and lead to flocculation. Hence, micelles made by abundant natural resources through simple procedures should be taken into consideration.
Rosin derives from the secretions of living pine, and it is abundant in nature and available throughout the world. Rosin acids are mainly monobasic carboxylic acids containing the phenanthrene skeleton with 20 carbon atoms in the molecule.19 The double bonds and carboxyl group of rosin acid made it available for synthesizing a series of unique surfactant with excellent performance by introducing various hydrophilic groups.20 Thus, novel nano-micelles based on rosin would be bestowed with unique advantages. Besides, rosin-based surfactants aroused wide interest, and recently, rosin and its derivatives have drawn increasing attention in pharmaceutical applications.21
In this paper, a novel, stable nano-micelle based on a rosin derivative via the conjugation of tetraethylenepentamine (TEPA) with maleopimaric acid anhydride (MPA), was prepared. It depicted outstanding absorption capabilities. Metal ions were bound with the amine imine and carboxyl groups of the derivative by sharing lone pairs of electrons resulting in the formation of metal-chelated complexes, which precipitate due to the reduction of the hydrophilicity of groups of micelles. The complexes can be filtered, redissolved and regenerated easily by various economical methods to separate the metal ions and recycle the adsorbent.
Experimental section
Preparation of the adsorbent
The synthesis process is shown in Fig. 1. Dried rosin and maleic anhydride (molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were mixed in an ampoule bottle, after being vacuum degassed, the reaction bottle was sealed. The reaction mixture was kept at 140 °C until all the components melted, it was then reacted for 4 h at 125 °C to obtain maleopimaric acid anhydride (MPA).22 Afterwards, MPA was taken out and mixed with TEPA solution (weight ratio 1
1) were mixed in an ampoule bottle, after being vacuum degassed, the reaction bottle was sealed. The reaction mixture was kept at 140 °C until all the components melted, it was then reacted for 4 h at 125 °C to obtain maleopimaric acid anhydride (MPA).22 Afterwards, MPA was taken out and mixed with TEPA solution (weight ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in a three-necked round-bottom flask equipped with a thermometer, a condenser and a magnetic stirrer. The reaction was conducted at 60 °C for 12 h. Finally, the resultant solution was precipitated by acetone, and the deposit was washed three times with alcohol then lyophilized to get TEPA-MPA.
1) in a three-necked round-bottom flask equipped with a thermometer, a condenser and a magnetic stirrer. The reaction was conducted at 60 °C for 12 h. Finally, the resultant solution was precipitated by acetone, and the deposit was washed three times with alcohol then lyophilized to get TEPA-MPA.
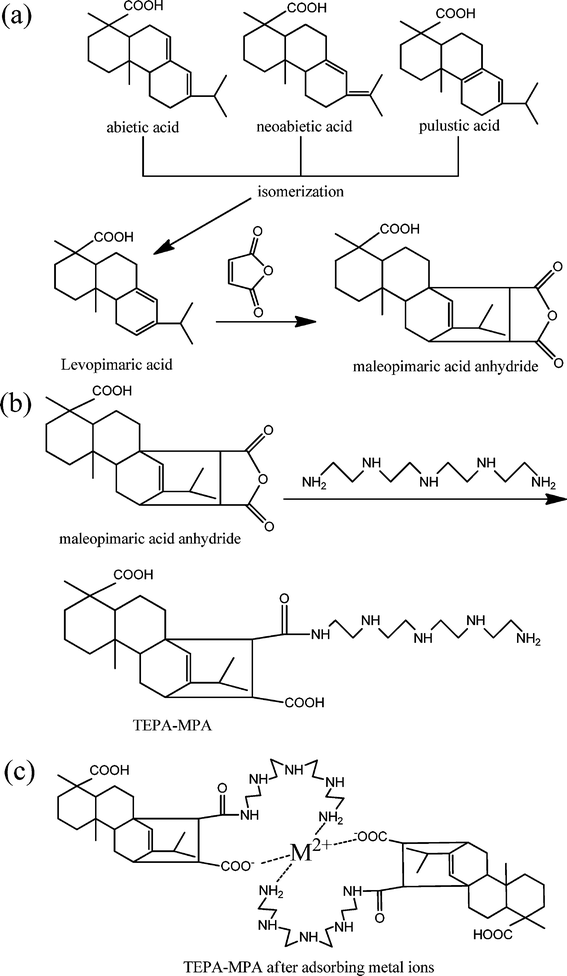 | ||
| Fig. 1 (a) Synthesis of maleopimaric acid anhydride (MPA) from rosin and maleic anhydride; (b) synthesis of TEPA-MPA from MPA and tetraethylenepentamine; (c) TEPA-MPA after absorbing metal ions. | ||
Characterisation
Pyrene was first dissolved in tetrahydrofuran (THF) to get a solution of 3.0 × 10−2 M, and the pyrene/THF solution was then diluted in triple distilled water to make pyrene concentration of 1.2 × 10−7 M. To remove THF, the diluted solution was kept under vacuum at 30 °C for 3 h. In the fluorometric measurement, the pyrene solution was further diluted to pyrene concentration of 6.0 × 10−7 M by adding the same volume of sample solution (with TEPA-MPA concentration from 5.12 × 10−5 mg mL−1 to 2 mg mL−1). The fluorescence emission spectra of pyrene in the presence of various concentration of TEPA-MPA were recorded at an excitation wavelength of 330 nm. The slit widths were set at 5.0 nm for both emission and excitation, and the excitation and emission wavelengths were 360 and 450 nm, respectively.
Result and discussion
Synthesis of MPA
Although 90% of the constituents of resin are acidic, the significant structures of rosin include abietic acid with conjugated double bonds, and pimaric acid with non-conjugated double bonds; only levopimaric acid can directly react with maleic anhydride to obtain MPA via the Diels–Alder cycloaddition reaction because of its conjugated double bonds. Other acids were transferred into levopimaric acid through isomerization, which was performed in a vacuum sealed tube at high temperature. As soon as the transfer process finished, it would react with maleic anhydride to form the intermediate MPA (maleopimaric acid anhydride).FT-IR analysis of TEPA-MPA
Fig. 2 shows the FT-IR spectra of rosin, MPA and TEPA-MPA. The peak of rosin at 1697 cm−1 is assigned to the stretching vibrations of the carboxylic carbonyl groups (Fig. 2a). It is notable that after reacting with maleic anhydride, two peaks at 1849 and 1780 cm−1 appear on the spectrum of MPA, which correspond to the anhydride C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch (Fig. 2b). The conjugation of TEPA with MPA results in the production of a large number of free imine groups (–NH2). As can be seen from the spectrum of TEPA-MPA, the significant bands of 1631 and 1572 cm−1 are assigned to primary (VC
O stretch (Fig. 2b). The conjugation of TEPA with MPA results in the production of a large number of free imine groups (–NH2). As can be seen from the spectrum of TEPA-MPA, the significant bands of 1631 and 1572 cm−1 are assigned to primary (VC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and secondary amide peaks, respectively; the increasing intensity of the peek at 3433 cm−1 is assigned to the stretching vibrations of –NH2 overlapped with that of –OH. Furthermore, the new peak at 1128 cm−1 corresponds to the stretching vibrations of C–N of TEPA, and new peaks at 817 and 770 cm−1 are attributed to the wag vibrations of N–H. These characteristic peaks indicate that TEPA-MPA was synthesized successfully. The introduction of a carboxyl group and abundant free amine groups not only improves the solubility of rosin, but also serves as a functional group for adsorbents.
O) and secondary amide peaks, respectively; the increasing intensity of the peek at 3433 cm−1 is assigned to the stretching vibrations of –NH2 overlapped with that of –OH. Furthermore, the new peak at 1128 cm−1 corresponds to the stretching vibrations of C–N of TEPA, and new peaks at 817 and 770 cm−1 are attributed to the wag vibrations of N–H. These characteristic peaks indicate that TEPA-MPA was synthesized successfully. The introduction of a carboxyl group and abundant free amine groups not only improves the solubility of rosin, but also serves as a functional group for adsorbents.
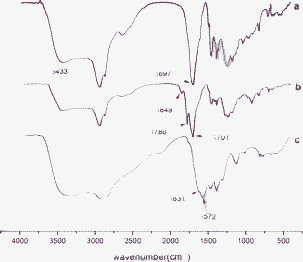 | ||
| Fig. 2 FT-IR spectra of (a) rosin; (b) MPA; (c) TEPA-MPA. | ||
Size and morphology
Fig. 3 presents the sizes of the micelles examined by DLS. The sizes of the micelles have a narrow distribution with a peak at 96.5 nm. The morphology of TEPA-MPA micelles is depicted by TEM images as displayed in Fig. 4(A). As can be seen from the TEM image, the micelles have a shape of approximately regular spherical with sizes in the range of 10 to 30 nm. The reason for the small size observed in TEM is that the samples are dehydrated during the preparation process of TEM, and the micelles shrank.24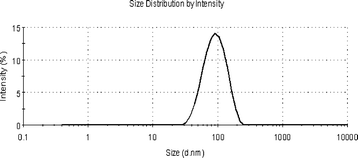 | ||
| Fig. 3 The size of TEPA-MPA. | ||
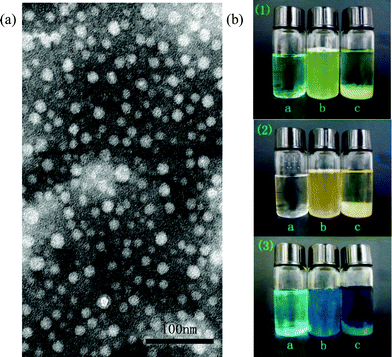 | ||
| Fig. 4 (A) TEM image of TEPA-MPA, (B) photos of adsorption experiments: (1) Ni(II); (2) Cd(II); (3) Cu(II); and (a) metal ions solution; (b) the moment of addition TEPA-MPA; (c) after standing overnight. | ||
Fig. 4(B) presents optical images of three metal ion solutions before and after the addition of TEPM-MPA. From top to bottom, the metal ions presented are Ni(II), Cd(II) and Cu(II), respectively. In each photo, bottle (a) was loaded with a pure metal ion solution. After the addition of TEPA-MPA solution, the solutions became opaque, and darker colours were presented compared to those of the corresponding pure metal solutions (Fig. 4(B) (b). The opaque appearance of the mixed solution is attributed to the formation of a coordination compound of metal ions and TEPA-MPA. Once the adsorbent contacts with metal ions, the joint bond between metal ions and adsorbent functional groups (–NH3+ and –COOH) on TEPA-MPA are formed by lone pair electrons, resulting in large-sized coordination compound precipitating in the solution. The darker colour of the mixed solution can be ascribed to the tan color of the TEPA-MPA solution. After 16 h, the adsorbate precipitated and the solution recovered to pellucid again with a darker colour than that of the pure metal solution (Fig. 4(B) (c). The sedimentation can be visually observed on the bottom of the bottle.
CMC measurement
The critical micelle concentration (CMC) of TEPA-MPA in water was determined by using 6.0 × 10−7 M pyrene as fluorescence probe. The pyrene displays weak fluorescence intensity in polar media (i.e. water) due to its poor solubility. In the presence of TEPA-MPA, when TEPA-MPA micelles formed in water, the pyrene accumulates in the hydrophobic cores of micelles, which exhibits strong emission, thus the CMC of TEPA-MPA can be determined. Due to the sensitivity of fluorescent spectra, subtle change of its surrounding can be perceived. The intensity of the first vibrational peak of pyrene at 372 nm to the third vibrational peak at 383 nm, I372/I383 can be used to determine the value of CMC.25,26 The fluorescence emission spectra of pyrene in various concentrations of TEPA-MPA at 25 °C were recorded (experimental data were not shown). The I372/I383 value versus concentration of TEPA-MPA was plotted in Fig. 5. As shown in the figure, the I372/I383 values are relatively stable in the low concentration TEPA-MPA solution. As soon as the concentration reaches the value of CMC, the values decrease greatly with increasing concentration. Therefore, the intersection of two straight lines (I372/I383) is used to determine the CMC of micelles.26 The CMC value of TEPA-MPA is determined to be 6.575 × 10−4 mg mL−1. The low CMC value of TEPA-MPA reveals its great capability of self-aggregation and excellent stability in aqueous solution. This may indicate that the derivative could form stable nano-micelles, even in low concentrations. Generally, surfactants based on small molecules have no definite CMC value, and their nano-size self-assemby morphology can not be observed by TEM images. The bulky ring structure and methyl groups on TEPA-MPA result in intense intermolecular hydrophobic interaction that leads to a solid, compact core of micelles, and the hydrophilic imine, amino and carboxyl groups stretch in water, making the micelles stable in water. The compact, stable structure of micelles may contribute to the formation of stable nano-micelles, even at low concentration.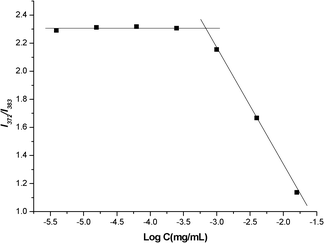 | ||
| Fig. 5 Change of intensity ratio (I372/I383) for pyrene in water with various concentrations of TEPA-MPA. (Change of fluorescence intensity ratio (I372/I383) of pyrene in water as a function of TEPA-MPA concentration.) | ||
FT-IR analysis of TEPA-MPA before and after adsorbing metal ions
The metal-chelated deposits were filtrated, dried and diluted with KBr for FT-IR measurement. There were significant changes in the FT-IR spectra after adsorbing metal ions as can be seen in Fig. 6. The peak of N–H at 1574 cm−1 shifts to a lower wavenumber, and the intensity of the N–H bending vibration peaks at 817 cm−1 and 770 cm−1 decreases noticeably. Besides, the C–N stretching adsorption at 1128 cm−1 is hardly observed. These results indicate that the –NH2– participates in the adsorbing of metal ion. On the other hand, the peak of anti-symmetric stretching vibration of –COOH at 1631 cm−1 shifts to higher frequency (1695–1701 cm−1) due to the electronic effect (I effect) between metal ions and TEPA-MPA. The I effect changes the distribution of the electron cloud as well as the force constant, which finally leads to the shift of characteristic peaks. Moreover, the more electronegative the atom or group augmentations are, the stronger the I effect becomes. It is known that the electronegativities of these three metal ions display an increasing succession Cd(II) < Ni(II) ≈ Cu(II).27 This characteristic property of the metal ions can also be testified by the differences in the FT-IR spectra: both Cu(II) and Ni(II) have strong peaks at 1701 cm−1 while the intensity of this peak of Cd(II) is normal.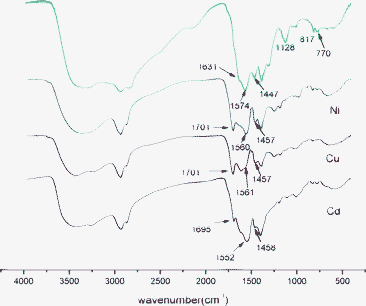 | ||
| Fig. 6 FT-IR spectra of TEPA-MPA before and after adsorbing metal ions. | ||
The wavenumber difference (Δvalues) between anti-symmetric stretching vibration (1631–1701 cm−1) and symmetric stretching vibration (around 1447 cm−1) of carboxyl group is calculated by subtracting the symmetric wavenumber from the anti-symmetric wavenumber, which can be used to determine the structure of carboxylates that exists in the TEPA-MPA–metal ions complex. For the unloaded TEPA-MPA, and the Ni, Cu and Cd loaded TEPA-MPA, the Δvalues are 184 cm−1, 244 cm−1, 244 cm−1 and 237 cm−1, respectively. It is significant that the Δvalue of the initial TEPA-MPA (184 cm−1) is smaller than those of metal ion adsorbed TEPA-MPA (244 cm−1, 244 cm−1 and 237 cm−1), indicating the existence of unidentate carboxylates rather than bidentate carboxylates in the complex after metal adsorption, which is attributed to the involvement of amine in adsorbing behaviour.28 It is known that a metal ion (or a proton) can be bound to N and O atoms via sharing electron pair, since the nucleus of N atoms has a weaker attraction to lone pair of electrons, N atoms have higher tendency than O atoms to donate lone pair of electrons to share with metal ions to form a complex, which leads to dominant adsorption between amine and metal ions in the system, and the binding between metal ion and COOH is reduced.29
XPS studies
The wide scan XPS spectra of TEPA-MPA before and after metal ions absorption were recorded, which are displayed in Fig. 7. The presence of new peaks at 856.03, 934.71 and 577.30 eV in the spectra of TEPA-MPA after metal ions adsorption are ascribed to Ni(II), Cu(II) and Cr(III), suggesting that metal ions are adsorbed.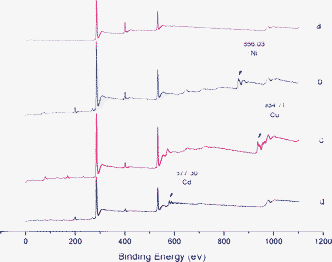 | ||
| Fig. 7 The wide scan XPS spectra of (a) TEPA-MPA; (b) TEPA-MPA after adsorption of Ni(II); (c) TEPA-MPA after adsorption of Cu(II); (d) TEPA-MPA after adsorption of Cr(II). | ||
Fig. 8 depicts the high resolution XPS spectra of N 1 s. As shown in Fig. 8a, 8b, 8c and 8d, the peaks at 398.89 and 400.95 eV correspond to the N atom of –NH2– and amino group of TEPA in the deconvoluted N 1 s spectrum of TEPA-MPA. The content of the –NH2– group is stronger than that of the amino group because the amount of –NH2– is larger than that of NH3+ in TEPA. Clearly, the peaks at 398.89 and 400.95 eV shift to higher BE values after adsorbing metal ions, the reason might be that the covalent bond between metal ions and N, which is formed by accepting a lone pair of electrons from the N atom, results in the decrease of the electron cloud density of the N atom.13,29,30 The structure of R-NH2M2+ is likely to exist after metal sorption, which is shown as follows:
| R-NH2 + M2+ → R-NH2M2+ | (1) |
| R-NH3+ + M2+ → R-NH2M2+ + H+ | (2) |
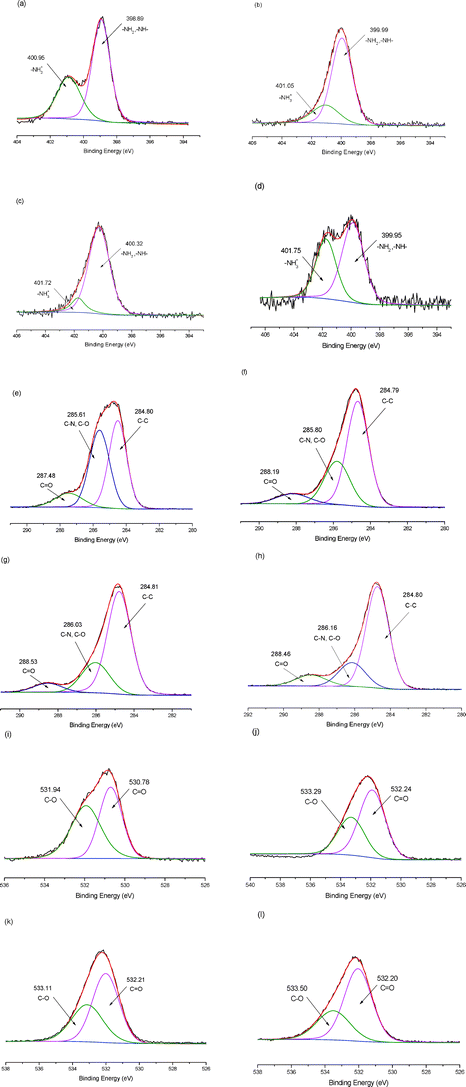 | ||
| Fig. 8 XPS spectra of adsorbent: (a) N 1s of TEPA-MPA; (b) N 1s of TEPA-MPA after adsorption of Ni(II); (c) N 1s of TEPA-MPA after adsorption of Cu(II); (d) N 1s of TEPA-MPA after adsorption of Cr(III); (e) C 1s of TEPA-MPA; (f) C 1s of TEPA-MPA after adsorption of Ni(II); (g) C 1s of TEPA-MPA after adsorption of Cu(II); (h) C 1s of TEPA-MPA after adsorption of Cr(III); (i) O 1s of TEPA-MPA; (j) O 1s of TEPA-MPA after adsorption of Ni(II); (k) O 1s of TEPA-MPA after adsorption of Cu(II); (l) O 1s of TEPA-MPA after adsorption of Cr(III). | ||
Fig. 8e, 8f, 8g and 8h depict the deconvolution of C 1 s spectra of samples. The peaks of TEPA-MPA at 284.80 eV, 285.63 eV and 287.5 eV are attributed to the carbon atom in forms of C–C, C–N and C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively. It is noticeable that both the peaks of C–O and C
O, respectively. It is noticeable that both the peaks of C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O which are ascribed to –COOH, move to higher BE peaks significantly after adsorption of metal ions. But the peak of C–C almost does not alter, and the peaks of C–N and C–O become broad. Besides, as shown in Table S1 (ESI†), the alteration of related content of C–O (43.78% to 28.87%, 22.90% and 18.78%) is larger than that of C
O which are ascribed to –COOH, move to higher BE peaks significantly after adsorption of metal ions. But the peak of C–C almost does not alter, and the peaks of C–N and C–O become broad. Besides, as shown in Table S1 (ESI†), the alteration of related content of C–O (43.78% to 28.87%, 22.90% and 18.78%) is larger than that of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (10.7% to 8.61%, 7.01% and 11.35%). This might be attributable to the decrease of the electron cloud density of the adjacent carbon atom in C
O (10.7% to 8.61%, 7.01% and 11.35%). This might be attributable to the decrease of the electron cloud density of the adjacent carbon atom in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O, caused by the oxygen atom electron provision to metal ions; the tendency of metal ions that bond to the C–O is much stronger than that of C
O and C–O, caused by the oxygen atom electron provision to metal ions; the tendency of metal ions that bond to the C–O is much stronger than that of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, because the tendency of metal ions to bind with O atoms is weaker than that of N atoms in the presence of –NH2– and NH3+, resulting in the formation of unidentate carboxylates. This agrees with results of FTIR.31 Hence, –COOH is functional in metal ion adsorption as well.
O, because the tendency of metal ions to bind with O atoms is weaker than that of N atoms in the presence of –NH2– and NH3+, resulting in the formation of unidentate carboxylates. This agrees with results of FTIR.31 Hence, –COOH is functional in metal ion adsorption as well.
Fig. 8h, 8i, 8j and 8k display the O 1 s spectra of samples. The peaks at 530.78 eV and 531.94 eV, which are assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O or OH, that originated from oxygen atoms move to a markedly higher BE value.30 The relative content of peaks of C–O decrease and the peaks become broad. These changes are similar to that of O 1 s spectra because the bond between the metal ion and O atoms of C–O share the lone pair electrons from O atoms and lead to the decrease of electron density. This is in accordance with the results of the C 1 s study and FTIR analysis.
O and C–O or OH, that originated from oxygen atoms move to a markedly higher BE value.30 The relative content of peaks of C–O decrease and the peaks become broad. These changes are similar to that of O 1 s spectra because the bond between the metal ion and O atoms of C–O share the lone pair electrons from O atoms and lead to the decrease of electron density. This is in accordance with the results of the C 1 s study and FTIR analysis.
We have also investigated the difference of valence states of metal ion (II, III) adsorption in the XPS study. In the N 1 s spectra of TEPA-MPA after adsorption of Ni(II) and Cu(II), the peak of NH3+ becomes broad and its related content (24.37%, 12.2%) is smaller than that of original adsorbent (35.85%), suggesting that the R-NH2M2+ structure of eqn (2) plays a dominate role in adsorbent behavior. But after adsorption of Cr(III), the peak of NH2 becomes stronger and its related content (40.92%) is also bigger than that of the original adsorbent (35.85%). This might be because there are several different kinds of ligand structures in solution such as [CrCl2(H2O)4)]+, [CrCl(H2O)5]2+ and [CrCl3(H2O)3]+. In view of the stronger electronegativity between Cr(III) and TEPA-MPA, as well as the reasons mentioned in the FTIR analysis and C 1 s, there is a stronger tendency to bind to –NH2– than to –NH3+ to form a stable metal–absorbent complex. As compared with C 1 s and O 1 s spectra of TEPA-MPA, after adsorption of Ni(II) and Cu(II), it can be also found that the related content of peak of C–O of Cr(III) decreases more significantly, and the peak becomes much broader than that of metal ions (II), due to its stronger electronegativity and higher electron cloud density.
Adsorption experiments
The adsorption capacity of TEPA-MPA for Ni(II), Cu(II) and Cd(II) ions in different initial metal element concentration solutions is shown in Table 1. The adsorbent has different affinities to these three ions. TEPA-MPA has a slightly higher removal efficiency for Ni(II) than for Cu(II) and Cd(II), while for the latter two ions, TEPA-MPA exhibits quite similar adsorption capacities. There are only slight decreases of removal efficiency (%) when the metal ion concentration increases. This is because that part of heavy metal ions might bind with water,32 as shown in eqn (3), resulting in the formation of insoluble oxidation. Hence, it is difficult to remove the metal ion completely even though excess absorbent is applied| 2M2+ + 3H2O = M2O3 + 6H+ + 2e− | (3) |
| Initial metal element concentration (mg L−1) | Adsorption capacity (mg g−1) | Removal efficiency (%) | ||||
|---|---|---|---|---|---|---|
| Ni(II) | Cu(II) | Cd(II) | Ni(II) | Cu(II) | Cd(II) | |
| a Adsorbent concentration = 1 g L−1, contact time = 24 h, T = 303 K, pH = 5. | ||||||
| 40 | 348.16 | 306.88 | 293.74 | 87.08 | 76.72 | 73.44 |
| 60 | 510.43 | 448.50 | 432.58 | 85.07 | 74.75 | 72.10 |
| 80 | 680.67 | 588.34 | 574.82 | 85.08 | 73.54 | 71.85 |
| 100 | 842.16 | 732.28 | 712.54 | 84.22 | 73.23 | 71.25 |
In fact, it also indicates that the adsorbent can keep the high adsorption rate as long as the residual metal ion concentration is above the threshold of saturated adsorption, and the novel micelle is prone to absorb large amounts of metal ions from highly concentrated metal solutions because of the abundant functional groups that distribute on the surface. Therefore, the absorbent is vigorous to deal with high concentrations of metal ions in waste water.
Generally, the solution pH value plays a dominant role in the adsorption due to its effect on ionic modality. The pH values of the solutions were set from 3 to 7, and the adsorption results are presented in Table 2. Contrary to general thought, the variations of Ni(II) and Cu(II) adsorption at different pH are not significant, whereas Cd(II) adsorption has a slight increase when the pH value changes from pH 3 to pH 7. It illustrates that the adsorption of metal ions by TEPA-MPA is not pH dependent. The pH independence of the adsorption is contributed to by the amphiphilic property of TEPA-MPA. TEPA-MPA contains both cationic groups (amino and imino) and anionic groups (carboxyl). At low pH value, the moiety imine would be fully protonated so that adsorption of metal ions is mainly attributed to ion-exchange reaction.33 In addition, the existing H+ prevents –COOH from ionizing, resulting in competition with M2+. Consequently, these lead to low removal efficiency of metal ions.34 In the range of pH 5 to 7, –NH2– is not protonated due to low concentration of H+, while –COOH is easy to ionized, therefore the reaction between –COOH and metal ions becomes the main binding reaction. Moreover, the maximum adsorption may be partly attributed to the partial hydrolysis of metal ions caused by the formation of M(OH)+ and M(OH)2. With the increase of pH, the metal exists in the form of M(OH)2 in the solution, which leads to the enhancement of metal ion adsorption.31 Therefore, with an appropriate pH, the adsorption mechanism of TEPA-MPA is comprised of a synergistic effect of coordination reaction, ion-exchange and metal hydroxide precipitation.
| pH | Adsorption capacity (mg g−1) | ||
|---|---|---|---|
| Ni(II) | Cu(II) | Cd(II) | |
| a Adsorbent concentration = 1 g L−1, initial metal element concentration = 100 mg L−1, contact time = 24 h, T = 303 K | |||
| 3 | 770.89 | 681.64 | 564.53 |
| 4 | 792.51 | 702.56 | 582.13 |
| 5 | 816.22 | 721.70 | 653.36 |
| 6 | 826.26 | 742.68 | 704.63 |
| 7 | 829.05 | 761.25 | 723.45 |
As seen from Table 3, the adsorbent dosage also influences the adsorption of metal ions. With increasing adsorbent concentration, the removal efficiency (%) raises slightly. The reason is similar to that of the incomplete removal of metal ions. The insoluble oxidate of the metal ion, which originated from the combination of metal ions and water molecules, is difficult to be bound by the functional groups on the adsorbent surface, as a result, the concentrations of residual metal ions in the solution after filtration of the metal–adsorbent complex do not decrease significantly, even with increasing the dosage of adsorbent. The removal efficiency also follows the sequence Ni(II) > Cu(II) > Cd(II). Abundant –NH2– and –COOH groups provide great availability of ion-exchange and abundant ligand sites for the removal of metal ions.35 However, the fast equilibrium ability of TEPA-MPA made less exchanges between adsorptions.
| Adsorbent concentration (g L−1) | Adsorption capacity (mg g−1) | Removal efficiency (%) | ||||
|---|---|---|---|---|---|---|
| Ni(II) | Cu(II) | Cd(II) | Ni(II) | Cu(II) | Cd(II) | |
| a Initial metal element concentration = 100 mg L−1, contact time = 24 h, T = 303 K, pH = 5. | ||||||
| 0.5 | 1655.08 | 1443.70 | 1388.48 | 82.75 | 72.18 | 69.42 |
| 1.0 | 845.36 | 736.76 | 712.54 | 84.53 | 73.68 | 71.25 |
| 1.5 | 572.99 | 502.15 | 491.64 | 85.95 | 75.32 | 73.74 |
The adsorption of Ni(II), Cu(II) and Cd(II) was investigated at different time and temperature intervals. As shown in Fig. 9, the adsorption of metal ions is increased when the contact time increases, and the maximum are approached at 48 h (the data over 48 h is not shown). The 72 hour-long experimental data which is not displayed in the figure indicates negligible adsorption after 48 h. The adsorbate molecules are diffused sufficiently on the adsorbent during the short standing time. With the gradual binding of adsorbent functional groups with metal ions, the availability of adsorption sites decreases, and the adsorption process reaches kinetic equilibrium.36 The adsorption capability diminishes as temperature rises, which is contrary to the normal theory that higher temperature leads to accelerated diffusion of solute and thus stronger adsorption.37 It is well known that micelles are more stable at low temperature, as the temperature rises, the amount of active adsorption on TEPA-MPA decreases, which in turn leads to a slightly decreased adsorption ability.
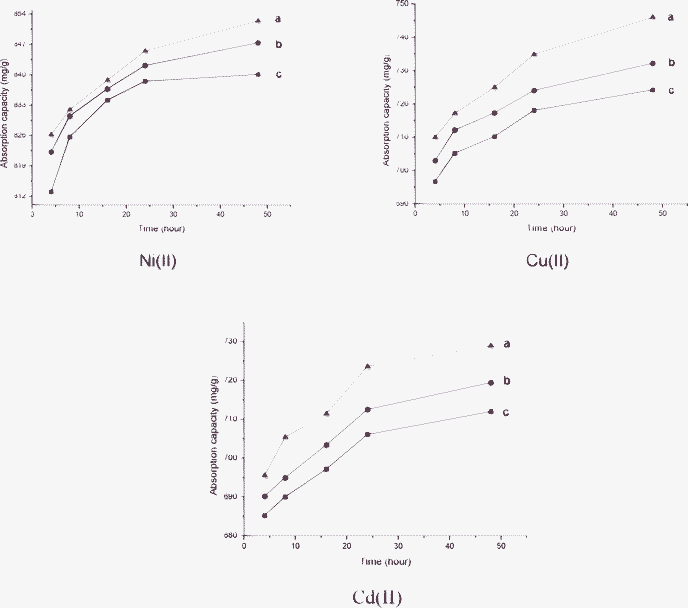 | ||
| Fig. 9 Effect of contact time and temperature on TEPA-MPA adsorption: (a) 288 K; (b) 303 K; (a) 318 K (adsorbent concentration = 1 g L−1, initial concentration = 100 mg L−1, pH = 5). | ||
Table 4 presents the results of competitive adsorption of Ni(II), Cu(II) and Cd(II) ions by TEPA-MPA from a mixed metal solution. TEPA-MPA showed the highest affinity for Ni(II) with the lowest Cd(II) adsorption. It is noted that the more charge and smaller ionic semidiameter the metal ion has, the stronger the affinity is between the adsorbent and adsorbate.38 The ions used have the same charge density, but the ionic semidiameters are different, with a succession Ni(II) < Cu(II) < Cd(II). Thus, in a mixed metal solution, Ni(II) has the strongest affinity to the TEPA-MPA, followed by Cu(II) and Cd(II).
| Metal ions | Initial metal element concentration (mg mL−1) | Removal efficiency (%) |
|---|---|---|
| a Adsorbent concentration = 1 g L−1, contact time = 24 h, T = 303 K, pH = 5. | ||
| Ni(II) | 35.542 | 93.136 |
| Cu(II) | 33.326 | 72.340 |
| Cd(II) | 34.510 | 64.600 |
As expected, the adsorption capacities range from 293.74 mg g−1 to 1655.08 mg g−1 in different experiment conditions (Table 1–4). All the data suggests that TEPA-MPA has an outstanding adsorption capacity for metals. They are higher than that of Fe3O4 magnetic nanoparticles with humic acid (46.3 to 97.7 mg g−1, size: 140 nm).11 They are also much higher than that of adsorbents based on natural materials, such as chitosan (153.85 mg g−1)8 and marine algae (285 mg g−1).10 This might be because of the tremendous surface area provided by small sized micelles, containing plentiful amine, imine and carboxyl groups on the surface to serve as functional groups to bond with large amount of metals.
Conclusions
In this paper, a micelle based on a rosin derivative (TEPA-MPA) was used to remove metal ions. It has minute size (96.5 nm) and low CMC value (6.575 × 10−4 mg mL−1). Its stable nano-size self-assembly morphology, observed by TEM, indicates that this kind of rosin derivative, unlike other low molecular surfactants, possesses great self-assembly capabilities and the excellent stability of micelles in aqueous solution. This also suggests that the derivative could form stable nano-micelles even in low concentrations. Its structure was characterized by FT-IR. The adsorbent mechanism of Cu(II), Ni(II), Cd(II) and Cr(III) by TEPA-MPA were investigated by FT-IR and XPS, revealing that both carboxyl groups and amino groups were involved in metal adsorption.The metal absorption experiment data show that it has outstanding absorption capacities which range from 293.74 to 1655.08 mg g−1 in different experiment conditions. All the results depict that the adsorption followed the sequence Ni(II) > Cu(II) > Cd(II), and Ni(II) has the highest adsorption in the mixed metal ion solution. Besides, the results prove that low metal ion and adsorbent concentration are beneficial to the adsorption, and the adsorbtion ability of TEPA-MPA has less dependence on the solution pH value, contact time, and temperature. It can be concluded that TEPA-MPA has a high stability and excellent metal absorption capacities, which can be a potent potential absorbent for the removal of metal ions.
Acknowledgements
The authors thanks financial support provided by the National Science Foundation of China (20874032) and the Team Program of the Natural Science Foundation of Guangdong Province, China (S2011030003134).References
- J. O. Nriagu and J. M. Pacyna, Quantitative assessment of worldwide contamination of air, water and soils by trace metals, Nature, 1988, 333, 134–139 CrossRef CAS
.
- W. Li, H. Zhao, P. R. Teasdale, R. John and S. Zhang, Application of a cellulose phosphate ionexchangemembrane as a bindingphase inthe diffusive gradients in thin films technique for measurement of trace metals, Anal. Chim. Acta, 2002, 464, 331–339 CrossRef CAS
.
- N. Meunier, P. Drogui, C. Montané, R. Hausler, G. Mercier and J. F. Blais, Comparison between electrocoagulation and chemical precipitation for metals removal from acidic soil leachate, J. Hazard. Mater., 2006, 137, 581–590 CrossRef CAS
.
- M. Alkan and M. Dogan, Adsorption of copper(II) onto perlite, J. Colloid Interface Sci., 2001, 243, 280–291 CrossRef CAS
.
- S. Bag, P. N. Trikalitis, P. J. Chupas, G. S. Armatas and M. G. Kanatzidis, Porous semiconducting gels and aerogels from chalcoenide clusters, Science, 2007, 317, 490–493 CrossRef CAS
.
- M. J. Manos, C. D. Malliakas and M. G. Kanatzidis, Heavy-metal-ion capture, ion-exchange, and exceptional acid stability of the open-framework chalcogenide (NH4)4In12Se20, Chem.–Eur. J., 2007, 13, 51–58 CrossRef CAS
.
- S. H. Hasan, K. K. Singh, O. Prakash, M. Talat and Y. S. Ho, Removal of Cr(VI) from aqueous solutions using agricultural waste ‘maize bran’, J. Hazard. Mater., 2008, 152, 356–365 CrossRef CAS
.
- V. M. Boddu, K. Abburi, J. L. Talbott and E. D. Smith, Removal of hexavalent chromium from wastewater using a new composite chitosan biosorbent, Environ. Sci. Technol., 2003, 37(19), 4449–4456 CrossRef CAS
.
- R. Celis, M. C. HermsÍn and J. Cornejo, Heavy metal adsorption by functionalized clays, Environ. Sci. Technol., 2000, 34(21), 4593–4599 CrossRef CAS
.
- R. Jalali, H. Ghafourian, Y. Asef, S. J. Davarpanah and S. Sepehr, Removal and recovery of lead using nonliving biomass of marine algae, J. Hazard. Mater., 2002, 92(3), 253–262 CrossRef CAS
.
- X. Wang, Y. Zheng and Y. Wang, Fast removal of copper ions from aqueous solution by chitosan-g-poly(acrylic acid)/attapulgite composites, J. Hazard. Mater., 2009, 168, 970–977 CrossRef CAS
.
- G. Para, E. Jarek and P. Warszynski, The Hofmeister series effect in adsorption of cationic surfactants—theoretical description and experimental results, Adv. Colloid Interface Sci., 2006, 122, 39–55 CrossRef CAS
.
- J. F. Liu, Z. S. Zhao and G. B. Jiang, Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water, Environ. Sci. Technol., 2008, 42, 6949–6954 CrossRef CAS
.
- G. B. Jiang, D. Quan, K. Liao and H. Wang, Novel polymer micelles prepared from chitosan grafted hydrophobic palmitoyl groups for drug delivery, Mol. Pharmaceutics, 2006, 3(2), 152–160 CrossRef CAS
.
- F. Henselwood, G. Wang and G. Liu, Removal of perylene from water using block copolymer nanospheres or micelles, J. Appl. Polym. Sci., 1998, 70, 397–408 CrossRef CAS
.
- M. Porras-Rodriguez and F. I. Talens-Alesson, Removal of 2,4-Dichlorophenoxyacetic Acid from Water by Adsorptive Micellar Flocculation, Environ. Sci. Technol., 1999, 33, 3206–3209 CrossRef CAS
.
- C. N. Mulligan, R. N. Yong, B. F. Gibbs, S. James and H. P. J. Bennett, Metal removal from contaminated soil and sediments by the biosurfactant surfactin, Environ. Sci. Technol., 1999, 33(21), 3812–3820 CrossRef CAS
.
- K. D. Pennell, L. M. Abriola and W. J. Weber, Jr., Surfactant-enhanced solubilization of residual dodecane in soil columns. 1. Experimental investigation, Environ. Sci. Technol., 1993, 27(12), 2332–2340 CrossRef CAS
.
-
P. N. Hemmings and L. Wang, Rosin ester derivative as surfactants, United States Patent, 1996, No. 5, 552, p. 519 Search PubMed
.
- P. M. Satturwar, S. V. Fulzele, J. Panyamb, P. M. Mandaogade, D. R. Mundhada, B. B. Gogte, V. Labhasetwar and A. K. Dorle, Evaluation of new rosin derivatives for pharmaceutical coating, Int. J. Pharm., 2004, 270, 27–36 CrossRef CAS
.
- C. E. Beneke, A. M. Viljoen and J. H. Hamman, Polymeric plant-derived excipients in drug delivery, Molecules, 2009, 14, 2602–2620 CrossRef
.
- J. S. Lee and S. I. Hong, Synthesis of acrylic rosin derivatives and application as negative photoresist, Eur. Polym. J., 2002, 38(2), 387–392 CrossRef CAS
.
- S. Kwon, J. H. Park, H. Chung, I. C. Kown and S. Y. Jeong, Physicochemical characteristics of self-assembled nanoparticles based on glycol chitosan bearing 5β-cholanic acid, Langmuir, 2003, 19(24), 10188–10193 CrossRef CAS
.
- Y. S. Wang, L. R. Liu, J. Weng and Q. Q. Zhang, Preparation and characterization of self-aggregated nanoparticles of cholesterol-modified O-carboxymethyl chitosan conjugates, Carbohydr. Polym., 2007, 69(3), 597–606 CrossRef CAS
.
- C. L. Zhao, M. A. Winnik, G. Riess and M. D. Croucher, Fluorescence probe techniques used to study micelle formation in water-soluble block copolymers, Langmuir, 1990, 6, 514–516 CrossRef CAS
.
- M. Whihelm, C. L. Zhao, Y. Wang, R. Xu, M. A. Winnik, J. L. Mura, G. Riess and M. D. Croucher, Poly(styrene ethyleneoxide) block copolymer micelle formation in water: a fluorescence probe study, Macromolecules, 1991, 24, 1033–1040 CrossRef
.
- T. B. Kinraide and U. A. Yermiyahu, A scale of metal ion binding strengths correlating with ionic charge, Pauling electronegativity, toxicity, and other physiological effects, J. Inorg. Biochem., 2007, 101, 1201–1213 CrossRef CAS
.
- C. Oldham, Complexes of simple carboxylic acids, Prog. Inorg. Chem., 1968, 10, 223–250 CrossRef CAS
.
- L. Jin and R. Bai, Mechanisms of lead adsorption on chitosan/PVA hydrogel beads, Langmuir, 2002, 18(25), 9765–9770 CrossRef CAS
.
- H. Liu, F. Yang, Y. Zheng, J. Kang, J. Qu and J. P. Chen, Improvement of metal adsorption onto chitosan/sargassum sp. composite sorbent by an innovative ion-imprint technology, Water Res., 2011, 45, 145–154 CrossRef CAS
.
- S. F. Lim, Y. M. Zheng, S. W. Zou and J. P. Chen, Characterization of copper adsorption onto an alginate encapsulated magnetic sorbent by a combined FT-IR, XPS, and mathematical modeling study, Environ. Sci. Technol., 2008, 42, 2551–2556 CrossRef CAS
.
- Z. Shi, J. T. Nurmi and P. G. Tratnyek, Effects of nano zero-valent iron on oxidation–reduction potential, Tratnyek, Environ. Sci. Technol., 2011, 45(4), 1586–1592 CrossRef CAS
.
- B. J. Rappoli and D. A. Rowley, The sorption kinetics of copper (II) on chemically modified controlled pore glass, J. Colloid Interface Sci., 2000, 226, 218–221 CrossRef CAS
.
- K. Kadirvelu, K. Thamaraiselvi and C. Namasivayam, Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste, Bioresour. Technol., 2001, 76, 63–65 CrossRef CAS
.
- A. K. Meena, G. K. Mishra, P. K. Rai, C. Rajagopal and P. N. Nagar, Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent, J. Hazard. Mater., 2005, 122, 161–170 CrossRef CAS
.
- B. Yu, Y. Zhang, A. Shukla, S. S. Shukla and K. L. Dorris, The removal of heavy metal from aqueous solutions by sawdust adsorption—removal of copper, J. Hazard. Mater., 2000, 80, 33–42 CrossRef CAS
.
- A. M. El-Kamash, A. A. Zaki and M. A. El-Geleel, Modeling batch kinetics and thermodynamics of zinc and cadmium ions removal from waste solutions using synthetic zeolite A, J. Hazard. Mater., 2005, 127, 211–220 CrossRef CAS
.
- R. Han, J. Zhang, W. Zou, H. Xiao, J. Shi and H. Liu, Biosorption of copper(II) and lead(II) from aqueous solution by chaff in a fixed-bed column, J. Hazard. Mater., 2006, 133(1–3), 262–268 CrossRef CAS
.
Footnotes |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra20767b/ |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2012 |
