Energy storage in in vivo synthesizable biominerals†
Sung-Wook
Kim
ab,
Kyu-Young
Park
c,
Jungki
Ryu
d,
Jong Wan
Ko
e,
Woosuk
Cho
f,
Sang-Min
Kim
f,
Chan Beum
Park
e and
Kisuk
Kang
*c
aSustainable Energy Technologies Department, Brookhaven National Laboratory, Upton, NY 11973, United States of America
bDepartment of Chemistry, State University of New York at Stony Brook, Stony Brook, NY11794, United States of America
cDepartment of Materials Science and Engineering, Seoul National University, Seoul 151-742, Republic of Korea. E-mail: matlgen1@snu.ac.kr; Tel: +82-2-880-7088
dDepartment of Materials Science and Engineering, Massachusetts Institute of Technology, Cambridge, MA 20139-4307, United States of America
eDepartment of Materials Science and Engineering, Daejeon 305-701, Republic of Korea
fAdvanced Batteries Research Center, Korea Electronics Technology Institute, Seongnam, Gyeonggi 463-816, Republic of Korea
First published on 18th April 2012
Abstract
With the move toward the use of greener materials for powered vehicles, environmentally-benign synthesis of energy materials is becoming important. Here, the energy storage capability of biominerals from the jaws of a marine bloodworm, Glycera dibranchiate, is demonstrated, implying the possibility of a bio-factory (or in vivo synthesis) for energy storage.
Over the past decade, biomaterials have been extensively studied for medical applications, such as new pharmaceuticals, tissue engineering, and artificial organs, due to their excellent biocompatibility.1–4 Nowadays, biomaterials further expand their boundaries to various functionalities in semiconductors, sensors, and display devices.5–10 Mimicking the structure and synthetic route of biomaterials in living organisms or direct use of the biomaterials often provides insights in enhancing the performance of devices due to similarities in the material requirements for functional devices and those of living organisms.
The feasibility of biological materials as a template for the synthesis of energy storage materials has recently been investigated.11–13 Pioneering works done by Nam et al. showed that organic biomolecules can be used as a structural template to provide growth sites for electrode materials in batteries.11 In this study, Co3O4 nanoparticles were grown onto genetically modified M13 viruses as templates, resulting in good electrochemical performance. In vitro synthesized, self-assembled peptides were also used as templates for the fabrication of battery electrodes.14–16 Facile control of the peptide nanostructures by self-assembly could result in ideal electrode nanostructures for Li rechargeable batteries. Further evidence of this approach was reported by Ryu et al. who fabricated Fe phosphate electrode materials on peptide nanostructured templates by mimicking the natural biomineralization process of bone formation in which Ca phosphates form on collagen.16
Recently, Chen et al. reported that a biomaterial itself can function as an active electrode for Li batteries.17 Organic Li2C6O6 extracted from biomass was capable of storing four Li ions, delivering a specific capacity of 580 mAh g−1, which is substantially higher than that of conventional cathode materials (∼170 mAh g−1). Such organic electrodes can potentially offer sustainable life cycles from production to consumption without generating additional CO2. Thus, the use of such chemistry is believed to be able to result in greener and more sustainable batteries.
We envision that electrode materials can be produced from ‘bio-factories,’ once electrochemically active biomaterials are identified from the living organisms. Fig. 1 briefly illustrates the concept of the bio-factory. The living organisms that possess electrochemically active biominerals are farm-bred on a large scale. The biominerals are extracted for use in battery fabrication and the productivity of biominerals from the living organisms can be optimized by genetic engineering. The production of battery materials from bio-factories has several advantages over production from a ‘real’ factory, including environmental friendliness, non-toxicity to humans, non-necessity of large building sites, and potentially lower production cost. The greener production of Li batteries also reduces the environmental burdens of large-volume Li battery production.
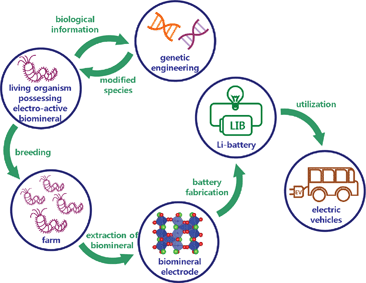 | ||
| Fig. 1 Schematic illustration of a ‘bio-factory’. Electrochemcially active biominerals can be extracted from the living organisms and used as electrodes for Li rechargeable batteries. | ||
In this study, we discovered that Cu2Cl(OH)3, a Cu-based natural biomineral found in the jaws of a marine bloodworm Glycera dibranchiate, can efficiently convert electric energy into chemical energy and vice versa through a reversible electrochemical reaction with Li ions. Cu2Cl(OH)3 is the only Cu-based biomineral known to exist in nature, while Ca-, Si-, and Fe-based biominerals are commonly observed in other living organisms.18–20 Although previous studies have shown that some organic biomaterials can be used as active electrode materials, the intrinsic inferiority of organic materials with regard to long-term stability is a major drawback in their application to long-life energy devices.21–24 This study reveals that an inorganic biomineral, Cu2Cl(OH)3, can be a promising electrode material for Li rechargeable batteries. This material reversibly stores and releases Li ions via a conversion reaction that is widely observed in transition metal oxide, nitride, and fluoride electrodes.25–29
We chemically synthesized Cu2Cl(OH)3 to examine the energy storage capability of the Cu2Cl(OH)3 biomineral-based electrode. A detailed description of the synthesis is provided in the Supplementary Information.† The X-ray diffraction (XRD) pattern shown in Fig. 2a indicates that the synthesized Cu2Cl(OH)3 is composed of two phases with similar structures, atacamite and clinoatacamite. Although atacamite is the most common crystalline form of Cu2Cl(OH)3, clinoatacamite can be regarded as a distorted structure of atacamite with similar atomic arrangements (see Supplementary Fig. S1a and b†). The crystal information for both phases is summarized in Fig. S1.†Fig. 2b shows that the synthesized Cu2Cl(OH)3 is a polyhedral shape of a few hundred nanometers in dimension. Magnified transmission electron microscope (TEM) images reveal that individual particles are composed of a number of well-formed crystalline primary nanoparticles. (Fig. S2†).
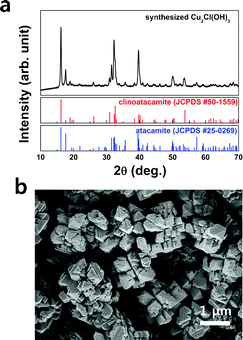 | ||
| Fig. 2 (a) XRD pattern and (b) SEM image of the synthesized Cu2Cl(OH)3. | ||
The electrochemical properties of Cu2Cl(OH)3 were examined using a CR2016-type coin cell with elemental Li as a counter electrode. Fig. 3a shows charge–discharge profiles of the Cu2Cl(OH)3 electrode operated in the 0.01–3 V range at a current rate of 100 mA g−1. An abnormally high specific capacity was obtained at the first discharge (∼1100 mAh g−1), indicating that a significant amount of Li was used to irreversibly form surface passivation layers. After several subsequent cycles, stable cycling was observed with a specific capacity of about 500 mAh g−1, which is comparable to the theoretical capacity of Cu2Cl(OH)3 based on the 2Cu2+/Cu0 redox reaction.
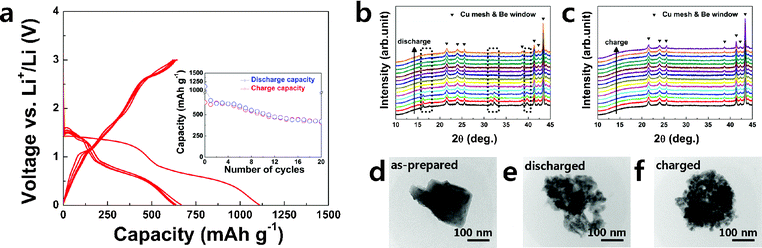 | ||
| Fig. 3 Electrochemistry of Cu2Cl(OH)3: (a) charge–discharge profiles for the initial 5 cycles and (inset) capacity retention, in situ XRD patterns during (b) discharge and (c) charge, and ex situ TEM images of (d) as-prepared, (e) discharged, and (f) charged electrodes. | ||
To verify the detailed Li storage mechanism in Cu2Cl(OH)3, XRD analysis was performed on the electrode during both the discharge and charge processes using an in situ electrochemical cell. The evolution of the XRD patterns during the first discharge process in Fig. 3b reveals that the original Cu2Cl(OH)3 structure completely disappears, as indicated in the dotted box. Furthermore, the crystal structure is not recovered during subsequent charging, as shown in Fig. 3c. This behavior is similar to the phase behavior generally observed for an electrode that undergoes a conversion reaction.27–29 When the conversion reaction occurs, the pristine crystalline phase deforms into a nanocomposite of the amorphous or nanosized LinX (X = anion) and metal, which is hardly observable with XRD. Ex situ TEM analysis (Fig. 3d–f) of the electrode shows that a corresponding microscopic structural change occurred during the electrochemical reaction. Single Cu2Cl(OH)3 particles, a few hundred nanometers in size, were destroyed to a size of tens of nanometers after discharge. The morphology of the particles was not recovered after subsequent charge, consistent with the XRD results. A magnified images of the discharged state (Supplementary Fig. S3†) shows that the nanoparticles are embedded in the matrix, as is commonly observed in conversion reaction compounds in which metal nanoparticles are embedded in the LinX matrix.27–29 From the XRD and TEM analysis, it is reasonable to assume that Cu2Cl(OH)3 underwent a conversion reaction: however, the precise identification of reaction products was not successful. Further study of the reaction mechanism is necessary and currently under way.
In summary, the possibility of energy storage in the Cu-based biomineral, Cu2Cl(OH)3, was examined to assess its use as an electrode material in Li rechargeable batteries. Cu2Cl(OH)3 delivered approximately 500 mAh g−1 through the conversion reaction. It is noteworthy that Cu2Cl(OH)3 is a novel-class of electrode material, containing Cl− and (OH)− as anions, which are not common for electrode materials. Chemically synthesized Cu2Cl(OH)3 was investigated in this study, hence further study on ‘real’ Cu2Cl(OH)3 biomineral from the marine bloodworm should be followed to demonstrate the feasiliby of the bio-factory. In addition, purification and scale-up of the biomineral electrode still remains a challenge. There are numerous natural biominerals that contain other transition metal ions, such as Fe and Mn, which can serve as excellent redox elements. Thus, significant unexplored opportunities for energy device applications exist in natural biominerals with different electrochemical properties.
This research was supported by the Energy Efficiency and Resources R&D program (20112020100070), Human Resources Development of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant (20114010203120) funded by the Ministry of Knowledge Economy, and the National Research Laboratory (ROA-2008-000-20041-0) program of the National Research Foundation (NRF), Republic of Korea. The work was also supported by the Northeastern Center for Chemical Energy Storage, and Energy Frontier Research Center funded by the U.S. DOE, BES under award No. DE-SC0001294.
References
- N. Huebsch and D. J. Mooney, Nature, 2009, 462, 426–432 CrossRef CAS.
- N. A. Peppas and R. Langer, Science, 1994, 263, 1715–1720 CAS.
- K. Ishihara, Sci. Technol. Adv. Mater., 2000, 1, 131–138 CrossRef CAS.
- F. Watari, A. Yokoyama, M. Omori, T. Hirai, H. Kondo, M. Uo and T. Kawasaki, Compos. Sci. Technol., 2004, 64, 893–908 CrossRef CAS.
- I. Willner, Science, 2002, 298, 2407–2408 CrossRef CAS.
- S. Bayliss, L. Buckberry, P. Harris and C. Rousseau, Thin Solid Films, 1997, 297, 308–310 CrossRef CAS.
- K. Itoga, J. Kobayashi, M. Yamato, A. Kikuchi and T. Okano, Biomaterials, 2006, 27, 3005–3009 CrossRef CAS.
- C. J. Bettinger and Z. Bao, Adv. Mater., 2010, 22, 651–655 CrossRef CAS.
- J. L. Rouge, B. E. Eaton and D. L. Feldheim, Energy Environ. Sci., 2011, 4, 398–402 CAS.
- C. Jeffryes, J. Campbell, H. Li, J. Jiao and G. Rorrer, Energy Environ. Sci., 2011, 4, 3930–3941 CAS.
- K. T. Nam, D.-W. Kim, P. J. Yoo, C.-Y. Chiang, N. Meethong, P. T. Hammond, Y.-M. Chiang and A. M. Belcher, Science, 2006, 312, 885–888 CrossRef CAS.
- Y. J. Lee, H. Yi, W.-J. Kim, K. Kang, D. S. Yun, M. S. Strano, G. Ceder and A. M. Belcher, Science, 2009, 324, 1051–1055 CAS.
- X. Chen, K. Gerasopoulos, J. Guo, A. Brown, C. Wang, R. Ghodssi and J. N. Culver, ACS Nano, 2010, 4, 5366–5372 CrossRef CAS.
- S.-W. Kim, T. H. Han, J. Kim, H. Gwon, H.-S. Moon, S.-W. Kang, S. O. Kim and K. Kang, ACS Nano, 2009, 3, 1085–1090 CrossRef CAS.
- J. Ryu, S.-W. Kim, K. Kang and C. B. Park, ACS Nano, 2009, 4, 159–164 CrossRef.
- J. Ryu, S.-W. Kim, K. Kang and C. B. Park, Adv. Mater., 2010, 22, 5537–5541 CrossRef CAS.
- H. Chen, M. Armand, G. Demailly, F. Dolhem, P. Poizot and J. M. Tarascon, Chem. Sus. Chem, 2008, 1, 348–355 CrossRef CAS.
- H. C. Lichtenegger, T. Schöberl, M. H. Bartl, H. Waite and G. D. Stucky, Science, 2002, 298, 389–392 CrossRef CAS.
- S. Weiner and L. Addadi, Science, 2002, 298, 375–376 CrossRef CAS.
- H. C. Lichtenegger, H. Birkedal, D. M. Casa, J. O. Cross, S. M. Heald, J. H. Waite and G. D. Stucky, Chem. Mater., 2005, 17, 2927–2931 CrossRef CAS.
- P. Novák, K. Müller, K. S. V. Santhanam and O. Haas, Chem. Rev., 1997, 97, 207–282 CrossRef.
- T. Le Gall, K. H. Reiman, M. C. Grossel and J. R. Owen, J. Power Sources, 2003, 119–121, 316–320 CrossRef CAS.
- J. Qu, T. Katsumata, M. Satoh, J. Wada, J. Igarashi, K. Mizoguchi and T. Masuda, Chem.–Eur. J., 2007, 13, 7965–7973 CrossRef CAS.
- K. Nakahara, S. Iwasa, M. Satoh, Y. Morioka, J. Iriyama, M. Suguro and E. Hasegawa, Chem. Phys. Lett., 2002, 359, 351–354 CrossRef CAS.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 2000, 407, 496–499 CrossRef CAS.
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS.
- M. R. Palacin, Chem. Soc. Rev., 2009, 38, 2565–2575 RSC.
- P. G. Bruce, B. Scrosati and J.-M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930–2946 CrossRef CAS.
- R. Malini, U. Uma, T. Sheela, M. Ganesan and N. Renganathan, Ionics, 2009, 15, 301–307 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: detailed description on experiments, crystal structures of atacamite and clinoatacamite, and TEM images. See DOI: 10.1039/c2ra20671d/ |
| This journal is © The Royal Society of Chemistry 2012 |
