A quasi-solid-state dye-sensitized solar cell based on sol–gel derived in situ gelation of a siloxane hybrid electrolyte†
Jun-Young
Bae
a,
DaeSup
Lim
a,
Ho-Gyeong
Yun
b,
Myoung
Kim
bc,
JungHo
Jin
a and
Byeong-Soo
Bae
*a
aLaboratory of Optical Materials and Coating (LOMC), Department of Materials Science and Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, 305-701, Republic Korea. E-mail: bsbae@kaist.ac.kr; Fax: +82-42-350-3310; Tel: +82-42-350-4119
bConvergence Components & Materials Research Laboratory, Electronics and Telecommunications Research Institute (ETRI), Daejeon, 305-700, Republic of Korea
cSchool of Advanced Materials Engineering and Research center for Advanced Materials Department, Chonbuk National University, Chonju, 561-756, Republic of Korea
First published on 25th April 2012
Abstract
A stable dye-sensitized solar cell (DSSC) was fabricated by a novel in situ gelation of the electrolyte. A highly condensed oligosiloxane gel was formed by sol–gel reaction of silane monomers. The oligosiloxane gel DSSC showed reduced charge recombination, and an efficiency of 5.8% with long-term stability.
Dye-sensitized solar cells (DSSCs) have attracted widespread attention as an alternative to conventional silicon-based solar cells due to their high efficiency, simple and cost-effective fabrication process.1,2 Currently, a solar-to-electricity conversion efficiency of up to 12.3% has been achieved by employing a liquid electrolyte composed of cobalt based redox shuttles.3,4 However, liquid electrolytes have disadvantages such as the evaporation and leakage of the solvent under practical long-term operation of devices.
In an effort to replace these electrolytes, less volatile gel electrolytes such as polymer gel electrolytes (PGEs),5,6 room temperature ionic liquids (RTILs),7–9 and hole transport materials (HTMs)10 have been extensively studied. Among them, PGE can be simply made by gelation of a liquid electrolyte using polymers such as poly(ethylene glycol),11 poly(vinylidene fluoride-co-hexafluoropropylene),12 and poly(acrylonitrile-co-vinyl acetate).13 A polymer matrix can effectively trap the liquid solvent, thereby inhibiting evaporation and offering a transfer channel for redox shuttles. On the other hand, polymers have a high molecular weight and require an elevated temperature for casting or injection into the cell, which results in poor interfacial contact between the electrolyte and TiO2.13,14 In response to these issues, in situ gelation has been studied, as it affords thermal stability and effective penetration of the electrolyte, thereby providing good cell performance. Meanwhile, several gel electrolytes, composed of nano-sized organic and inorganic components, have been fabricated by the sol–gel process.15–18
Building on these works, we introduced a novel in situ chemical gelation through the sol–gel reaction of silane monomers to form less volatile gel electrolytes for stable DSSCs (Fig. 1a). Moreover, we designed a highly condensed oligosiloxane structure near the TiO2 surface to retard the recombination of electrons in the TiO2 particles with redox shuttles. The oligosiloxane gel electrolyte (OGE) was synthesized by a non-hydrolytic sol–gel condensation reaction of 3,4-epoxy cyclohexylethyl trimethoxysilane (ECTS), 3,3,3-trifluoropropyl trimethoxysilane (FTMS), and diphenylsilanediol (DPSD) at 80 °C for 4 h. We aimed to synthesize a highly viscous gel electrolyte with no remaining water. Thus, DPSD containing hydroxyl (–OH) and bulky phenyl groups was selected as the precursor in order to promote a non-hydrolytic condensation reaction and form highly condensed oligosiloxane. We used FTMS for homogeneous mixing of iodide ions with silanes due to the polarity of the fluoro groups. In addition, ECTS was added to balance the polarity and prevent phase separation of iodide ions. The fabricated electrolyte was composed of 0.25 M ECTS, 0.75 M FTMS, 1.5 M DPSD, 0.7 M 1-butyl-3-methyl imidazolium iodide (BMII), 0.14 M I2, 0.1 M lithium iodide (LiI), and 0.25 M 4-tert-butylpyridine (tBP) in 3-methoxypropionitrile (MPN). The composition of reference liquid electrolyte was 0.7 M BMII, 0.14 M I2, 0.1 M LiI, and 0.25 M tBP in MPN. The silane composition (FTMS : ECTS : DPSD = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) was optimized for the homogeneity of the mixed solution and the highest condensation degree of oligosiloxane. We used an increased content of I2 compared with that of the reported liquid electrolyte. A high I3− content is needed for the best ionic conductivity and efficiency in the presence of the highly viscous oligosiloxane.
6) was optimized for the homogeneity of the mixed solution and the highest condensation degree of oligosiloxane. We used an increased content of I2 compared with that of the reported liquid electrolyte. A high I3− content is needed for the best ionic conductivity and efficiency in the presence of the highly viscous oligosiloxane.
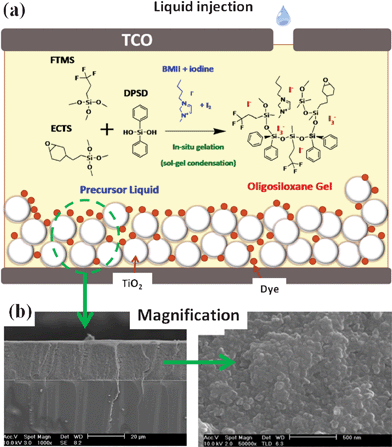 | ||
| Fig. 1 (a) Scheme for the fabrication of the DSSC using an in situ sol–gel reaction of silane monomers in a pre-filled liquid electrolyte. (b) Cross-sectional scanning electron microscopy (SEM) images of the TiO2 film containing the gelled oligosiloxane electrolyte. | ||
The DSSCs were fabricated using an electrode coated with a ruthenium dyed 12 μm TiO2 film with a particle size of 20 nm. The liquid state nano-sized monomers can fully penetrate the TiO2 pores and achieve intimate contact at room temperature. Through in situ gelation inside the cell at elevated temperature, the electrolyte became a highly viscous gel by formation of branched linear oligomers (Fig. S1†), containing I−/I3− redox shuttles (Fig. S2†). Fig. 1b shows the cross-sectional SEM photographs of the TiO2 particles filled with the OGE. After the pore filling and in situ gelation of the precursors, the internal nanoparticles were homogeneously covered with the gelled electrolyte. Also, the photocurrent of the fabricated DSSC using OGE increases with increasing TiO2 thickness, which is consistent with the infiltration of the electrolyte. (Table S1†). Therefore, nano-sized oligomers were successfully formed inside the TiO2 pores, promoting good interfacial contact between the TiO2 and the electrolyte.19
Here, the FT-IR spectra were measured to confirm the formation of an oligosiloxane structure inside the gel electrolyte (Fig. 2a). The bands at 1212 and 1263 cm−1 are assigned to C–F (fluoro groups). The alkoxy groups (R′–Si(OR)3) of FTMS and ECTS react with the silanol groups (Si–OH) of DPSD to form siloxane (Si–O–Si) bonds. The broad bands at 1020∼1070 cm−1 represent the siloxane network, which appears after the formation of an oligosiloxane gel electrolyte.20 These results suggest that silane monomers were successfully condensed to siloxane oligomers in the presence of iodide ions.
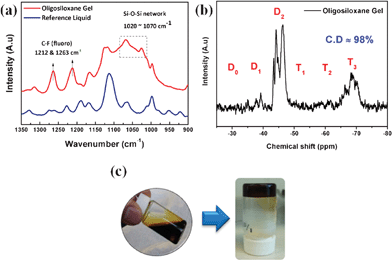 | ||
| Fig. 2 (a) FT-IR spectra of the reference liquid (before gelation) and the oligosiloxane gel (after gelation) electrolyte. (b) 29Si NMR spectra and condensation degree of the oilgosiloxane gel electrolyte. (c) Photographs of the precursor liquid electrolyte (left) and the oligosiloxane gel electrolyte (right) in a vial. | ||
The formation of siloxane bonds and the high degree of condensation (98%) were also investigated using 29Si NMR spectroscopy (Fig. 2b). The chemical shifts of the condensed trialkoxysilanes (R′–Si(OR)3) were from −50 to −70 ppm, while the shifts of condensed diphenylsilandiol were from −29 to −47 ppm. Those are denoted by Tn and Dn, respectively, where n represents the number of siloxane bonds attached to a silicon atom. The OGE was characterized by two major D2 and T3 signals, which were assigned to Si species with no remaining methoxy (–OMe) groups, confirming that the condensation reaction of the silane monomers was fully carried out.21,22
Fig. 2c shows photographs of two vials containing the precursor liquid electrolyte (left) and the OGE (right). We obtained homogeneous and stable liquid state electrolytes by stirring the silane precursors, iodides, and additives in solvent. Thus, the electrolytes could effectively penetrate the TiO2 film from the top surface. After the sol–gel reaction, the precursor liquid electrolyte became a viscous gel and showed little flow upon turning the vial upside down.
The photovoltaic parameters and J–V curves of the DSSCs using the liquid electrolytes and OGE are shown in Table 1 and Fig. 3.
 | ||
| Fig. 3 The photocurrent–voltage curve of the DSSCs using the reference liquid, precursor liquid and oligosiloxane gel electrolyte measured under (a) an illumination state and (b) a dark state. | ||
| Electrolyte | J sc (mA cm−2) | V oc (V) | FF | n (%) |
|---|---|---|---|---|
| Reference liquid | 13.8 | 0.71 | 0.63 | 6.16 |
| Precursor liquid | 12.6 | 0.71 | 0.67 | 6.00 |
| Oligosiloxane gel | 10.9 | 0.79 | 0.68 | 5.83 |
The parameters of the DSSC employing the optimized OGE were 10.9 mA cm−2 (Jsc, short circuit current density), 0.79 V (Voc, open circuit voltage), and 0.68 (FF, fill factor). We confirmed a reliable oligosiloxane gel state at fixed reaction conditions and obtained reproducible viscosity, redox shuttle formation and ionic conductivity, indicating consistent efficiency and parameters for each of the devices (Fig. S2,S3 and Table S2†). An optimized gel DSSC using a TiO2 layer with a thickness of 12 μm showed the best conversion efficiency of 5.83% (Table S1†).
The limitation of the Jsc of the DSSC using the OGE was mainly owing to the retarded ion conduction imposed by the highly condensed Si–O–Si network. On the other hand, the Voc of the oligosiloxane gel DSSC was significantly increased compared with that (0.71 V) of the liquid DSSCs. The increase in the Voc is due to a shift in the conduction band caused by the presence of blocking materials near the TiO2 surface. In general, the low triiodide mobility of the gel electrolyte causes a build-up near the TiO2 and increases the probability of electron recombination. However, three dimensional oligosiloxane adsorbed on the TiO2 surface suppresses the electron transfer from the conduction band of the TiO2 to I3− in the electrolyte. This is because the highly condensed oligosiloxane structure inhibits the access of the redox shuttle to the bare TiO2 surface and the bulky phenyl functional groups prevent molecular aggregation.23–25 The fluoro groups also suppress the recombination due to their large electronegativity.26 These combined effects lead to a reduced electron–hole recombination rate and an increased electron lifetime at the TiO2/electrolyte interface, and thereby an improved Voc.24,25
Dark current–voltage analysis also supported the decreased interfacial charge recombination (Fig. 3b). The dark current onset of the oligosiloxane gel DSSC was shifted to a higher voltage compared to that of the liquid DSSC. Also, the dark current density of the DSSC using the oligosiloxane gel electrolyte was decreased, showing that the electron recombination was decreased. It shows a higher FF (0.68) than that of the liquid DSSCs, which is consistent with the low dark current of the oligosiloxane gel DSSC.27Therefore, the efficiency of the oligosiloxane gel DSSC (5.83%) was close to the efficiency of the DSSC (6.16%) using reference liquid electrolyte. Due to the enhanced electron liftetime as a result of the retarded electron recombination, the Voc and FF increased and compensated for the drop in the Jsc.
Furthermore, we measured the open circuit voltage decay to compare the interfacial recombination rates between the oligosiloxane gel and the reference liquid DSSC. Each Voc was measured under illumination and in a dark state over time. After the light was switched off, we could evaluate the Voc decay rate, which is inversely proportional to the electron lifetime and thus proportional to the electron recombination. The decreased Voc decay rate in the DSSC using OGE suggests a slow rate of electron recombination and a long electron lifetime at the TiO2/electrolyte interface.28,29 (Fig. 4a) The fluoro oligosiloxane matrix effectively reduced the electron recombination, playing a role similar to the polymer by acting as a barrier to decrease the interfacial back electron transfer.
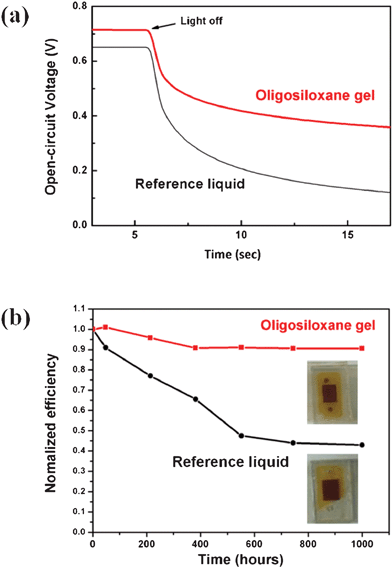 | ||
| Fig. 4 (a) Open circuit voltage decay measurement of the DSSCs using the reference liquid and the oligosiloxane gel electrolyte. (b) Variation of the normalized conversion efficiency of the DSSCs using the reference liquid and the oligosiloxane gel electrolyte at 50 °C in the dark sate convection oven. | ||
We have investigated the thermal stability of DSSCs (Fig. 4b). The oligosiloxane gel DSSC shows improved long-term stability compared to the reference liquid DSSC. However, the stability did not reach that of reported DSSCs using polymer gel electrolytes at high temperatures and light-soaking conditions. We consider that it is due to the nature of oligomer structure, which has a poorer solvent trapping effect compared to the highly cross-linked polymer structure with a high molecular weight. After 1000 h at 50 °C, the DSSC using the OGE maintained 91% of its initial efficiency, while the efficiency of the liquid DSSC gradually dropped due to a significant decrease in the Jsc, indicating solvent leakage at elevated temperatures. This result shows that the chemically bonded oligosiloxane matrix can efficiently trap the solvent, thereby inhibiting evaporation.
Conclusions
We fabricated a stable DSSC employing an oligosiloxane gel electrolyte by introducing a novel in situ gelation of a liquid electrolyte. The alkoxysilane monomers are capable of gelling the liquid electrolyte through a sol–gel reaction, resulting in effective infiltration and contact. The existence of a fluoro functional group and the formation of the three dimensional oligosiloxane structure in the gel electrolyte decreased the electron recombination rate at the electrolyte/TiO2 interface and thereby improved the Voc and FF of the DSSC. Finally, a conversion efficiency of up to 5.83% was achieved with a good long-term stability.Experimental
3,4-Epoxy cyclohexylethyl trimethoxysilane (ECTS, Aldrich), 3,3,3-trifluoropropyl trimethoxysilane (FTMS,TCI) and diphenylsilanediol (DPSD, Gelest) were used as the silane precursors. Barium hydroxide monohydrate (BH, Aldrich) was added as a catalyst to promote a reaction. 1-Butyl-3-methyl imidazolium iodide (BMII, C-TRI), iodine (I2, Aldrich), additives and solvent were mixed with the silane precursors in a vial by stirring the solution at room temperature for 6 h. An oligosilxane gel electrolyte was synthesized by a sol–gel condensation reaction of the silane precursors inside the mixed liquid electrolyte at 80 °C for 4 h.Anatase TiO2 particles were synthesized using a reported sol–gel method30 and the diameter of the TiO2 particles was adjusted to 20 nm. The synthesized TiO2 particles were converted into a paste using an organic vehicle.31 The paste was deposited on fluorine doped tin oxide (FTO) coated glass by the doctor blade method. The glass was heat treated at 550 °C for 30 min under an air atmosphere. The 12 μm TiO2-coated FTO glass was immersed in an anhydrous ethanolic solution of 0.3 mM N719 dye (Solaronix SA) at room temperature for 24 h. A counter electrode was fabricated by spin-coating with 5 mM hydrogen hexachloro palatinate(IV) hydrate (H2PtCl6·H2O) in 2-propanol, then annealed at 450 °C for 30 min. The electrode and counter electrode were sealed with a Surlyn (50 μm, Solaronix SA) under a pressure of 200 kPa cm−2 at 100 °C. The liquid electrolyte was injected into the space between the electrode and the Pt counter electrode through a pre-drilled hole at room temperature. The oligosiloxane gel DSSC was fabricated using in situ gelation by heating the cell inside an oven at 80 °C for 4 h. The hole was sealed with a cover glass heated onto Surlyn.
ATR IR spectra were measured using a Fourier transform infrared (FT-IR) spectrometer (Bruker) in the wavenumber range from 900 to 1350 cm−1. 29Si NMR spectra (Bruker FT 600 MHz) were measured with a sample consisting of 30 vol% of the oligosiloxane gel electrolyte in chloroform-d Chromium(III) acetylacetonate (CDCl3). Cross-sectional SEM images of the TiO2 electrodes were obtained using a scanning electron microscope (Phillips, xl30).
The photocurrent-voltage (J–V) characteristics of the cells were measured using a Keithley 2400 source meter using an AM 1.5 G solar simulator with a 1000 W xenon lamp (Oriel, 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 193) as a light source. The light intensity was adjusted with a reference solar cell composed of a crystalline Si capped with a KG-5 glass to 1 sun light intensity of 100 mW cm−2. The Voc was measured using a potentiostat/galvanostat (Gamry, Reference 600) and AM 1.5 G solar simulator with a 1000 W xenon lamp (Oriel, 91
193) as a light source. The light intensity was adjusted with a reference solar cell composed of a crystalline Si capped with a KG-5 glass to 1 sun light intensity of 100 mW cm−2. The Voc was measured using a potentiostat/galvanostat (Gamry, Reference 600) and AM 1.5 G solar simulator with a 1000 W xenon lamp (Oriel, 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 193). The DSSCs operated under 1 sun light intensity and the Voc was measured as a function of time. After few seconds, the light was turned off and the Voc decay rate was measured as a function of time. To test the thermal stabilities of the cells, the precursor liquid and oligosiloxane gel DSSCs were stored in a dark state at 50 °C for 1000 h.
193). The DSSCs operated under 1 sun light intensity and the Voc was measured as a function of time. After few seconds, the light was turned off and the Voc decay rate was measured as a function of time. To test the thermal stabilities of the cells, the precursor liquid and oligosiloxane gel DSSCs were stored in a dark state at 50 °C for 1000 h.
Acknowledgements
This work was supported by Mid-career Researcher Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 20100013811). This work was also supported by the Ministry of Knowledge and Economy and the Small & Medium Business Administration, Republic of Korea [SL122749].References
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef CAS.
- M. Grätzel, Acc. Chem. Res., 2009, 42, 1788 CrossRef.
- A. Yella, H.-W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W.-G. Diau, C.-Y. Yeh, S. M. Zakeeruddin and M. Grätzel, Science, 2011, 334, 629 CrossRef CAS.
- Q. Yu, C. Yu, F. Guo, J. Wang, S. Jiao, S. Hao, H. Li and L. Zhao, Energy Environ. Sci., 2012, 5, 6151 CAS.
- P. Wang, S. M. Zakeeruddin, I. Exnar and M. Grätzel, Chem. Commun., 2002, 2972 RSC.
- J. Wu, S. Hao, Z. lan, J. Lin, M. Huang, y. Huang, L. Fang, S. Yin and T. Sato, Adv. Funct. Mater., 2007, 17, 2645 CrossRef CAS.
- P. Wang, S. M. Zakeeruddin, P. Comte, I. Exnar and M. Grätzel, J. Am. Chem. Soc., 2003, 125, 1166 CrossRef CAS.
- Y. Bai, Y. Cao, J. Zhang, M. Wang, R. Li, P. Wang, S. M. Zakeeruddin and M. Grätzel, Nat. Mater., 2008, 7, 626 CrossRef CAS.
- S. M. Zakeeruddin and M. Grätzel, Adv. Funct. Mater., 2009, 19, 2187 CrossRef CAS.
- U. Bach, D. Lupo, P. comte, J. E. Moser, F. Weissörtel, J. Salbeck, H. Spreitzer and M. Grätzel, Nature, 1998, 395, 583 CrossRef CAS.
- Y. J. Kim, J. H. Kim, M. S. Kang, M. J. Lee, J. Won, J. C. Lee and Y. S. Kang, Adv. Mater., 2004, 16, 1753 CrossRef CAS.
- S. R. Scully, M. T. Lloyd, R. Herrera, E. P. Giannelis and G. G. Malliaras, Synth. Met., 2004, 144, 291 CrossRef CAS.
- C. L. Chen, H. Teng and Y. L. Lee, Adv. Mater., 2011, 23, 4199 CrossRef CAS.
- K. Suzuki, M. Yamaguchi, S. Hotta, N. Tanabe and S. Yanagida, J. Photochem. Photobiol., A, 2004, 164, 81 CrossRef CAS.
- E. Stathatos, P. Lianos, U. Lavrencic-Stangar and B. Orel, Adv. Mater., 2002, 14, 354 CrossRef CAS.
- E. Stathatos, P. Lianos, S. M. Zakeeruddin, P. Liska and M. Grätzel, Chem. Mater., 2003, 15, 1825 CrossRef CAS.
- L. Jin, Z. Wu, T. Wei, J. Zhai and X. Zhang, Chem. Commun., 2011, 47, 997 RSC.
- K. H. Jung, J.-Y. Bae, H.-G. Yun, M. G. Kang and B.-S. Bae, ACS Appl. Mater. Interfaces, 2011, 3, 293 CAS.
- M. S. Kang, J. H. Kim, Y. J. Kim, J. O. Won, N. G. Park and Y. S. Kang, Chem. Commun., 2005, 889 RSC.
- J. H. Oh, S. Y. Kwak, S. C. Yang and B.-S. Bae, ACS Appl. Mater. Interfaces, 2010, 2, 913 CAS.
- D. Hoebbel, T. Reinert and H. Schmidt, J. Sol-Gel Sci. Technol., 1996, 7, 217 CrossRef CAS.
- J.Y. Bae, S. C. Yang, J. H. Jin, K. H. Jung, J. S. Kim and B.-S. Bae, J. Sol-Gel Sci. Technol., 2010, 58, 114 CrossRef.
- S. Ito, S. M. Zakeeruddin, P. Comte, P. Liska, D. Kuang and M. Grätzel, Nat. Photonics, 2008, 2, 693 CrossRef CAS.
- S. Kambe, S. Nakade, T. Kitamura, Y. Wada and S. Yanagida, J. Phys. Chem. B, 2002, 106, 2967 CrossRef CAS.
- R. Komiya, L. Han, R. Yamanaka, A. Islam and T. Mitate, J. Photochem. Photobiol., A, 2004, 164, 123 CrossRef CAS.
- D. Saikia, C. C. Han and Y. W. Chen-Yang, J. Power Sources, 2008, 185, 570 CrossRef CAS.
- J. N. de Freitas, A. de Souza Goncalves, M. de Paoli, J. R. Durrant and A. F. Nogueira, Electrochim. Acta, 2008, 53, 7166 CrossRef.
- H.-G. Yun, J. H. Park, B.-S. Bae and M. G. Kang, J. Mater. Chem., 2011, 21, 3558 RSC.
- H.-G. Yun, B.-S. Bae and M. G. Kang, Adv. Energy Mater., 2011, 1, 337 CrossRef CAS.
- S. H. Lee, Y. S. Jun, K. J. Kim and D. H. Kim, Sol. Energy Mater. Sol. Cells, 2001, 65, 193 CrossRef CAS.
- M. C. Carotta, M. Ferroni, V. Guidi and G. Martinelli, Adv. Mater., 1999, 11, 943 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section and supplementary figures. See DOI: 10.1039/c2ra20320k |
| This journal is © The Royal Society of Chemistry 2012 |
