Enhanced energy storage and rate performance induced by dense nanocavities inside MnWO4 nanobars†
En
Zhang‡
b,
Zheng
Xing
a,
Ji
Wang
b,
Zhicheng
Ju
*a and
Yitai
Qian
ab
aHefei National Laboratory for Physical Sciences at Microscale and Department of Chemistry, University of Science and Technology of China, Hefei, Anhui 230026, P. R. China. E-mail: juzc@ustc.edu.cn; Fax: +86-551-360-7402; Tel: +86-551-360-1589
bSchool of Chemistry and Chemical Engineering, Shandong University, Jinan, 250100, P. R. China
First published on 6th July 2012
Abstract
MnWO4 nanobars with dense nanocavities have been synthesized through a one pot hydrothermal route. Galvanostatic cycling of the MnWO4 lithium battery anode exhibits an excellent rate performance. The standout electrochemical performance could be attributed to the unique geometry nanocavities and improved accommodation of the transformation strains during cycling.
Introduction
Over the past few decades, great progress has been made in the field of lithium-ion batteries (LIB). However, the commercially available graphitic anodes of LIB with only a theoretical capacity of 372 mAh g−1 could not meet the growing demand for high energy density and high power density batteries.1 Therefore, many scientists are constantly looking for some new anode materials with high specific capacity, high energy densities and excellent capacity retention.2–4 As we all know, the electrochemical properties of electrode material is greatly associated with the particle properties, such as size, morphology, specific surface area and so on. To improve electrochemical performances, nanostructured electrode materials were applied to these batteries due to their small particle size with large surface area leading to shorter Li+ insertion/extraction pathways and higher electrode/electrolyte contact area which is beneficial for achieving high rate capability and good cycling performance.5 Thus, designing and tailoring the configuration and morphology of materials from the micron to nanometer scale is the major challenging issue to materials scientists.6Transition metal oxides have been considered as promising alternative anode materials owing to their high specific capacity, lower operating voltage, environmental friendliness and they may even have the advantage of being more economical.7 However, the large volume changes resulted in poor capacity retention and low energy efficiency during the electrochemical conversion process, which has hindered their applications in LIB.8 Hollow nanostructured materials could enhance structural integrity with sufficient interior space for buffering against the local volume variation during the Li+ insertion/extraction, which can effectively alleviate pulverization and aggregation of the electrode materials, hence improving the capacity retention.9 Therefore, the hollow nanostructures can provide a good opportunity for developing Li-ion storage technologies.
MnWO4 materials have attracted increasing attention because of their humidity sensing, magnetic properties and shows potential application ranging from medicine, meteorology, to agriculture.10,11 However, the study into their electric capability has rarely been reported. MnWO4 belongs to the monoclinic P2/c space group, and it can be described as the Mn and W atoms each being in an approximately octahedral coordination surrounded by six nearest neighbor oxygen atom sites, similar to ternary transition metal oxides. Therefore, MnWO4 is a potential anode material for LIB, because both Mn and W are electrochemically active metals, just like Li metal. In addition, MnWO4 exhibits an excellent electrochemical property—ionic conductivity for hydronium ions12—which may also be conducive to lithium-ion and electron conduction. MnWO4 materials have usually been synthesized by recrystallization or chemical reaction in a molten salt media, mechanical grinding in a mill, and high-temperature thermal decomposition processes.13 Recently, wet chemical methods have been developed to prepare MnWO4 nanorods, nanofibers, nanoplates, etc., which made a substantial progress.14,15
Nanocavities are isolated entities inside an organic or inorganic solid materials and, thus, are very different from nanopores, which connect together and are open to the surface.16 Moreover, not only do nanocavities provided a framework similar to hollow structures, but also they may supply a useful environment for absorption or reaction. So, internal surfaces of nanocavities are exceptionally useful for enhancing spontaneous emission rate,17 light emission18 or optical absorption.16 Generally, nanocavities were made by neutron irradiation,19 gas ions,20 thermal decomposition16 and so on.21 However, the materials with nanocavities in the LIB field have not yet attracted much attention.22 Herein, we report an effective hydrothermal route to prepare MnWO4 nanobars with dense nanocavities. We found that these dense nanocavities significantly enhance the specific capacity and rate performance, thereby providing a new approach to increase the energy storage in the LIB field. The insightful information will benefit the design of the anode materials in LIB.
Results and discussion
The crystal structure of the as-obtained products were identified by X-ray diffraction (XRD) patterns. Fig. 1 shows the XRD pattern of the product synthesized through a typical reaction. All reflection peaks can be indexed as the monoclinc phase of MnWO4 (JCPDS Card No. 13-0434, space group P2/c). The lattice parameters calculated from the pattern (a = 4.822 Å, b = 5.761 Å and c = 4.986 Å) were in good agreement with the report value (a = 4.829 Å, b = 5.756 Å and c = 4.998 Å). The strong intensity of the sharp diffraction peaks suggests that the MnWO4 is highly crystallized. No peaks from the impurities could be found in this pattern, which indicates that the samples obtained were pure-phase MnWO4 materials.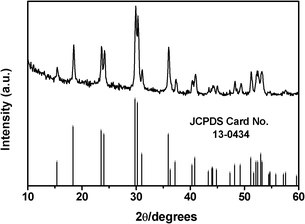 | ||
| Fig. 1 A typical XRD pattern of MnWO4 nanobars. | ||
The morphology of the as-prepared MnWO4 is characterized by transmission electron microscopy (TEM) (Fig. 2). Overall TEM observation show that the nanobars have similar shape and size distributions in the range of 20–30 nm (Fig. 2a). Furthermore, these nanobars are single crystals and with dense nanocavities inside. The typical size of the nanocavities is a statistical distribution in the range of 5–8 nm, and with a sharp polyhedral shape, as shown in the high-magnification image viewed along the principle directions (Fig. 2b). The high-resolution TEM (HRTEM) image recorded from the middle part of a randomly selected nanobar clearly shows that the spacing between any two adjacent lattice fringes is 2.95 Å, corresponding to that of the (111) planes of the monoclinic MnWO4 phase. The corresponding energy-dispersive X-ray spectroscopy (EDS) showed that the element here is mainly W, Mn and O (Fig. S1, see ESI for details†). The electron diffraction pattern (Fig. 2c) taken from the whole nanobar was indexed as the [010] zone axis of MnWO4, indicating that the nanobar is still a single crystal phase. Fig. 2d shows a typical MnWO4 crystal structure which belongs to the monoclinic P2/c space group, and consists of edge-sharing [MnO6] and [WO6] octahedra that form zigzag chains along the c-axis; tungsten atoms and manganese atoms are arranged in alternating sheets parallel to (100). Interestingly, the nanocavities inside the MnWO4 nanobars are rarely present near the edge of the nanobars, which is similar to the TiO2 nanorods from the thermal decomposition of H2Ti3O7.16 The process of formation of the dense nanocavities in the MnWO4 nanobars is analogous to the mechanism of inside-out Ostwald ripening,23 that is, selective dissolution of the interior and re-deposition on the shell caused by the supersaturation and vacancy. Hematite spindles,24 SnO2 hollow spheres,25 Cu2O nanocubes,26etc., are observed to undergo similar selective dissolution of the interiors under hydrothermal or solvothermal conditions with the exterior protected by adsorbed inorganic salt anions.
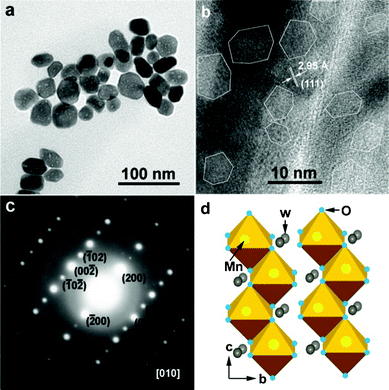 | ||
| Fig. 2 TEM image of MnWO4 nanobars with dense nanocavities (a), HRTEM image taken on randomly selected nanobars (b), a typical selected area electron diffraction pattern (c), schematic structure of MnWO4 crystal (d). | ||
To gain insight into electrochemical reaction mechanism, the cyclic voltammograms (CV) of the MnWO4 are measured and illustrated in Fig. 3. The CV curves of MnWO4 electrode carried out at a scan rate of 0.1 mV s−1 in the voltage range of 0.01–3.0 V versus Li/Li+. The first discharge cycle differs from the other charge/discharge cycles because of the irreversible destruction of the MnWO4 structure. In the first cathodic scan, two obvious reduction peaks are observed at around 1.17 and 0.63 V, possibly corresponding to the reduction of Mn2+, W6+, and the formation of Mn, W (Fig. S2, see ESI for details†) and amorphous Li2O, respectively. Moreover, stepwise redox processes are readily observed in the charge profile. The subsequent charge cycles showed anodic peaks located in 1.1–1.3 V, which could be attributed to the formation of MnO27 and WO3.28,29 The following cycle showed a pair of redox peaks at around 1.56 and 0.34 V in the discharge cycle and at around 1.20 and 2.0 V in the charge cycle. The oxidation peak at 2.0 V that disappeared in the subsequent cycles could be attributed to an irreversible process. Furthermore, a positive shift and increased intensity of the pairs of redox peaks in Fig. 3 could be attributed to the conversion reaction of WO3 with Li, which is similar to the ZnWO4 anode materials.30 Therefore, we expected the following electrochemical reaction mechanism would be responsible for the reaction of MnWO4 with Li.
| MnWO4 + 8Li+ + 8e− → Mn + W + 4Li2O | (1) |
| Mn + W + 4Li2O ↔ MnO + WO3 + 8Li+ + 8e− | (2) |
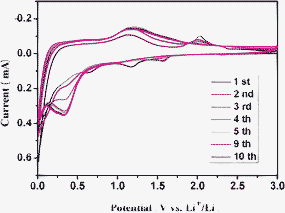 | ||
| Fig. 3 Cyclic voltammetry of the MnWO4 nanobars with dense nanocavities at a scan rate of 0.1 mV s−1 in the voltage range of 0.01–3.0 V vs. Li+/Li. | ||
Electrochemical performance of the sample was also examined at a current rate of 100 mA g−1, at room temperature (25 °C), in the potential window range from 0.01 V to 3 V with Li foil as a counter electrode. As shown in Fig. 4a, in the initial discharge process, the voltage drops rapidly down to 0.7 V, then exhibits a long plateau at 0.68 V, followed by a sloping profile until the lower cutoff limit of 0.01 V for the MnWO4, possibly attributed to the reduction of MnWO4 to Mn0, W0 and the formation of amorphous Li2O. In the first discharge cycle, the voltage plateau corresponded well with the cathodic peak of the CV profile. The first-charge curve, up to 3.0 V shown in Fig. 4a, is smooth for MnWO4 up to about 2.0 V with a slight indication of a plateau at 1.5–2.0 V. Above 2 V, the voltage rises rapidly, indicating little capacity contribution above this voltage. The initial discharge and charge specific capacities are up to about 1230 mAh g−1 and 810 mAh g−1, respectively. The extra capacity over the theoretical specific capacity (708 mAh g−1) might arise from the insertion of lithium ions into acetylene black and interfacial storage. Subsequent discharge–charge cycles at 100 mA g−1 show poor capacity retention, which could be attributed to the irreversible formation of a stable SEI layer on the electrode surface as well as establishment of an intimate electric contact of the “composite” with the current collector.31 The capacities slightly increased after 60 cycles, which is a similar phenomenon to ZnWO4.30 Hereafter, the electrode displays an excellent cycling performance and the coulombic efficiency is higher than 98%. The electrode still maintains a capacity of 600 mAh g−1 after 160 cycles at a current density of 100 mA g−1, which is better than ZnWO4 and molybdate salt electrode materials with the same structure.30,32,33 The excellent performance of the MnWO4 nanobars is derived from its dense inner nanocavities structure, endowing them with a hollow texture, providing shorter electron transport pathways, large surface-to-volume ratio, more efficient lithium-ion storage sites, and effectively relieving the volume change capability when lithium ions insert into/out the electrode. This strategy is widely adapted in sulfur–porous carbon composites for Li/S batteries,34,35 and even lithium/iodine batteries.36
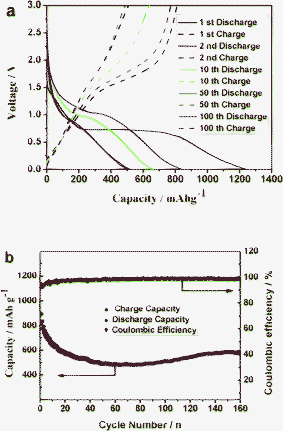 | ||
| Fig. 4 (a) Discharge/charge curves of the electrode made from the as-prepared MnWO4 nanobars with dense nanocavities, (b) Plot of capacity versus cycle number for the MnWO4 nanobar electrode in the voltage range 0.01–3.0 V at a current rate of 100 mA g−1. | ||
Besides the high specific capacity and good cycle performance, the rate capabilities are also a very important evaluation method for LIB. The rate capabilities of the prepared MnWO4 nanobars are also researched at different current densities (Fig. 5). It was investigated using five cycles at 100, 200, 500, 1000, and 2000 mA g−1, and the average discharge capacities were 680, 540, 380, 300 and 220 mAh g−1, respectively. Additionally, after reaching a huge discharge–charge current density, the specific capacity was still be able to return to more than 570 mAh g−1 when the current density was reduced to 100 mA g−1. The initial specific capacity could almost be recovered, which implies that the structure of the electrode is stable even during the high current density. The enhanced cycle performance and rate capability could be attributed to the unique nanocavity geometry and improved accommodation of the transformation strains during cycling. The above results indicate that the as-prepared MnWO4 nanobars with dense nanocavities are promising anode candidates to replace commercial graphite as the LIB anode.
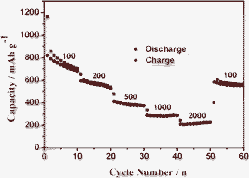 | ||
| Fig. 5 Rate capabilities of the MnWO4 nanobars at different current rates. | ||
Conclusions
The MnWO4 nanobars with dense nanocavities were successfully synthesized through an effective hydrothermal route. The formation mechanism of the nanocavity structures inside the nanobars is analogous to the process of inside-out Ostwald ripening. The prepared MnWO4 nanobars with dense nanocavities show excellent rate performance and high specific capacity in LIB. The outstanding electrochemical performance could be ascribed to the unique nanocavity geometry and improved accommodation of the transformation strains during cycling, which allow for efficient electrode–electrolyte contact and short electron transport pathways, at the same time offering sufficient lithium-ion storage sites during the Li+ insertion/extraction.Acknowledgements
This work was supported by the 973 Project of China (No. 2011CB935901), the National Nature Science Foundations of China (No. 11179043, Grant No. 91022033), and China Postdoctoral Science Foundation (2012M511927).References
- M. S. Whittingham, Dalton Trans., 2008, 5424 RSC
.
- H. Ma, F. Cheng, J. Y. Chen, J. Z. Zhao, C. S. Li, Z. L. Tao and J. Liang, Adv. Mater., 2007, 19, 4067 CrossRef CAS
.
- A. Manthiram and D. Applestone, RSC Adv., 2012, 2, 5411 RSC
.
- J. Ma and A. Manthiram, RSC Adv., 2012, 2, 3187 RSC
.
- A. Magasinski, P. Dixon, B. Hertzberg, A. Kvit, J. Ayala and G. Yushin, Nat. Mater., 2010, 9, 353 CrossRef CAS
.
- Y.-G. Guo, J.-S. Hu and L.-J. Wan, Adv. Mater., 2008, 20, 2878 CrossRef CAS
.
- J. Liu, Y. Zhou, F. Liu, C. Liu, J. Wang, Y. Pan and D. Xue, RSC Adv., 2012, 2, 2262 RSC
.
- J. Cabana, L. Monconduit, D. Larcher and M. R. Palacín, Adv. Mater., 2010, 22, E170 CrossRef CAS
.
- X. W. Lou, D. Deng, J. Y. Lee and L. A. Archer, Chem. Mater., 2008, 20, 6562 CrossRef CAS
.
- W. Qu, W. Wlodarski and J.-U. Meyer, Sens. Actuators, B, 2000, 64, 76 CrossRef
.
- Y.-X. Zhou, Q. Zhang, J.-Y. Gong and S.-H. Yu, J. Phys. Chem. C, 2008, 112, 13383 CAS
.
- L. Zhang, C. Lu, Y. Wang and Y. Cheng, Mater. Chem. Phys., 2007, 103, 433 CrossRef CAS
.
- M. Jang, T. J. R. Weakley and K. M. Doxsee, Chem. Mater., 2001, 13, 519 CrossRef CAS
.
- S. H. Yu, B. Liu, M. S. Mo, J. H. Huang, X. M. Liu and Y. T. Qian, Adv. Funct. Mater., 2003, 13, 639 CrossRef CAS
.
- S.-J. Chen, X.-T. Chen, Z. Xue, J.-H. Zhou, J. Li, J.-M. Hong and X.-Z. You, J. Mater. Chem., 2003, 13, 1132 RSC
.
- W. Q. Han, L. J. Wu, R. F. Klie and Y. M. Zhu, Adv. Mater., 2007, 19, 2525 CrossRef CAS
.
- E. J. A. Kroekenstoel, E. Verhagen, R. J. Walters, L. Kuipers and A. Polman, Appl. Phys. Lett., 2009, 95, 263106 CrossRef
.
- M. Makarova, V. Sih, J. Warga, R. Li, L. D. Negro and J. Vuckovic, Appl. Phys. Lett., 2008, 92, 161107 CrossRef
.
- J. H. Evans, Nature, 1971, 229, 403 CrossRef CAS
.
- H. Seel and R. Brendel, Thin Solid Films, 2004, 451–452, 608 CrossRef CAS
.
- W.-Q. Han and A. Zettl, Appl. Phys. Lett., 2004, 84, 2644 CrossRef CAS
.
- Q. Li, J. Zhang, B. Liu, M. Li, R. Liu, X. Li, H. Ma, S. Yu, L. Wang, Y. Zou, Z. Li, B. Zou, T. Cui and G. Zou, Inorg. Chem., 2008, 47, 9870 CrossRef CAS
.
- Y. Xiong, B. Wiley, J. Chen, Z.-Y. Li, Y. Yin and Y. Xia, Angew. Chem., Int. Ed., 2005, 44, 7913 CrossRef CAS
.
- C.-J. Jia, L.-D. Sun, Z.-G. Yan, L.-P. You, F. Luo, X.-D. Han, Y.-C. Pang, Z. Zhang and C.-H. Yan, Angew. Chem., Int. Ed., 2005, 44, 4328 CrossRef CAS
.
- X. W. Lou, Y. Wang, C. Yuan, J. Y. Lee and L. A. Archer, Adv. Mater., 2006, 18, 2325 CrossRef CAS
.
- J. J. Teo, Y. Chang and H. C. Zeng, Langmuir, 2006, 22, 7369 CrossRef CAS
.
- Y. Deng, S. Tang, Q. Zhang, Z. Shi, L. Zhang, S. Zhan and G. Chen, J. Mater. Chem., 2011, 21, 11987 RSC
.
- N. Sharma, G. V. S. Rao and B. V. R. Chowdari, Electrochim. Acta, 2005, 50, 5305 CrossRef CAS
.
- W.-J. Li and Z.-W. Fu, Appl. Surf. Sci., 2010, 256, 2447 CrossRef CAS
.
- H.-W. Shim, I.-S. Cho, K. S. Hong, A.-H. Lim and D.-W. Kim, J. Phys. Chem. C, 2011, 115, 16228 CAS
.
- G. Binotto, D. Larcher, A. S. Prakash, R. H. Urbina, M. S. Hegde and J. M. Tarascon, Chem. Mater., 2007, 19, 3032 CrossRef CAS
.
- N. Sharma, K. M. Shaju, G. V. S. Rao, B. V. R. Chowdari, Z. L. Dong and T. J. White, Chem. Mater., 2003, 16, 504 CrossRef
.
- W. Xiao, J. S. Chen, C. M. Li, R. Xu and X. W. Lou, Chem. Mater., 2009, 22, 746 CrossRef
.
- X.-P. Gao and H.-X. Yang, Energy Environ. Sci., 2010, 3, 174 CAS
.
- B. Zhang, X. Qin, G. R. Li and X. P. Gao, Energy Environ. Sci., 2010, 3, 1531 CAS
.
- Y. L. Wang, Q. L. Sun, Q. Q. Zhao, J. S. Cao and S. H. Ye, Energy Environ. Sci., 2011, 4, 3947 CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/c2ra20813j |
| ‡ En Zhang contributed equally with Zheng Xing, and they are co-first authors for this paper. |
| This journal is © The Royal Society of Chemistry 2012 |
