Ligand-free coupling of phenols and alcohols with aryl halides by a recyclable heterogeneous copper catalyst†
Man
Wang
,
Bizhen
Yuan
,
Tongmei
Ma
,
Huanfeng
Jiang
and
Yingwei
Li
*
School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou, 510640, China. E-mail: liyw@scut.edu.cn
First published on 24th May 2012
Abstract
A novel and efficient heterogeneous catalyst system based on CuI immobilized on a MOF material for the O-arylation of phenols and alcohols with aryl halides under ligand-free conditions is reported.
Diaryl ethers and alkyl aryl ethers are common structural units found in many natural products, important pharmaceutically active compounds, and polymers in the material science industries.1 Over the past few decades, the experimental synthesis of diaryl ethers and alkyl aryl ethers has attracted tremendous attention.2 The most straightforward way to synthesize these ethers involves the direct formation of C–O bonds by the coupling of aryl halides with alcohols/phenols. Efficient palladium-catalyzed C–O bond forming cross-couplings have been developed with the assistance of tunable ligands.3 However, the use of expensive palladium, and noncommercial and highly oxophilic phosphine ligands limits the applicability of these processes.
Towards the commercialization of these protocols, copper salts have been widely investigated as attractive alternatives to palladium catalysts in the synthesis of diaryl ethers and alkyl aryl ethers in spite of their relatively low activities as compared to the latter. During the past decade, a great deal of research effort has been devoted to the development of efficient Cu–ligand systems for these transformations.4 A number of organic additives, such as 1-naphthoic acid,5 1,10-phenanthroline,6 N,N-dimethylglycine,7 neocuproine,8 β-ketoester,9 tripod ligands,10 and 2,2,6,6-tetramethylheptane-3,5-dione,11 have been shown to be able to enhance the reaction rate with a large substrate scope. However, these homogeneous protocols suffer from the major drawback of the recyclability of the catalysts and ligands. Moreover, the presence of impurities in the final products is also a major issue regarding purification.
From economic and industrial viewpoints, heterogeneous catalysts are desirable because of the reusability of the catalysts and the easy purification of the products as compared to homogeneous catalysts. However, up to the present there are only a few reports on the employment of unsupported copper nanoparticles or immobilized copper species on solid materials (e.g. Al–hydrotalcite, Al2O3, and silica gel) as recyclable catalysts for the C–O cross-coupling reactions under ligand-free conditions.12 Moreover, these heterogeneous systems showed a limited scope of substrates for alcohols.
Herein, we report a novel, highly efficient and reusable heterogeneous catalytic system for the C–O cross-coupling of a variety of phenols and alcohols with aryl halides without the assistance of any ligands. The catalyst is based on CuI immobilized on a metal–organic framework (MOF). MOFs are known as a new class of porous materials that have exhibited interesting catalytic properties in a number of chemical conversions.13 Nevertheless, a literature survey shows that there is no example using MOFs as the catalyst in C–O bond formation between phenols/alcohols and aryl halides.
MOF-253 (Al(OH)(bpydc)) was prepared from the hydrothermal reaction of AlCl3 and 2,2′-bipyridine-5,5′-dicarboxylic acid (bpydc) (see the ESI†).14,15 In the MOF structure, the 2,2′-bipyridine (bpy) moieties are not coordinated with any metal ions. The activated MOF-253 was then soaked in a acetonitrile solution of CuI to afford MOF-253·xCuI (x, molar ratio of CuI to bpy; x = 0.5), as determined by elemental analysis (Fig. 1). The intermolecular interaction between the MOF and CuI was investigated by X-ray photoelectron spectroscopy (XPS). The binding energy of the N 1s peak of MOF-253·0.5CuI was shifted remarkably toward higher binding energy (by ca. 1.2 eV), compared to that of the pristine MOF-253. Such a big shift reflected a decrease in the electron density of the N atom,16 which may be attributed to the strong coordination between the bpy and Cu atom in the MOF-253·0.5CuI.
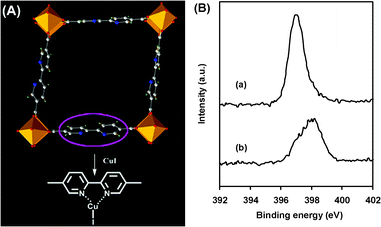 | ||
| Fig. 1 (A) Immobilization of CuI in MOF-253. The orange octahedra represent the Al atoms. Oxygen, red; nitrogen, blue; carbon, grey; hydrogen, green. (B) XPS spectra of the N 1s region for (a) MOF-253 and (b) MOF-253·0.5CuI. | ||
The reaction conditions were first optimized by using the model reaction of 4-methoxyiodobenzene with p-cresol in the presence of 20 mol% MOF-253·0.5CuI catalyst. The results presented in Table 1 indicated that both the solvent and base had a significant effect on the product yield of the reaction. When using dimethyl sulfoxide (DMSO) as solvent and Cs2CO3 as base, the reaction gave the highest yield (ca. 96%) of the cross-coupling product at 80 °C and 20 h (Table 1, entry 2). However, only a trace of the coupling product was obtained when CuI or MOF-253 was used alone under the same reaction conditions (Table 1, entries 9 and 10). The results suggest a significant promoting effect of the MOF-253 on the CuI active species. Furthermore, the catalytic efficiency was also observed to be reduced remarkably when the same amount of bpy was used to replace MOF-253 in the presence of CuI (Table 1, entry 11). This could be related to the difference in the electron configuration of the bpy motifs in the MOF from that of the single bpy molecules scattered in solution because of the presence of charge transfers between adjacent ligands and metals in the MOFs.15 The reaction could also proceed well at lower catalyst loadings (Table 1, entry 12), although a longer reaction time would be required to achieve a complete conversion.
| Entry | Catalyst | Base | Solvent | Yield (%)b |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.4 mmol), 2a (0.6 mmol), catalyst (20 mol%), base (0.8 mmol), solvent (2 mL), 80 °C, 20 h. b Yields were determined by GC-MS analysis. c Catalyst (10 mol%). | ||||
| 1 | MOF-253·0.5CuI | Cs2CO3 | acetonitrile | 2 |
| 2 | MOF-253·0.5CuI | Cs2CO3 | DMSO | 96 |
| 3 | MOF-253·0.5CuI | Cs2CO3 | toluene | 49 |
| 4 | MOF-253·0.5CuI | Cs2CO3 | 1,4-dioxane | 56 |
| 5 | MOF-253·0.5CuI | Cs2CO3 | DMA | 18 |
| 6 | MOF-253·0.5CuI | Cs2CO3 | DMF | 35 |
| 7 | MOF-253·0.5CuI | K3PO4 | DMSO | 18 |
| 8 | MOF-253·0.5CuI | t-BuOK | DMSO | 28 |
| 9 | MOF-253 | Cs2CO3 | DMSO | — |
| 10 | CuI | Cs2CO3 | DMSO | 3 |
| 11 | bpy + CuI | Cs2CO3 | DMSO | 51 |
| 12c | MOF-253·0.5CuI | Cs2CO3 | DMSO | 65 |
Under the optimized reaction conditions, the scope of this novel MOF-253·0.5CuI-catalyzed coupling of a variety of aryl iodides/bromides with phenols was investigated. As shown in Table 2, all the reactions proceeded smoothly and afforded the desired products in good to excellent yields under mild reaction conditions. The electronic and steric factors of the substituents played an important role in determining the product yield. In general, the electron-deficient phenols provided the coupling products in lower yields (e.g., entries 2 vs. 10). However, the electron-deficient aryl halides exhibited a higher activity than the electron-rich ones (e.g., entries 2 vs. 3). An increase in steric hindrance led to a slight decrease in the product yield (Table 1, entries 4–6). Generally, aryl bromides were less reactive than aryl iodides (Table 1, entries 11–15) under identical conditions. The use of aryl chlorides did not furnish the desired products under the reaction conditions.
| Entry | Aryl halide | Phenol | Product | Yield (%)b |
|---|---|---|---|---|
| a Reaction conditions: 1 (0.4 mmol), 2 (0.6 mmol), Cs2CO3 (0.8 mmol), MOF-253·0.5CuI (20 mol%), DMSO (2 mL), 80 °C, 24 h. b Isolated yield. c Reaction performed at 120 °C for 36 h. | ||||
| 1 |
 1b
1b
|
 2b
2b
|
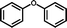 3b
3b
|
97 |
| 2 |
 1a
1a
|
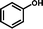 2b
2b
|
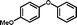 3c
3c
|
76 |
| 3 |
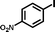 1c
1c
|
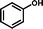 2b
2b
|
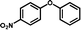 3d
3d
|
98 |
| 4 |
 1b
1b
|
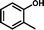 2c
2c
|
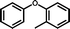 3e
3e
|
90 |
| 5 |
 1b
1b
|
 2d
2d
|
 3f
3f
|
96 |
| 6 |
 1b
1b
|
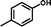 2a
2a
|
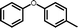 3g
3g
|
98 |
| 7c |
 1b
1b
|
 2e
2e
|
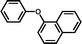 3h
3h
|
80 |
| 8 |
 1b
1b
|
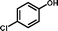 2f
2f
|
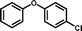 3i
3i
|
90 |
| 9 |
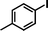 1d
1d
|
 2a
2a
|
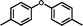 3j
3j
|
98 |
| 10 |
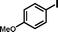 1a
1a
|
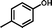 2a
2a
|
 3a
3a
|
94 |
| 11 |
 1e
1e
|
 2a
2a
|
 3g
3g
|
81 |
| 12 |
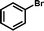 1e
1e
|
 2b
2b
|
 3b
3b
|
77 |
| 13c |
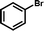 1e
1e
|
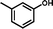 2d
2d
|
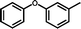 3f
3f
|
77 |
| 14c |
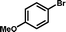 1f
1f
|
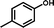 2a
2a
|
 3a
3a
|
68 |
| 15c |
 1g
1g
|
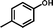 2a
2a
|
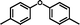 3j
3j
|
75 |
We further investigated the coupling of aryl iodides with alcohols using DMSO as solvent. It is well known that the O-arylation reaction of alcohols is much more difficult than phenols, especially in the presence of solvents.3–12 By using our catalytic system, various aliphatic alcohols, such as methanol, ethanol, n-butanol, and n-octanol, could be successfully coupled with iodobenzenes in good to excellent yields (Table 3, entries 1–6). As the carbon chain length of the alcohol increased, a slight reduction in the product yield was observed (Table 3, entries 1–4). The alcohols with aromatic rings or aliphatic rings also underwent the O-arylation smoothly and furnished good yields of the desired coupling products (Table 3, entries 7–9).
| Entry | Aryl halide | Alcohol | Product | Yield (%)b |
|---|---|---|---|---|
| a Reactions conditions: 1 (0.4 mmol), 2 (2 mmol), Cs2CO3 (0.8 mmol), MOF-253·0.5CuI (20 mol%), DMSO (2 mL), 100 °C, 24 h. b Isolated yield. c Reaction performed at 120 °C for 36 h. | ||||
| 1 |
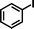 1b
1b
|
CH3OH 2g |
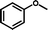 3k
3k
|
98 |
| 2 |
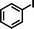 1b
1b
|
C2H5OH 2h |
 3l
3l
|
96 |
| 3 |
 1b
1b
|
n-BuOH 2i |
 3m
3m
|
86 |
| 4 |
 1b
1b
|
n-octanol 2j |
 3n
3n
|
80 |
| 5 |
 1d
1d
|
C2H5OH 2k |
 3o
3o
|
90 |
| 6 |
 1c
1c
|
C2H5OH 2l |
 3p
3p
|
97 |
| 7 |
 1b
1b
|
 2m
2m
|
 3q
3q
|
70 |
| 8c |
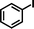 1b
1b
|
 2n
2n
|
 3r
3r
|
89 |
| 9c |
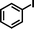 1b
1b
|
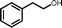 2o
2o
|
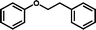 3s
3s
|
81 |
Finally, the reusability of the catalyst system was investigated (see the ESI†). After the reaction, the supernatant liquid was decanted and the catalyst was allowed to settle down. The solid catalyst was filtered and then reused in subsequent runs under identical reaction conditions. No significant efficiency loss was observed after three runs. These findings were in good agreement with AAS experiments on the reaction mixture for which a very low Cu leaching amount (<0.1% of the total Cu) was detected in solution as well as with the X-ray diffraction (XRD) experiments of the catalyst after the third run which showed no significant changes in the crystalline structure of the MOF after reactions (see the ESI†).
In summary, we have developed a novel and efficient heterogeneous catalyst system based on CuI immobilized on a MOF material, for the C–O bond forming cross-coupling of a variety of phenols/alcohols with aryl halides under ligand-free conditions. Furthermore, the catalyst can be recovered and reused without any significant loss of catalytic activity. This synthetic approach can be further applied to different catalytically active metal species, leading to highly efficient and recyclable heterogeneous catalysts for a variety of chemical reactions.
This work was supported by the NSF of China (20 803 024, 20 936 001, and 21 073 065), the Doctoral Fund of the Ministry of Education of China (200 805 611 045), Guangdong Science Foundation (S2 011 020 002 397, 2011B090 400 406, and 10 351 064 101 000 000), the Fundamental Research Funds for the Central Universities (2011ZG0009), and the program for New Century Excellent Talents in Universities (NCET-08-0203).
References
- (a) K. E. Torraca, X. Huang, C. A. Parrish and S. L. Buchwald, J. Am. Chem. Soc., 2001, 123, 10770 CrossRef CAS; (b) A. W. Czarnik, Acc. Chem. Res., 1996, 29, 112 CrossRef CAS.
- (a) F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 6954 CrossRef CAS; (b) A. W. Thomas and S. V. Ley, Angew. Chem., Int. Ed., 2003, 42, 5641 CrossRef; (c) R. Arundhathi, D. Damodara and P. R. Likhar, Adv. Synth. Catal., 2011, 353, 1591 CrossRef CAS; (d) O. Bistri, A. Correa and C. Bolm, Angew. Chem., 2008, 120, 596 CrossRef; (e) S. Kuwabe, K. E. Torraca and S. L. Buchwald, J. Am. Chem. Soc., 2001, 123, 12202 CrossRef CAS; (f) G. Mann and J. F. Hartwig, J. Am. Chem. Soc., 1996, 118, 13109 CrossRef CAS.
- (a) A. V. Vorogushin, X. Huang and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 8146 CrossRef CAS; (b) S. Gowrisankar, A. G. Sergeev, P. Anbarasan, A. Spannenberg, H. Neumann and M. Beller, J. Am. Chem. Soc., 2010, 132, 11592 CrossRef CAS; (c) A. Aranyos, D. W. Old, A. Kiyomori, J. P. Wolf, J. P. Sadighi and S. L. Buchwald, J. Am. Chem. Soc., 1999, 121, 4369 CrossRef CAS; (d) N. Kataoka, Q. Shelby, J. P. Stambuli and J. F. Hartwig, J. Org. Chem., 2002, 67, 5553 CrossRef CAS; (e) G. S. Chen, A. S. C. Chan and F. Y. Kwong, Tetrahedron Lett., 2007, 48, 473 CrossRef CAS.
- (a) A. Tlili, F. Monnier and M. Taillefer, Chem.–Eur. J., 2010, 16, 12299 CrossRef CAS; (b) H. J. Cristau, P. P. Cellier, S. Hamada, J. F. Spindler and M. Taillefer, Org. Lett., 2004, 6, 913 CrossRef CAS; (c) D. Maiti and S. L. Buchwald, J. Org. Chem., 2010, 75, 1791 CrossRef CAS; (d) R. A. Altman, A. Shafir, A. Choi, P. A. Lichtor and S. L. Buchwald, J. Org. Chem., 2008, 73, 284 CrossRef CAS; (e) A. B. Naidu, E. A. Jaseer and G. Sekar, J. Org. Chem., 2009, 74, 3675 CrossRef CAS.
- J. F. Marcoux, S. Doye and S. L. Buchwald, J. Am. Chem. Soc., 1997, 119, 10539 CrossRef CAS.
- M. Wolter, G. Nordmann, G. E. Job and S. L. Buchwald, Org. Lett., 2002, 4, 973 CrossRef CAS.
- D. Ma and Q. Cai, Org. Lett., 2003, 5, 3799 CrossRef CAS.
- R. K. Gujadhur, C. G. Bates and D. Venkataraman, Org. Lett., 2001, 3, 4315 CrossRef CAS.
- X. Lv and W. Bao, J. Org. Chem., 2007, 72, 3863 CrossRef CAS.
- Y. J. Chen and H. H. Chen, Org. Lett., 2006, 8, 5609 CrossRef CAS.
- E. Buck, Z. J. Song, D. Tschaen, P. G. Dormer, R. P. Volante and P. J. Reider, Org. Lett., 2002, 4, 1623 CrossRef CAS.
- (a) B. Sreedhar, R. Arundhathi, M. A. Reddy and M. L. Kantam, Synthesis, 2009, 483 CrossRef CAS; (b) S. Kokkirala, N. M. Sabbavarapu and V. D. N. Yadavalli, Org. Biomol. Chem., 2011, 9, 5978 RSC; (c) T. Miao and L. Wang, Tetrahedron Lett., 2007, 48, 95 CrossRef CAS; (d) J. Y. Kim, J. C. Park, A. Kim, A.Y. Kim, H. J. Lee, H. Song and K. H. Park, Eur. J. Inorg. Chem., 2009, 4219 CrossRef CAS; (e) R. Zhang, J. Liu, S. Wang, J. Niu, C. Xia and W. Sun, Chem Cat Chem, 2011, 3, 146 CAS; (f) B. Sreedhar, R. Arundhathi, P. L. Reddy and M. L. Kantam, J. Org. Chem., 2009, 74, 7951 CrossRef CAS.
- (a) A. Corma, H. Garcia and F. X. Llabrés Xamena, Chem. Rev., 2010, 110, 4606 CrossRef CAS; (b) J.-Y. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450 RSC; (c) A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Catal. Sci. Technol., 2011, 1, 856 RSC; (d) F. Schröder and R. A. Fischer, Top. Curr. Chem., 2010, 293, 77 CrossRef; (e) D. Farrusseng, S. Aguado and C. Pinel, Angew. Chem., Int. Ed., 2009, 48, 7502 CrossRef CAS; (f) H.-L. Jiang and Q. Xu, Chem. Commun., 2011, 47, 3351 RSC; (g) B. Z. Yuan, Y. Y. Pan, Y. W. Li, B. L. Yin and H. F. Jiang, Angew. Chem., Int. Ed., 2010, 49, 4054 CrossRef CAS.
- E. D. Bloch, D. Britt, C. Lee, C. J. Doonan, F. J. Uribe-Romo, H. Furukawa, J. R. Long and O. M. Yaghi, J. Am. Chem. Soc., 2010, 132, 14382 CrossRef CAS.
- (a) M. D. Allendorf, C. A. Bauer, R. K. Bhakta and R. J. T. Houk, Chem. Soc. Rev., 2009, 38, 1330 RSC; (b) H. L. Liu, B. L. Yin, Z. Q. Gao, Y. W. Li and H. F. Jiang, Chem. Commun., 2012, 48, 2033 RSC.
- J. M. Lindquistt and J. C. Hemminger, Chem. Mater., 1989, 1, 72 CrossRef.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental details, catalyst characterization and additional reaction results, and spectra data for the products. See DOI: 10.1039/c2ra20730c/ |
| This journal is © The Royal Society of Chemistry 2012 |