Conformation and cytotoxicity of a tetrapeptide constellated with alternative D- and L-proline†
Biswadip
Banerji
*a,
Sumit Kumar
Pramanik
a,
Uttam
Pal
b and
Nakul Chandra
Maiti
b
aDepartment of Chemistry, Indian Institute of Chemical Biology, 4 Raja S.C. Mullick Road, Kolkata, India 700032. E-mail: biswadip.banerji@gmail.com; Fax: (+) 91 33 24735197; Fax: 91 33 24723967; Tel: (+) 91 33 24995709
bStructural Biology & Bioinformatics Division, Indian Institute of Chemical Biology, 4 Raja S.C. Mullick Road, Kolkata, India 700032
First published on 5th July 2012
Abstract
Proline containing peptides are highly important due to their natural abundance in various secondary structural elements like turns (β turn and γ turn etc.) in proteins. Here the conformation, cytotoxicity and structure of a unique tetrapeptide composed of alternative D- and L-proline residues are discussed. The peptide showed a polyproline II like conformation in dilute aqueous solution. The aqueous solution of the peptide self-assembled to form spheroidal oligomers with a diameter of ∼90 nm. The morphological features were confirmed by bright field confocal images, TEM analysis and AFM. The alternative D- and L-proline residues in the peptide showed toxicity towards cancer cell lines and ∼50% cell death was recorded against three different types of cancer cells (Neura 2a, HEK 293 and Hep G2).
Proline, a naturally occurring amino acid, possesses a unique cyclic conformation that enables it to play a critical role in many important biological processes including signal transduction, protein folding and stability of natively unfolded protein in the solution state.1–3 Due to its ideal five member heterocyclic ring chemistry, small size and unique conformational flexibility, there is a growing interest to develop biologically active oligo-peptide based drugs against diseases such as cancer and other pathological disorders.4 Here we report the structure and oligomer formation of a unique tetra-peptide made of alternative D- and L-proline residues. The solution state conformation of the peptide was defined by CD spectroscopy and the conversion of the peptide in aqueous environment into self-assambled nano-size oligomers was characterized by TEM, AFM and confocal microscopy. Phase contrast imaging, JC-1 mediated mitochondrial dysfunction and confocal imaging unambiguously confirm that the peptide is cytotoxic to different cancer cell lines.
The tetrapeptide has been synthesized by solution phase peptide synthesis (ESI†). The heterochiral sequence appears to be unique in nature in terms of its conformation and structural propensity. Owing to the puckered ring of the proline residue, the alternate chirality at CHα renders a further twist/turn in the overall structure.
A synergic investigation by Fourier transform infrared (FT-IR), circular dichroism (CD) and computational modelling revealed an extended conformation similar to poly-L-proline, often termed as polyproline II (PPII). The CD spectra of the tetra-peptide in water are shown in Fig. 1. The peptide in dilute conditions (less than 25 μM) showed a negative CD band at ∼199 nm. However, with an increase in peptide concentration, the CD absorption band red-shifted to longer wavelengths, a CD band appeared at ∼215 nm at peptide concentration ∼200 μM. The CD signatures implied that at very dilute conditions the peptide remained very flexible with PPII conformations (CD peaks usually appeared at 195–210 nm for a non-hydrogen bonded PPII structure).5 The possibility of the formation of a monomeric α-helical structure was excluded as the CD signal was not altered to signatory α-helical bands at ∼207 and 222 nm. The α-helical structure could be restricted since the cyclic proline ring potentially imposes strain to (Φ, Ψ) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, which prefer to be exposed towards solvents such as water.6
O bonds, which prefer to be exposed towards solvents such as water.6
![(A) CD spectrum of the peptide at different concentrations [(a) 100 μM, (b) 50 μM, (c) 25 μM, (d) 12.5 μM, (e) 6.5 μM, (f) 3.25 μM]. (B) FT-IR spectrum of a zwitterionic tetrapeptide in a KBr pellet.](/image/article/2012/RA/c2ra20616a/c2ra20616a-f1.gif) | ||
| Fig. 1 (A) CD spectrum of the peptide at different concentrations [(a) 100 μM, (b) 50 μM, (c) 25 μM, (d) 12.5 μM, (e) 6.5 μM, (f) 3.25 μM]. (B) FT-IR spectrum of a zwitterionic tetrapeptide in a KBr pellet. | ||
The PPII conformation has been found as the secondary structure element in the ensemble of many natively unfolded proteins and peptides.7 However, its stability depends on the orientation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group with respect to the backbone axis and hydrogen bond it can form with the solvent molecules. Interestingly, we observed a red shift in the CD signature (band position) upon an increase in the peptide concentration when the oligomeric structure was formed. It indicates that at a higher concentration of the peptide and due to the formation of oligomer, the amide geometry may be changed.
O group with respect to the backbone axis and hydrogen bond it can form with the solvent molecules. Interestingly, we observed a red shift in the CD signature (band position) upon an increase in the peptide concentration when the oligomeric structure was formed. It indicates that at a higher concentration of the peptide and due to the formation of oligomer, the amide geometry may be changed.
The conformation of the peptide was further deduced from FT-IR data in D2O and in the solid state. The solid state infra-red spectra of the peptide in KBr crystals is shown in Fig. 1(b) and different stretching modes are assigned in Table 1 (see ESI†). The IR band at 1649 cm−1 was assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration. Peptides containing more than four L-proline residues and many other small peptides are often characterized with an amide I vibration peak in the range 1640–1650 cm−1 and multiple investigations associated with this peak designate the polyprolin II conformation to this peptide backbone.8 Thus the peptide in the solid state occupies the most preferred PPII conformation. The C
O stretching vibration. Peptides containing more than four L-proline residues and many other small peptides are often characterized with an amide I vibration peak in the range 1640–1650 cm−1 and multiple investigations associated with this peak designate the polyprolin II conformation to this peptide backbone.8 Thus the peptide in the solid state occupies the most preferred PPII conformation. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching mode further indicated that the peptide group was planner.
O stretching mode further indicated that the peptide group was planner.
L-Proline containing peptides can form two stable conformations in aqueous solution and in the solid state as (i) left handed polyproline II (PPII) and (ii) a right handed polyprolin I (PPI).9 However, the PPII structure was the most predominant. PPII is a left-handed helix with an axial translation of 3.2 Å per residue, composing three prolyl residues per turn. It lacks strong intra-residue hydrogen bonding and all the peptide bonds are trans in nature. ψ angles for PPII and PPI were 145° and 158° respectively, and ϕ angles varied between −75° and −85° for both the conformations.10 The energy minimized model conformation of the tetrapeptide showed a conformation with an axial translation of 11.5 Å having 4 residues per turn (Fig. 2D). It indicates planarity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O structure in the prolyl moiety, raising the possibility of forming hydrogen bonds with the surrounding molecules. The C
O structure in the prolyl moiety, raising the possibility of forming hydrogen bonds with the surrounding molecules. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequency indicated extended PPII conformation of the peptide in the solid state without much puckering of the prolyl ring, preserving the planarity of the C
O stretching frequency indicated extended PPII conformation of the peptide in the solid state without much puckering of the prolyl ring, preserving the planarity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group.
O group.
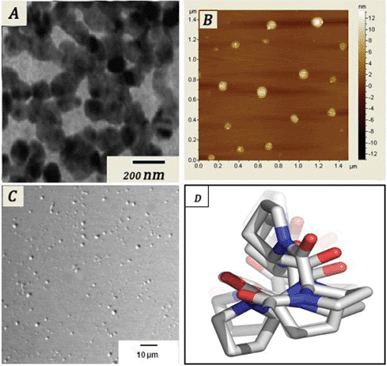 | ||
| Fig. 2 (A) TEM, (B) AFM, (C) confocal images of the self-assembled tetrapeptide; (D) energy minimized structure. | ||
Another important aspect about the peptide is that in aqueous solution (3 mg mL−1) it forms a self assembled structure. The morphology and other microscopic features of the self assembled peptide were characterized by transmission electron microscopy (TEM), atomic force microscopy (AFM) and confocal microscopy. The TEM investigation of the aqueous solution (3 mg mL−1) of the tetrapeptide was carried out on a carbon coated copper grid (300 mesh) after slow evaporation and vacuum drying of the sample at 30 °C for 24 h.
The TEM image (Fig. 2A) of the oligomer shows spherical shapes of nearly 90–100 nm diameter. For AFM one drop of aqueous solution (3 mg mL−1) of this tetrapeptide was taken on a mica foil and evaporated to dryness under vacuum for 10 h. The AFM image of the oligomers also confirmed the formation of nano-size (90–100 nm) spherical balls. The differential interference contrast (DIC) image observed from the confocal microscope showed a uniform spherical structure in the aqueous phase. Furthermore DLS studies were performed to get the size distribution in solution, which was uniformly 90–100 nm in size.
The peptide showed substantial cytotoxicity and potentially killed cultured cancer cells. Neura 2a, HEK 293 and human hepatocellular carcinoma cell line Hep G2 were subjected to the tetrapeptide. The viable cells were evaluated using a MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay where the viability of the cells was determined by the reduction of the yellow MTT into the purple formazan product by mitochondrial dehydrogenase present in metabolically active cells.11 The absorbance obtained from treated cells was expressed as percentages of absorbance obtained from untreated cells and are reported as mean ± Sd (n = 3). Fig. 3 shows the cell viability of different cell lines after incubation of the control and with 10 μM concentration of this tetrapeptide.
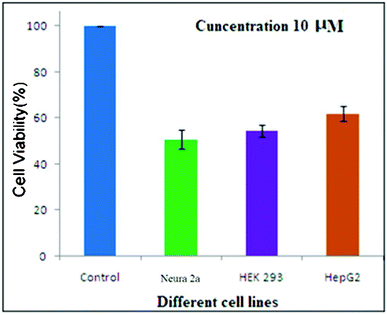 | ||
| Fig. 3 Cytotoxicity studies of the tetrapeptide against three different cell lines. | ||
After incubation, the cell viability was measured and estimated to be 50.64%, 54.3% and 61.9% for the Neura 2a, HEK 293 and Hep G2 respectively. The proliferation of these cancer cells was reduced significantly at a dose of 10 μM. The results of this study provide another big step for the eventual use of catalytic drugs, which could have a large innovative impact on human medicine and health care systems.
Furthermore, cells were also examined under an inverted phase contrast microscope. For example, Hek 293 cells were treated with this peptide for 48 h and phase contrast micrography was taken. As shown in Fig. 4, there was massive cell death in response to this tetrapeptide as compared to the control. It is worthy to mention here that the peptide under consideration is non-toxic to healthy cells.
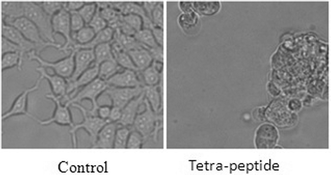 | ||
| Fig. 4 Phase contrast images showing cell death. | ||
Mostly, apoptosis happens through the disruption of active mitochondria. JC-1 dye is widely used to study mitochondrial health (i.e. indicator of mitochondrial membrane potential). This dye can exist as either a green fluorescent monomer at depolarized membrane potential or as a reddish orange fluorescent J-aggregate at hyperpolarized membrane potential.
Cells with good mitochondrial health show punctuated red staining along with green scattered staining, whereas the cells with mitochondrial dysfunction show only scattered green staining without any red punctuation. To check the mitochondrial health, cells were treated with this peptide.12 For this the tetra-peptide was treated with Hek 293 cells at 50 μM for 24 h. Cells were then stained with JC-1 dye (10 μg ml−1) for 15 min at 37 °C and live cells were imaged under a fluorescence microscope. As shown in Fig. 5, cells treated with this tetra-peptide result in loss of mitochondrial membrane potential as punctuated red staining is diminished compared to the control. That means the tetra-peptide causes cell death, at least in part, through mitochondrial dysfunction.
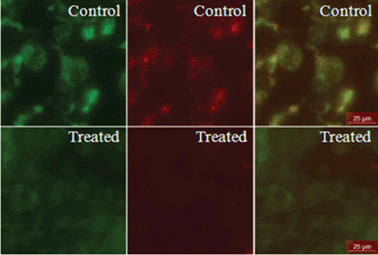 | ||
| Fig. 5 JC-1 staining of HEK 293 cells. Cells were treated with the tetra-peptide overnight, then stained with JC-1 dye for checking mitochondrial health. The cells were then studied and imaged under a fluorescence microscope. The second row shows control cells and cells treated with the tetra-peptide. The first column represents the green fluorescent monomer of JC-1, the second column represents the reddish orange fluorescence of the J-aggregate and the third column shows merged images. Scale bar represents 25 μm. | ||
Caspase 3 is cleaved in response to mitochondrial disruption and activated, hence a well known marker for apoptosis.13 We wanted to check whether this tetrapeptide activates caspase 3. To achieve this goal we treated HEK 293 cells with 50 μM of this tetra-peptide for 24 h. The cells were then fixed by 4% PFA and fixed cells were immunostained using a primary antibody, specific for cleaved caspase 3, and Alexa fluor 546 as a secondary antibody. The cells were imaged under confocal microscope. The result shows elevated active caspase 3 levels in treated cells (Fig. 6), which indicates the cell death.
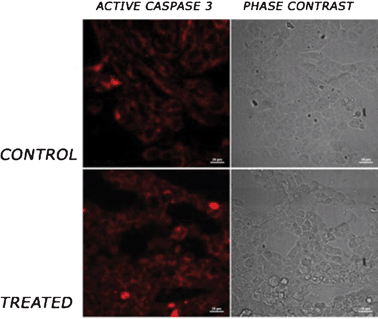 | ||
| Fig. 6 Active caspase 3 staining. HEK 293 cells were treated with the tetrapeptide overnight. The cells were fixed with 4% paraformaldehyde (PFA) and immunostained using a primary antibody against active caspase 3 and alexa-fluor 546 as the secondary antibody. The cells were imaged under a confocal microscope. First and second rows show control cells and cells treated with tetra-peptide. First and second columns show red staining for active caspase 3 and phase contrast micrographs of cells, respectively. Scale bar represents 20 μm. | ||
In conclusion this communication described the conformation, structure, self assembly aspects and cytotoxicity profile of a tetrapeptide constructed with heterochiral ‘D-pro-L-pro’ sequence. The FT-IR and CD data of the tetra peptide confirm a polyproline II structure, whereas its aqueous solution undergoes self association as recorded by TEM, AFM and confocal microscopy. Interestingly the tetra-peptide showed moderate cytotoxicity to different cancer cell lines and evidence comes from phase contrast and fluorescent microscopy, and confocal studies clearly show cell death.
Acknowledgements
S.P. thanks CSIR, New Delhi, India for a project assistantship, U.P. thanks DST India for financial support. We would also like to thank CSIR, India for providing a research grant through the Network-project, NWP0035.References
- T. Nanjo, M. Kobayashi, Y. Yoshiba, Y. Sanada, K. Wada, H. Tsukaya, Y. Kakubari, Y.-K. Shinozaki and K. Shinozaki, Biological functions of proline in morphogenesis and osmotolerance revealed in antisense transgenic Arabidopsis thaliana., Plant J., 1999, 18(2), 185–193 CrossRef CAS.
- B. K. Kay, M. P. Williamson and M. Sudol, The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains., The FASEB Journal, 2000, 14(2), 231–241 CAS.
- T. Nakano, L. C. Antonino, R. O. Fox and A. L. Fink, Effect of proline mutations on the stability and kinetics of folding of staphylococcal nuclease, Biochemistry, 1993, 32(10), 2534–2541 CrossRef CAS.
- N. Venkatesan and B. H. Kim, Peptide Conjugates of Oligonucleotides: Synthesis and Applications, Chem. Rev., 2006, 106(9), 3712–3761 CrossRef CAS.
- (a) A. L. Rucker and T. P. Creamer, Polyproline II helical structure in protein unfolded states: Lysine peptides revisited., Protein Science, 2002, 11(4), 980–985 CAS; (b) M. L. Tiffany and S. Krimm, Circular dichroism of poly-L-proline in an unordered conformation., Biopolymers, 1968, 6(12), 1767–1770 CrossRef CAS; (c) R. W. Woody, Circular dichroism spectrum of peptides in the poly(Pro)II conformation, J. Am. Chem. Soc., 2009, 131(23), 8234–45 CrossRef CAS.
- E. H. Strickland, M. Wilchek, J. Horwitz and C. Billups, Effects of Hydrogen Bonding and Temperature Upon the Near Ultraviolet Circular Dichroism and Absorption Spectra of Tyrosine and O-Methyl Tyrosine Derivatives, Journal of Biological Chemistry, 1972, 247(2), 572–580 CAS.
- A. V. Mikhonin and S. A. Asher, Uncoupled Peptide Bond Vibrations in α-Helical and Polyproline II Conformations of Polyalanine Peptides, J. Phys. Chem. B, 2005, 109(7), 3047–3052 CrossRef CAS.
- (a) M. L. Tiffany and S. Krimm, Biopolymers, 1972, 11, 2309 CrossRef CAS; (b) S. Krimm and A. Yasuaki, Proc. Natl. Acad. Sci. U. S. A., 1972, 69, 2788 CrossRef CAS; (c) S. Krimm and J. Bandekar, Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins, Adv. Protein Chem., 1986, 38, 181–364 CrossRef CAS.
- Y. K. Kang, J. S. Jhon and H. S. Park, Conformational Preferences of Proline Oligopeptides, J. Phys. Chem. B, 2006, 110(35), 17645–17655 CrossRef CAS.
- (a) S. Arnott and S. D. Dover, The structure of poly-l-proline II, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1968, 24(4), 599–601 CrossRef CAS; (b) A. A. Adzhubei and M. J. E. Sternberg, Left-handed Polyproline II Helices Commonly Occur in Globular Proteins, J. Mol. Biol., 1993, 229(2), 472–493 CrossRef CAS.
- B. Banerji, S. K. Pramanik, S. Mandal, N. C. Maiti and K. Chaudhuri, Synthesis, characterization and cytotoxicity study of magnetic (Fe3O4) nanoparticles and their drug conjugate, RSC Adv., 2012, 2(6), 2493–2497 RSC.
- T. Liu, B. Hannafon, L. Gill, W. Kelly and D. Benbrook, Flex-Hets differentially induce apoptosis in cancer over normal cells by directly targeting mitochondria, Mol. Cancer Ther., 2007, 6(6), 1814–22 CrossRef CAS.
- C. M. Troy and E. M. Ribe, Caspase-2: vestigial remnant or master regulator?, Sci. Signaling, 2008, 1(38), pe42 CrossRef.
Footnote |
| † Electronic Supplementary Information (ESI) available: The synthesis procedure, procedures for the FT-IR, CD-spectroscopy, AFM, TEM, confocal and cell culture studies are included. See DOI: 10.1039/c2ra20616a |
| This journal is © The Royal Society of Chemistry 2012 |
