Cotton-assisted preparation of mesoporous manganese oxide for supercapacitors†
Siheng
Li
ab,
Li
Qi
*a,
Lehui
Lu
a and
Hongyu
Wang
a
aState Key Laboratory of Electroanalytical Chemistry, and Jilin Provincial Key Laboratory of Advanced Low-carbon Chemical Power, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, 5625 Renmin St., Changchun, 130022, China
bGraduate University of Chinese Academy of Sciences, Beijing, 100039, China. E-mail: qil@ciac.jl.cn; Tel: +86 431 85262915; Fax: +86 431 85262287
First published on 30th May 2012
Abstract
A cotton-assisted preparation method of mesoporous manganese oxides for supercapacitors is reported. The samples were fully characterized to study the morphology, chemical composition, surface area and pore structure. The results of the electrochemical tests show that these electrode materials possessed good capacitive nature and long cycling life after 3000 cycles.
With the rapid depletion of fossil fuels and increasingly worsening environmental pollution being one of the most prevalent issues in this century, the development of clean and renewable energy as substitute fuels is becoming more and more urgent. Moreover, to tackle climate change, energy is one of the most important global topics in this century. Recently, supercapacitors (or electrochemical capacitors) have attracted keen interest worldwide mainly owing to their capability of providing higher power densities than batteries, and higher energy densities than conventional dielectric capacitors.1,2 In the supercapacitor family, pseudocapacitors based on redox processes have shown the potential to combine the high energy density of batteries and the high power capabilities of conventional electrostatic capacitors.3,4
In recent years, the research on manganese oxides as candidate electrode material for supercapacitors is growing vigorously probably due to their lower cost, earth abundance and environmentally benign nature.4–8 Various strategies have been proposed to prepare pure MnOx and their composites using carbon materials9–11 or metal oxides12–14 over the past decades. However, most of those methods are mainly achieved by the reduction of MnO4− in acidic solutions, oxidation of Mn2+ under basic condition or the redox reaction between MnO4− and Mn2+ or organics,15–19 which hardly meet the needs of clean industrialization. In this paper, we developed a facile and green strategy to prepare amorphous porous manganese oxides for supercapacitors. The merits are quite apparent. Firstly, it is a facile process based on a mild redox reaction between potassium permanganate and absorbent cotton without any other templates or surfactants under ambient conditions. Moreover, mesoporous structures were formed in one step, avoiding complicated apparatus and processes. Secondly, most of the reagents, like the water solvent and absorbent cotton, are environmentally benign. The obtained manganese oxides showed satisfactory capacitive behavior in neutral Na2SO4 aqueous electrolytes. Moreover, in galvanostatic cycling tests, the asymmetric capacitor composed with Maxsorb carbon negative MnO2 positive electrode showed a good cyclic stability after 3000 cycles. To the best of our knowledge, this is the first report on the preparation of MnO2 based electrode materials using cotton as a reducing agent. In addition, the aqueous electrolytes based devices are much safer and cleaner than the non-aqueous ones. Thus, we hope this procedure may be useful for practical industrial applications of manganese oxide electrode materials.
The synthesis of mesoporous manganese oxide was performed using the following procedures. At first, the cotton was pretreated according to the previous procedure with little modification.20 Then, a certain amount of absorbent cotton was added to 100 mL KMnO4 aqueous solution (0.04 mol L−1) with continuous vigorous stirring and ultrasonic irritation for 20 min. Finally, a dark brown precipitate was obtained when the mixed solution of cotton and KMnO4 was heated at 100 °C for some time. When the added cotton and reaction time were 50 mg and 12 h, the sample of product was labeled as M(50-12). Other products under different reaction conditions were also prepared for comparison (see ESI†). Dark brown precipitates were collected by centrifugation and washing several times with deionized water and ethanol and then dried in air at 80 °C for 12 h.
The morphology and crystal structure of products were examined using scanning electron microscopy (SEM, JEOL JSM-840) and X-ray diffraction (XRD) using a Rigaku D/max-IIB X-ray diffractometer. IR spectra were recorded in the range 400–4000 cm−1 on an Alpha Centauri FT/IR spectrophotometer. X-ray photoelectron spectroscopy (XPS) analysis has been done in order to obtain information about the chemical composition of the products. The nitrogen adsorption–desorption measurements were obtained using an Autosorb-1 (Quantachrome Instruments) analyzer at 77.3 K. Prior to the experiments, the samples were outgassed at 353 K for 24 h. The cyclic voltammetric and galvanostatic charge–discharge cycle measurements were similar to our previous report.21
Fig. 1 shows the characterization of manganese oxide when 50 mg cotton was added at 100 °C for 12, 24 and 48 h. The SEM image shows that the diameters were 250–300 nm. (Fig. 1a–c). In Fig. 1d, the broad XRD peak between 2θ = 20° and 30° was the peak of the glass substrate or residual carbon component. The other two main peaks at 37.2° and 66.2° can be indexed to the α-MnO2 (JCPDS NO. 44-0141). Broad and low intensity of the signals suggests the amorphous nature of MnO2.19 In the FTIR spectra, the broad band 400–800 cm−1 are attributed to the bending vibration of Mn–O (Fig. 1e).19,21
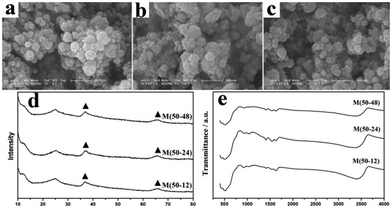 | ||
| Fig. 1 (a–c) SEM image, (d) XRD patterns and (e) FTIR spectra of M(50-12), M(50-24) and M(50-48). | ||
Moreover, the XPS analyses (Fig. 2) further confirmed the composition of manganese dioxide. For instance, M(50-12), two peaks located at 641.9 and 653.6 eV with a spin–energy separation of 11.7 eV in the Mn2p spectrum could be attributed to Mn2p3/2 and Mn2p1/2, respectively (Fig. 2a). The data agreed well with the reported results for MnO2 and indicated a tetravalence oxidation state for Mn. O1s spectrum of the samples could be deconvoluted into three components, which were related to the Mn–O–Mn bond for the tetravalent oxide, the Mn–O–H bond for a hydrated trivalent oxide, and the H–O–H bond for residual water.22 To study the porous nature, gas absorption measurements were carried out (see ESI†). It could be proved that those samples possessed mesoporous nature. Detailed results of SBET, pore size and pore volume of the samples are shown in Table 1.
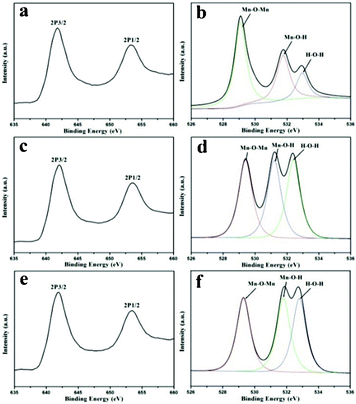 | ||
| Fig. 2 (a) Mn2p and (b) O1s XPS spectrum of M(50-12); (c) Mn2p and (d) O1s XPS spectrum of M(50-24); and (e) Mn2p and (f) O1s XPS spectrum of M(50-48). | ||
| Sample | S BET (m2 g−1) | Pore size (nm) | Pore volume (cm3 g−1) |
|---|---|---|---|
| M(50-12) | 45.6 | 51.2 | 0.585 |
| M(50-24) | 62.3 | 24.1 | 0.375 |
| M(50-48) | 37.9 | 27.6 | 0.262 |
| M(100-24) | 50.1 | 23.5 | 0.300 |
| M(100-48) | 92.1 | 17.0 | 0.392 |
| M(200-24) | 47.9 | 15.8 | 0.190 |
| M(200-48) | 24.1 | 18.6 | 0.113 |
| M(400-24) | 39.2 | 10.9 | 0.107 |
| M(400-48) | 39.2 | 10.8 | 0.105 |
| M(1000-24) | 15.6 | 16.6 | 0.065 |
| M(1000-48) | 19.7 | 14.9 | 0.074 |
Fig. 3a–c shows the cyclic voltammograms (CVs) of M(50-12), M(50-24) and M(50-48) in 1 mol L−1 Na2SO4 aqueous solutions at various scan rates. Relatively rectangular curves with redox peaks implied the ideal electrochemical reversibility and capacitive properties of the MnO2. As is well known, two mechanisms have been proposed for charge storage for MnO2 electrodes. One is based on adsorption/desorption of Na+ ions on the surface of electrode materials. The other involves the intercalation/deintercalation of H+ or Na+ ions in the electrode during reduction and oxidation. Thus, cycling the composite electrode at high rates will hinder the Na+ ions reaching the outer surface, resulting in a decrease in the pseudocapacitance. In CV curves, the redox peaks in CVs, which were also attributed to the deintercalation and intercalation of the cations in electrodes,6 were more apparent in curves at low scan rates, revealing the pseudocapacitive nature of the present MnO2. The corresponding specific capacitance (SC) values at the scan rate of 1 mV s−1 were 178.9, 177.4 and 175.1 F g−1, decreased with increasing dwell time. The overlapped CV curves of Maxsorb and M(50-48) in 1 mol L−1 Na2SO4 at 5 mV s−1 in the positive and negative portion are shown in Fig. 3d. Thus, the cutoff voltage of the asymmetric capacitors fabricated with Maxsorb and MnO2 as negative and positive electrodes, respectively, could reach 1.7 V when conducting the galvanostatic charge–discharge measurements.12 In addition, the calculated SC values from CVs are summarized in Table S1, and the detailed characterizations of other samples are also presented in the ESI.†
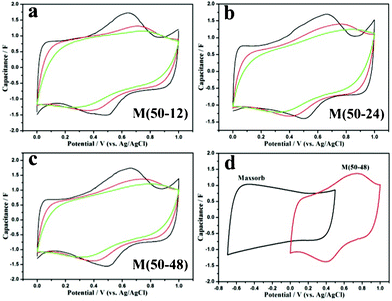 | ||
| Fig. 3 (a–c) CV curves of M(50-12), M(50-24) and M(50-48) in 1 mol L−1 Na2SO4 aqueous solutions at various scan rates (from inner to outer: 10, 5 and 1 mV s−1). (d) Overlapped CVs of Maxsorb and M(50-48) in 1 mol L−1 Na2SO4 at 5 mV s−1. | ||
Initial several charge–discharge profiles of the asymmetric capacitors Maxsorb/MnO2 at a current density of 166.67 mA g−1 are shown in Fig. 4a. The symmetrical trilateral curves and the linear relationship of the potential versus time revealed the ideal cyclic performance of the asymmetric capacitors. Long cycling measurements (Fig. 4b) of capacitors based on most MnO2 samples for 3000 charge–discharge cycles imply stable cycling performances and long cycling life. The reason for the poor cycling stability of M(1000-24) and M(1000-48) might be the excessive addition of cotton in the preparations, resulting in the low weight ratios of MnO2 in the final samples. To obtain detailed and specific information further studies are needed.
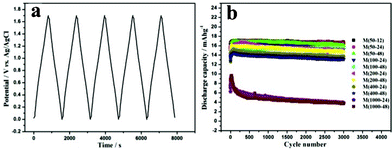 | ||
| Fig. 4 (a) Galvanostatic charge–discharge curves and (b) Long cycle performance of asymmetric capacitors Maxsorb/MnO2 in 1 mol L−1 Na2SO4 at a current density of 166.67 mA g−1 (from top to bottom: M(50-12), M(50-24), M(50-48), M(100-24), M(100-48), M(200-24), M(200-48), M(400-24), M(400-48), M(1000-24) and M(1000-48)). The discharge capacity values were calculated by taking into account the total mass of MnO2 (6 mg) and Maxsorb (6 mg). | ||
Conclusions
A green cotton-assisted preparation strategy of mesoporous manganese oxide for supercapacitors was reported. The results obtained via cyclic voltammetry and galvanostatic charge–discharge tests in Na2SO4 aqueous electrolyte showed that this mesoporous manganese oxide electrode material possessed ideal electrochemical capacitive performances and long cycling life after 3000 cycles within a working voltage of 1.7 V. As the method was achieved by a cotton-assisted route, we believe that this preparation method will surely play a key role in future industrial manufacture of MnO2 electrode material for supercapacitors.Acknowledgements
This project was supported by National Basic Research Program of China (2011CB935702), Hundred Talents Program of Chinese Academy of Sciences and Scientific Research Foundation for the Returned Overseas Chinese Scholars and State Education Ministry (SRF for ROCS, SEM).References
- B. E. Conway, Electrochemical supercapacitors: Scientific fundamentals and technological applications, Kluwer Plenum Pub. Co., New York, 1999 Search PubMed.
- S. Sarangapani, B. V. Tilak and C. P. Chen, J. Electrochem. Soc., 1996, 143, 3791 CrossRef CAS.
- J. P. Zheng, P. J. Cygan and T. R. Jow, J. Electrochem. Soc., 1995, 142, 2699 CrossRef CAS.
- X. H. Lu, G. M. Wang, T. Zhai, M. H. Yu, J. Y. Gan, Y. X. Tong and Y. Li, Nano Lett., 2012, 12, 1690 CrossRef CAS.
- M. Toupin, T. Brousse and D. Bélanger, Chem. Mater., 2004, 16, 3184 CrossRef CAS.
- T. Brousse, M. Toupin, R. Dugas, L. Athouël, O. Crosnier and D. Bélanger, J. Electrochem. Soc., 2006, 153, A2171 CrossRef CAS.
- H. Jiang, T. Sun, C. Z. Li and J. Ma, J. Mater. Chem., 2012, 22, 2751 RSC.
- X. H. Lu, D. Z. Zheng, T. Zhai, Z. Q. Liu, Y. Y. Huang, S. L. Xie and Y. X. Tong, Energy Environ. Sci., 2011, 4, 2915 CAS.
- J. Kim, K. H. Lee, L. J. Overzet and G. S. Lee, Nano Lett., 2011, 11, 2611 CrossRef CAS.
- L. Y. Yuan, X. H. Lu, X. Xiao, T. Zhai, J. J. Dai, F. C. Zhang, B. Hu, X. Wang, L. Gong, J. Chen, C. G. Hu, Y. X. Tong, J. Zhou and Z. L. Wang, ACS Nano, 2010, 4, 2822 CrossRef.
- R. R. Jiang, T. Huang, Y. Tang, J. Liu, L. G. Xue, J. H. Zhuang and A. H. Yu, Electrochim. Acta, 2009, 54, 7173 CrossRef CAS.
- X. H. Lu, T. Zhai, X. H. Zhang, Y. Q. Shen, L. Y. Yuan, B. Hu, L. Gong, J. Chen, Y. H. Gao, J. Zhou, Y. X. Tong and Z. L. Wang, Adv. Mater., 2012, 24, 938 CrossRef CAS.
- J. G. Wen, X. Y. Ruan and Z. T. Zhou, J. Phys. Chem. Solids, 2009, 70, 816 CrossRef CAS.
- E. H. Liu, W. Li, J. Li, X. Y. Meng, R. Ding and S. T. Tan, Mater. Res. Bull., 2009, 44, 1122 CrossRef CAS.
- O. A. Vargas, A. Caballero, L. Hernán and J. Morales, J. Power Sources, 2011, 196, 3350 CrossRef CAS.
- N. Tang, X. K. Tian, C. Yang, Z. B. Pi and Q. Han, J. Phys. Chem. Solids, 2010, 71, 258 CrossRef CAS.
- V. Subramanian, H. Zhu, R. Vajtai, P. M. Ajayan and B. Wei, J. Phys. Chem. B, 2005, 109, 20207 CrossRef CAS.
- P. Ragupathy, D. H. Park, G. Campet, H. N. Vasan, S. J. Hwang, J. H. Choy and N. Munichandraiah, J. Phys. Chem. C, 2009, 113, 6303 CAS.
- P. Ragupathy, H. N. Vasan and N. Munichandraiah, J. Electrochem. Soc., 2008, 155, A34 CrossRef CAS.
- Q. L. Li, C. R. Zhang, Z. S. Xue and J. Q. Li, Chin. J. Chem. Phys., 2010, 23, 207 CrossRef CAS.
- S. H. Li, L. Qi, L. H. Lu and H. Y. Wang, RSC Adv., 2012, 2, 3298–3308 RSC.
- B. Babakhani and D. G. Ivey, J. Power Sources, 2010, 195, 2110 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Characterization of other MnO2 samples under different reaction conditions: SEM images, XRD patterns, FTIR spectra, XPS spectra, N2 adsorption–desorption isotherms, CV curves; Table of summary for specific capacitance of various MnO2 samples. See DOI: 10.1039/c2ra20595e |
| This journal is © The Royal Society of Chemistry 2012 |
