High-connected strategy of polyoxometalates towards model of core–shell nanostructure†‡
Yanan
Yang
,
Jingjing
Gong
,
Weisong
Zhang
,
Hengchao
Zhang
,
Lili
Zhang
,
Yang
Liu
*,
Hui
Huang
,
Hailiang
Hu
* and
Zhenhui
Kang
*
Institute of Functional Nano & Soft Materials (FUNSOM) and Jiangsu Key Laboratory for Carbon Based Functional Materials & Devices, Soochow University, Suzhou, Jiangsu 215123, People's Republic of China. E-mail: zhkang@suda.edu.cn; Yangl@suda.edu.cn; hlhu@suda.edu.cn; Tel: +86 512 6588 0957;; Fax: +86 512 6588 2846
First published on 13th June 2012
Abstract
Cu-/Ag-modified polyoxometalates with 5/10/12 connections were obtained via a sulphur-assisted hydrothermal synthetic strategy.
Core–shell nanostructures has been one of the hottest topics in current materials science and chemistry, not only because of their great potential applications in adsorption, separation, catalysis and photonics, but also due to their easy alteration of properties by changing the diameter, chemical composition, and crystallinity.1–3 However, currently, there is still a lack of suitable practical models for understanding the essential properties and principles of core–shell nanostructures. As is known, in a single crystalline solid, nanosized clusters with various sizes, shapes, compositions, and structures can be regarded as ideal models for understanding the physical and chemical properties of nanoparticles and complex nanostructures.4–6 In light of this point, the design and construction of nanosized clusters in crystals will provide an efficient and straightforward way to get a reasonable model for core–shell nanostructures.
Polyoxometalates (POMs) have found widespread application, mainly in varied fields of analytical chemistry, catalysis, medicine and materials science.7–9 They have a versatile nature, homogeneous size, controllable architecture, large negative charges, possess multiple coordination sites and the great assembly ability to construct novel frameworks and nanosystems.10–12 Nowadays, POMs are usually modified to be ideal models for study in fundamental and applied science.13–15 Notably, for the modification of POMs, from the structural point of view, POMs can act as a core and then the connected metal–organic units (or metal atoms) may be regarded as a partial shell.16–19 Based on this structural characteristic, POMs with multiple coordination sites are the most optimal candidates to construct a practical crystal model for understanding the physical and chemical properties of the core–shell nanostructures. In order to attain this goal, high-connected modification is necessary to cover the whole surface of the POMs. To date, a number of examples about multi-connected modification of saturated Keggin-type POMs have been reported, yet the highest connection number of those polyanions is only eight.20,21 Consequently, to design and improve the connection numbers of transition metals coordinated with POMs is the urgent challenge.
Herein, we report the design and synthesis of three POM-based coordination polymers ([Cu3(C2H4N4)4] [PW12O40] (FUNSOM-3), [Ag8(C2H3N4S)4] [SiW12O40]·2H2O (FUNSOM-4), and [Ag6(C2H3N4S)4S2] [H3PW12O40]·2H2O (FUNSOM-5)) via a sulphur-assisted hydrothermal synthetic strategy. Structural analysis reveals that the polyanions in these compounds are modified by Cu and/or Ag atoms with different connection numbers, that is 5, 10, and 12-connected for FUNSOM-3, -4, and -5, respectively (Fig. 1). Significantly, this kind of high-connected metal-modification tends to form a continuous shell over the polyanion gradually, which will be a promising structural model towards core–shell nanostructures.
![Ball–stick view of the connection mode of POMs. The [TW12O40]n− anions function as inorganic ligands to connect with 5 Cu (a: T = P, n = 3), 10 Ag (b: T = Si, n = 4) and 12 Ag (c: T = P, n = 3) atoms within FUNSOM-3, -4 and -5, respectively.](/image/article/2012/RA/c2ra20605f/c2ra20605f-f1.gif) | ||
| Fig. 1 Ball–stick view of the connection mode of POMs. The [TW12O40]n− anions function as inorganic ligands to connect with 5 Cu (a: T = P, n = 3), 10 Ag (b: T = Si, n = 4) and 12 Ag (c: T = P, n = 3) atoms within FUNSOM-3, -4 and -5, respectively. | ||
To construct highly-connected POM-based compounds, in our initial experiments, 1-methyl-1,2,3,4-tetrazole (mt) with small steric hindrance and rich coordination sites was selected, as well as the copper salt due to its versatile coordination ability.22–24 A mixture of Cu(OAc)2·H2O, mt, H3PW12O40·xH2O and distilled water was kept at 160 °C for 3 days, leading to the formation of the orange crystal FUNSOM-3. Crystal structural analysis shows that the asymmetric unit of FUNSOM-3 consists of one [PW12O40]3− anion (PW12), three Cu ions, and four mt ligands (Fig. S1†). Analysis of the bond lengths, charge balance, and bond valence sum calculations (BVS) suggest all Cu ions are CuI in nature.25 Further analysis of the structure indicates that the four types of mt ligands adopt similar μ2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η1
η1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η1 coordination mode by using its two adjacent N atoms to bridge two Cu ions (Fig. S2†). The Cu1 and Cu3 atoms are simultaneously bridged by mt2 and mt4 ligands to form a binuclear unit. The Cu2 and Cu3 atoms are bridged by an mt3 ligand. Such two binuclear units are merged into trinuclear building units (SBUa) through sharing of the same bridge of the Cu3 atom (Fig. S3†). Furthermore, the mt1 ligand bridges adjacent SBUs, giving rise to right-/left-handed helical chains along the b-axes with a pitch of 14.9877 Å (Fig. S4†). Interestingly, the PW12 anions act as five-connected inorganic ligands to coordinate with five Cu atoms (Fig. 1a) from two adjacent chains with different spiral orientations, leading to the formation of a meso-helical structure with a pitch of 14.9877 Å (Fig. S5†). Up to now, such a symbiotic structure with left-, right-, and meso-helical has rarely been reported, especially in POM systems.26,27 Moreover, the overall result of such a structural organization leads to a two-dimensional (2D) metal–organic layer. However, the connection number (5) of [PW12O40]3− in FUNSOM-3 is too low to construct the ideal model for core–shell nanostructures, and choosing the proper organic ligands and metal salts to achieve compounds with higher connection number becomes the next task.
η1 coordination mode by using its two adjacent N atoms to bridge two Cu ions (Fig. S2†). The Cu1 and Cu3 atoms are simultaneously bridged by mt2 and mt4 ligands to form a binuclear unit. The Cu2 and Cu3 atoms are bridged by an mt3 ligand. Such two binuclear units are merged into trinuclear building units (SBUa) through sharing of the same bridge of the Cu3 atom (Fig. S3†). Furthermore, the mt1 ligand bridges adjacent SBUs, giving rise to right-/left-handed helical chains along the b-axes with a pitch of 14.9877 Å (Fig. S4†). Interestingly, the PW12 anions act as five-connected inorganic ligands to coordinate with five Cu atoms (Fig. 1a) from two adjacent chains with different spiral orientations, leading to the formation of a meso-helical structure with a pitch of 14.9877 Å (Fig. S5†). Up to now, such a symbiotic structure with left-, right-, and meso-helical has rarely been reported, especially in POM systems.26,27 Moreover, the overall result of such a structural organization leads to a two-dimensional (2D) metal–organic layer. However, the connection number (5) of [PW12O40]3− in FUNSOM-3 is too low to construct the ideal model for core–shell nanostructures, and choosing the proper organic ligands and metal salts to achieve compounds with higher connection number becomes the next task.
Inspired by previous reports, 1-methyl-5-mercapto-1,2,3,4-tetrazole (mmt) was introduced into the reaction system,28–30 and FUNSOM-4 was obtained through the hydrothermal reaction of AgOAc, mmt, H4SiW12O40·xH2O and distilled water. Crystal structure analysis reveals that FUNSOM-4 is a 3D metal–organic framework (Fig. 2a) with a 10-connected [SiW12O40]4− anion (SiW12) located in its straight channels (Fig. 2b). The asymmetric unit is shown in Fig. S6.† The basic building block of the cationic structure is the [Ag5(mmt)] fragment, which could be viewed as two isomers, SBUb and SBUc, due to their similar conformation. The two mmt ligands within SBUs adopt a similar μ5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η1
η1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η1
η1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η3 coordination fashion to coordinate with five Ag ions: two adjacent N atoms bridge two Ag ions, the soft S atom coordinates with three Ag ions (Fig. S7†). With the sharing of Ag ions, the two isomers SBUb and SBUc are linked alternately and arrayed in a point-to-face fashion, forming a 1D hybrid chain (Fig. S8†). Furthermore, these parallel chains are hexagonally arranged to form hexagonal cylinders with straight channels. Notably, the SiW12 anions function as 10-connected linkages (Fig. 1b) to coordinate with ten Ag ions through eight terminal O atoms and strongly cement into the straight channels (Fig. S11a†).
η3 coordination fashion to coordinate with five Ag ions: two adjacent N atoms bridge two Ag ions, the soft S atom coordinates with three Ag ions (Fig. S7†). With the sharing of Ag ions, the two isomers SBUb and SBUc are linked alternately and arrayed in a point-to-face fashion, forming a 1D hybrid chain (Fig. S8†). Furthermore, these parallel chains are hexagonally arranged to form hexagonal cylinders with straight channels. Notably, the SiW12 anions function as 10-connected linkages (Fig. 1b) to coordinate with ten Ag ions through eight terminal O atoms and strongly cement into the straight channels (Fig. S11a†).
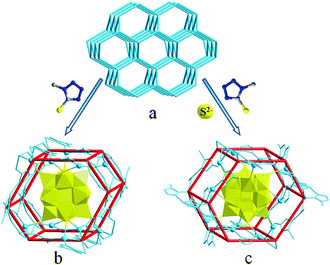 | ||
| Fig. 2 The 3D schematic representation of the metal–organic framework (a) and the coordination details of POMs (b, c) within FUNSOM-4 and -5. With the introduction of S element, the connection number of POMs increases step-by-step. | ||
Remarkably, comparison of the coordination mode between mt and mmt ligands indicates that the introduction of sulphur plays an important role in improving the connection number of POMs. To confirm the influence of sulphur within the high-connected strategy, as well as the achievement of higher connection number compounds, in our further experiments, besides the mmt ligand, the S2− anions were introduced into the reaction system, and a new compound FUNSOM-5 was synthesized (Fig. S9†). The host metal–organic framework is similar to FUNSOM-4. The basic building blocks of the framework are the [Ag4(mmt)] fragments, isomers SBUd and SBUe. The two mmt ligands within these SBUs adopt a similar μ4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η1
η1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η1
η1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η2 coordination fashion to coordinate with four Ag ions: two adjacent N atoms bridge two Ag ions, the soft S atom coordinates with two Ag ions (Fig. S10a–b†). Meanwhile, the PW12 anions use ten terminal O atoms to coordinate with twelve Ag ions (Fig. 1c) and finally incorporate into the hexagonal channels (Fig. 2c and Fig. S11b†). To the best of our knowledge, FUNSOM-5 represents the highest connection number of saturated Keggin-type POMs to date.20,21 Interestingly, the two S2− ions are combined in a S22− ion with S–S bond 1.943 Å. The S22− fragments fill in the void formed by SBUs (Fig. S10c†) and act as S-bridge to coordinate with two terminal Ag atoms in an interlocking manner, which could be recognized as a self-complementary of crystal stability. It is worth mentioning here that the introduction of S2− not only help to improve the connection number of POMs, but may also open up opportunities for the design of new POM-based compounds with novel structures.
η2 coordination fashion to coordinate with four Ag ions: two adjacent N atoms bridge two Ag ions, the soft S atom coordinates with two Ag ions (Fig. S10a–b†). Meanwhile, the PW12 anions use ten terminal O atoms to coordinate with twelve Ag ions (Fig. 1c) and finally incorporate into the hexagonal channels (Fig. 2c and Fig. S11b†). To the best of our knowledge, FUNSOM-5 represents the highest connection number of saturated Keggin-type POMs to date.20,21 Interestingly, the two S2− ions are combined in a S22− ion with S–S bond 1.943 Å. The S22− fragments fill in the void formed by SBUs (Fig. S10c†) and act as S-bridge to coordinate with two terminal Ag atoms in an interlocking manner, which could be recognized as a self-complementary of crystal stability. It is worth mentioning here that the introduction of S2− not only help to improve the connection number of POMs, but may also open up opportunities for the design of new POM-based compounds with novel structures.
For further comprehension on the present POM-based structural model for core–shell nanostructures, the following two questions are critical: How many metal atoms are needed to cover the whole surface of the polyanions? Could the saturated Keggin-type POM core afford enough coordination sites for those metal atoms? According to the mathematical analysis (Scheme S1†), about 23 Ag atoms are needed to form a rounded shell over the whole surface of the POMs. On the other hand, classical Keggin-type POMs (such as [PW12O40]3− and [SiW12O40]4−) have 12 terminal O atoms and 24 bridge O atoms, which could provide at least 36 coordination sites in principle. Therefore, it is reasonable to get a continuous and complete shell over the whole POM through improving the numbers of metal atoms. In practical synthetic cases, some factors (such as, the valid volume of the ligands; the topological and geometrical relations between the metal ions and the ligands, etc.) will affect the connection between the polyanions and the metal atoms, and then may further decrease the connection numbers of POMs. However, these negative influence would be conquered by carefully selecting the metals, POMs, organic ligands, and suitable additives. Based on above analyses, previous reports20,21,31,32 and our experimental results, there is still sufficient confidence in constructing the practical model of core–shell nanostructures by increasing the connection numbers (Fig. 3).
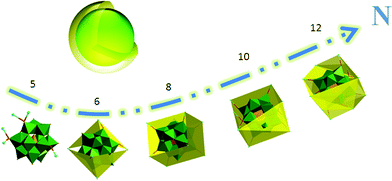 | ||
| Fig. 3 Scheme view of the POM-based highly-connected strategy towards core–shell model: as the number of transition-metals connected with POMs increases, the shell formed progressively. N, connection number. | ||
Here, we should also point out that the present synthetic system is endued with structural tunability by the introduction of the S element (a proposed mechanism is shown in Scheme S2†). It could be envisioned that the POMs with higher negative charges and/or the reintroduction of strong affinitive S element (such as Sx2−, x = 2–6), may further improve the above process, and further work on the optimization of the present sulphur-assisted hydrothermal synthetic method are ongoing.
In conclusion, the tetrazole-based ligand (mt/mmt) and the Keggin-type POMs ([PW12O40]3− and [SiW12O40]4−) have been applied to design and synthesize POM-based compounds with different connection numbers. Based on our present reaction system, the connection numbers can be tuned through introducing strong affinitive S atoms, and the 12-connected modification of saturated Keggin-type POMs towards core–shell model is achieved. Due to their versatile nature, these highly-connected POMs would be used as functional materials, such as catalysts, surface functionalization and photoluminescent devices. Relevant exploration is the subject of our ongoing research. This work, to some extent, indicates that the present synthetic strategy will open a new way for investigating essential properties and principles of the core–shell nanomaterials.
Acknowledgements
This work is supported by the National Basic Research Program of China (973 Program) (No. 2012CB825800, 2010CB934500), National Natural Science Foundation of China (NSFC) (No. 51132006, 21073127, 21071104), A Foundation for the Author of National Excellent Doctoral Dissertation of P R China (FANEDD) (No. 200929), A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and a Project supported by the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (Grant No. 11KJB150015).References
- D. Y. Zhao and Y. Wan, Chem. Rev., 2007, 107, 2821 CrossRef.
- P. Strasser, S. Koh, T. Anniyev, J. Greeley, K. More, C. Yu, Z. Liu, S. Kaya, D. Nordlund, H. Ogasawara, M. F. Toney and A. Nilsson, Nat. Chem., 2010, 2, 454 CrossRef CAS.
- M. B. Cortie and A. M. McDonagh, Chem. Rev., 2011, 111, 3713 CrossRef CAS.
- J. Qiao, K. Shi and Q. M. Wang, Angew. Chem., Int. Ed., 2010, 49, 1765 CrossRef CAS.
- J. J. Gong, W. S. Zhang, Y. Liu, H. C. Zhang, H. L. Hu and Z. H. kang, Dalton Trans., 2012, 41, 5468 RSC.
- T. Liu, M. L. K. Langston, D. Li, J. M. Pigga, C. Pichon, A. M. Todea and A. Mueller, Science, 2011, 331, 1590 CrossRef CAS.
- S. Liu and Z. Tang, Nano Today, 2010, 5, 267 CrossRef CAS.
- T. Yamase, Chem. Rev., 1998, 98, 307 CrossRef CAS.
- D. E. Katsoulis, Chem. Rev., 1998, 98, 359 CrossRef CAS.
- M. T. Pope and A. Muller, Angew. Chem., Int. Ed. Engl., 1991, 30, 34 CrossRef.
- M. Hutin, D. L. Long and L. Cronin, Isr. J. Chem., 2011, 51, 205 CrossRef CAS.
- B. Qin, H. Y. Chen, H. Liang, L. Fu, X. F. Liu, X. H. Qiu, S. Q. Liu, R. Song and Z. Y. Tang, J. Am. Chem. Soc., 2010, 132, 2886 CrossRef CAS.
- A. Dolbecq, E. Dumas, C. R. Mayer and P. Mialane, Chem. Rev., 2010, 110, 6009 CrossRef CAS.
- N. Casan-Pastor and P. Gomez-Romero, Front. Biosci., 2004, 9, 1759 CrossRef CAS.
- C. L. Hill, Chem. Rev., 1998, 98, 1 CrossRef CAS.
- D. L. Long, R. Tsunashima and L. Cronin, Angew. Chem., Int. Ed., 2010, 49, 1736 CrossRef CAS.
- A. Dolbecq, E. Dumas, C. R. Mayer and P. Mialane, Chem. Rev., 2010, 110, 6009 CrossRef CAS.
- S. Nlate, D. Astruc and R. Neumann, Adv. Synth. Catal., 2004, 346, 1445 CrossRef CAS.
- S. Nlate, L. Plault and D. Astruc, Chem.–Eur. J., 2006, 12, 903 CrossRef CAS.
- J. P. Zhang, Y. Y. Lin, X. C. Huang and X. M. Chen, J. Am. Chem. Soc., 2005, 127, 5495 CrossRef CAS.
- X. L. Wang, H. L. Hu and A. X. Tian, Cryst. Growth Des., 2010, 10, 4786 CAS.
- A. F. Stassen, E. M. Ferrero, C. Gimenez-Saiz, E. Coronado, J. G. Haasnoot and J. Reedijk, Monatsh. Chem., 2003, 134, 255 CrossRef CAS.
- Y. J. Kim, J. T. Han, W. S. Han and S. W. Lee, J. Chem. Soc., Dalton Trans., 2002, 3611 RSC.
- A. F. Stassen, M. Grunert, A. M. Mills, A. L. Spek, J. G. Haasnoot, J. Reedijk and W. Linert, Dalton Trans., 2003, 3628 RSC.
- I. D. Brown and D. Altermatt, Acta Crystallogr., Sect. B: Struct. Sci., 1985, 41, 244 CrossRef.
- C. Zhang, H. Pang, M. Hu, J. Li and Y. Chen, Solid State Commun., 2009, 182, 1772 CrossRef CAS.
- C. J. Zhang, H. J. Pang, Q. Tang, H. Y. Wang and Y. G. Chen, Inorg. Chem. Commun., 2011, 14, 731 CrossRef CAS.
- X. L. Wang, H. L. Hu, A. X. Tian, H. Y. Lin and J. Li, Inorg. Chem., 2010, 49, 10299 CrossRef CAS.
- M. Kurmoo, Chem. Soc. Rev., 2009, 38, 1353 RSC.
- M. N. Kozlova, S. Ferlay, N. Kyritsakas, M. W. Hosseini, S. E. Solovieva, I. S. Antipin and A. I. Konovalov, Chem. Commun., 2009, 2514 RSC.
- Y. Q. Lan, S. L. Li, Y. G. Li, Z. M. Su, K. Z. Shao and X. L. Wang, CrystEngComm, 2008, 10, 1129 RSC.
- X. Kuang, X. Wu, R. Yu, J. P. Donahue, J. Huang and C. Z. Lu, Nat. Chem., 2010, 2, 461 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC reference numbers 872046–872048. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra20605f |
‡ Crystal data for FUNSOM-3: Cu3C8N16H16PW12O40, M = 3404.07, monoclinic, space group P21/n, a = 16.9285(16), b = 14.9877(14), c = 19.0063(18) Å, β = 93.066(2)°, V = 4815.4(8) Å3, Z = 4, F(000) = 5944.0, Dc = 4.695 g cm−3, μ = 29.976 mm−1, 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 970 reflections measured, 9422 unique (Rint = 0.0386), final R1 = 0.0397, wR2 = 0.1272, GOF = 1.077, T = 296 K. CCDC number 872046.† FUNSOM-4: Ag8C8S4N16H16SiW12O42, M = 4233.83, monoclinic, space group P21/n, a = 10.0393(12), b = 13.9688(16), c = 19.893(2) Å, β = 102.369(2)°, V = 2725.0(6) Å3, Z = 2, F(000) = 3700.0, Dc = 5.155 g cm−3, μ = 28.293 mm−1, 19 970 reflections measured, 9422 unique (Rint = 0.0386), final R1 = 0.0397, wR2 = 0.1272, GOF = 1.077, T = 296 K. CCDC number 872046.† FUNSOM-4: Ag8C8S4N16H16SiW12O42, M = 4233.83, monoclinic, space group P21/n, a = 10.0393(12), b = 13.9688(16), c = 19.893(2) Å, β = 102.369(2)°, V = 2725.0(6) Å3, Z = 2, F(000) = 3700.0, Dc = 5.155 g cm−3, μ = 28.293 mm−1, 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 690 reflections measured, 4739 unique (Rint = 0.0560), final R1 = 0.0761, wR2 = 0.1511, GOF = 1.305, T = 296 K. CCDC number 872047.† FUNSOM-5: Ag6C8S6H19N16PW12O42, M = 4088.09, monoclinic, space group P21/n, a = 9.9493(12), b = 14.0796(17), c = 19.906(2) Å, β = 101.585(2)°, V = 2731.7(6) Å3, Z = 2, F(000) = 3578.0, Dc = 4.962 g cm−3, μ = 27.613 mm−1, 19 690 reflections measured, 4739 unique (Rint = 0.0560), final R1 = 0.0761, wR2 = 0.1511, GOF = 1.305, T = 296 K. CCDC number 872047.† FUNSOM-5: Ag6C8S6H19N16PW12O42, M = 4088.09, monoclinic, space group P21/n, a = 9.9493(12), b = 14.0796(17), c = 19.906(2) Å, β = 101.585(2)°, V = 2731.7(6) Å3, Z = 2, F(000) = 3578.0, Dc = 4.962 g cm−3, μ = 27.613 mm−1, 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 021 reflections measured, 4764 unique (Rint = 0.0427), final R1 = 0.0436, wR2 = 0.1101, GOF = 0.999, T = 296 K. CCDC number 872048.† 021 reflections measured, 4764 unique (Rint = 0.0427), final R1 = 0.0436, wR2 = 0.1101, GOF = 0.999, T = 296 K. CCDC number 872048.† |
| This journal is © The Royal Society of Chemistry 2012 |
