Redox-active alkaline electrolyte for carbon-based supercapacitor with pseudocapacitive performance and excellent cyclability
Haijun
Yu
,
Leqing
Fan
,
Jihuai
Wu
*,
Youzhen
Lin
,
Miaoliang
Huang
,
Jianming
Lin
and
Zhang
Lan
Engineering Research Center of Environment-Friendly Functional Materials, Ministry of Education; Institute of Materials Physical Chemistry, Huaqiao University, Quanzhou, Fujian 362021, P. R. China. E-mail: jhwu@hqu.edu.cn; Fax: (+86) 595-22692229; Tel: (+86) 595-22692229
First published on 13th June 2012
Abstract
A simple method has been implemented to prepare a stable and effective redox-active alkaline electrolyte by doping m-phenylenediamine into a conventional KOH electrolyte for a carbon-based supercapacitor. The quick electron transfer and reversible Faradic process in the new electrolyte system results in additional pseudocapacitive contribution for a carbon-based supercapacitor. The specific capacitance of the supercapacitor based on the new electrolyte is 78.01 F g−1, which is an increase of 114.16% over that of a supercapacitor based on a conventional KOH electrolyte (36.43 F g−1). Additionally, the supercapacitor exhibits an excellent cyclical stability.
Supercapacitors based on porous carbon materials, commonly known electric double-layer capacitors,1–3 have recently attracted considerable attention because they can provide a much larger capacity than conventional dielectric capacitors and much higher charge–discharge rate capability than primary/secondary batteries. Consequently, they are regarded as the most promising candidate devices for rapid storage and release of energy.4,5 However, the low capacitance of carbon materials, which is usually lower than 150 F g−1, based on the electric double-layer capacitance, depends upon the large specific areas of porous materials for many important applications and must be improved further.6,7
In order to improve the capacitance and performance of porous materials, intense research effort are aimed at increasing the energy density by optimizing the pore size distribution of nanoporous carbon materials or modifying carbon materials with pseudocapacitive components.8,9 The classic transition metal oxides (RuO2, Co3O4, MnO2) and conductive polymers have been developed and introduced into porous materials admirably in recent years due to their high pseudocapacitance caused by a fast redox reaction.10–12 Nevertheless, some negative effects come along with these strategies, such as poor stability or low performance/cost ratio, etc.13 An alternative design is urgently required for enhancing porous carbon-based supercapacitor systems.
Presently, a novel and highly-efficient strategy that improved the performance of porous carbon-based supercapacitors was reported through utilizing redox-active electrolytes, which brings additional pseudocapacitive attribution by the quick redox reactions of redox mediators. The electrochemical behavior and pseudocapacitive effects of Cu2+ and Fe2+ ions in porous carbons that worked in a H2SO4 electrolyte system were investigated;14 the capacity of the carbon electrode reached 223 mA h g−1 at a current density of 0.1 A g−1, which is higher than that of the H2SO4 system. Besides, the redox mediators, such as lignosulfonates15,16 and hydroquinone,17,18 have been added into acidic electrolyte to enhance the capacitive performance of carbon-based supercapacitors. However, as far as we know, there are few works investigating alkaline redox-active electrolytes in supercapacitors as an effective electrolyte, except introducing the Fe(CN)63−/Fe(CN)64− redox pairs to dope KOH electrolyte, which possesses a poor cyclical stability.19 Accordingly, it is important to explore fairly stable and high-effective redox mediators for alkaline solutions, which are the most common and effective electrolytes for supercapacitor applications.
In the current work, a direct joining method was applied to synthesize a novel redox-active alkaline electrolyte for carbon-based supercapacitors by adding a new redox mediator (m-phenylenediamine, MPD, Sinopharm Chemical Reagent Co., Ltd) into KOH aqueous solution. The quick reversible Faradic reactions of MPD at the electrode–electrolyte interface bring additional pseudocapacitive contribution for the system. Consequently, it is expected that the electrochemical performances of carbon-based supercapacitors can be improved prominently. A supercapacitor based on the redox-active electrolyte was fabricated, and a high specific capacitance of 78.01 F g−1 was reached, indicating an enhancement of 114.16% in comparison with that of a conventional KOH electrolyte assembled carbon-based supercapacitor.
The electrode composite materials are in the form of film, obtained by pressing a mixture of carbon material (85 wt.%, 1900 m2 g−1, Heda Shanghai carbon Co., Ltd), graphite (10 wt.%, Sinopharm Chemical Reagent Co., Ltd), PTFE binder (5 wt.% water suspension, Guangzhou Xingshengjie Science Technology Co. Ltd) and appropriate NMP (Sinopharm Chemical Reagent Co., Ltd) on a roller press.20 Subsequently, the carbon film was pressed to form a thin sheet by the Decal method.21 The sheet with a fixed surface area of 0.25 cm2 was pasted on a nickel-foam current collector under a pressure of 10 MPa. After being dried at 60 °C for 24 h, an activated-carbon electrode was obtained. Besides, each electrode possesses the same mass of 1.25 mg.
The redox-active electrolyte was prepared by the following procedure: different masses of MPD were added to a certain concentration (2 mol L−1) of KOH aqueous solution. After dissolving and homogeneously dispersing, a series of redox-active alkaline electrolytes were obtained. The conventional KOH electrolyte with the same concentration (2 mol L−1) of the redox-active alkaline electrolyte was applied for comparison.
The electrochemical performances of the composite carbon electrodes were tested with a two-electrode configuration using Swagelok-type cells.22 The electrochemical properties of the supercapacitors were determined on an electrochemical workstation (CHI660C, Shanghai Chenhua Instruments Co., Ltd) by cyclic voltammetry (CV, 5 mV s−1), electrochemical impedance spectroscopy (EIS, 10 mHz~100 kHz, 5 mV AC amplitude) and galvanostatic charge–discharge (GCD, 0.5∼10 A g−1). All the electrochemical measurements were performed at room temperature.
In order to get the optimum redox-active alkaline electrolyte, the specific capacitances of supercapacitors based on various electrolytes were calculated by the GCD test and the results are listed in Table 1. The specific capacitance of the supercapacitor can be calculated by using the equation:23
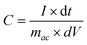 | (1) |
| Concentration of MPD in electrolyte | 0 | 0.025 | 0.050 | 0.075 | 0.010 |
|---|---|---|---|---|---|
| Specific capacitance (F g−1) | 36.43 | 55.88 | 78.01 | 72.59 | 69.06 |
It is observed that the specific capacitance of the supercapacitor changes with the increasing amount of MPD. When the MPD amount is 0.050 g, the specific capacitance reaches a high value (78.01 F g−1). Beyond 0.050 g, the specific capacitance drops with the increase of MPD concentration. The low concentration of MPD is not enough to improve efficiently the capacitive performance, but a higher concentration of MPD will lead to the aggregation of free ions and charges in system, which decrease the performances of supercapacitors.20 Consequently, the optimal concentration of redox-active alkaline electrolyte is a 2 mol L−1 KOH aqueous solution containing 0.050 g MPD, which is marked KOH+MPD.
In order to adequately and perfectly research the supercapacitor based on KOH and KOH+MPD electrolytes, the specific potential range −0.5∼0.5 V and the common potential range 0∼1 V are chosen in this study. The GCD tests were determined at the same current density of 0.5 A g−1 and the results are shown in Fig. 1A. All the curves reveal a symmetric shape (the discharge time (Tc) and charge time (Td) are almost equivalent), indicating the good reversibility during the charge–discharge process. From the red charge–discharge curve detected in the potential range −0.5∼0.5 V, it can be seen that the supercapacitor with KOH+MPD electrolyte exhibits an inclined section in the charging potential window from −0.1 to 0.1 V and the discharging potential window from 0.1 to −0.1 V indicates that redox reactions are superimposed in the charge–discharge process, which is consistent with the cyclic voltammograms in Fig. 2. But in the potential range 0∼1 V, the blue charge–discharge curve is nearly linearly symmetrical except for a little bump in the terminal of the discharge line. That's why the specific capacitance is lower in the potential range 0∼1 V. Besides, the supercapacitor with the KOH electrolyte shows a more pronounced iRdrop at the beginning of the discharge process. The potential drop is attributed to the internal resistance of the electrode associated with the electrical connection resistance, bulk solution resistance, and resistance of ion migration in electrode materials.24 The equivalent series resistance (ESR) of system could be roughly estimated from the iRdrop, and the relation is shown in following equation:20
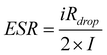 | (2) |
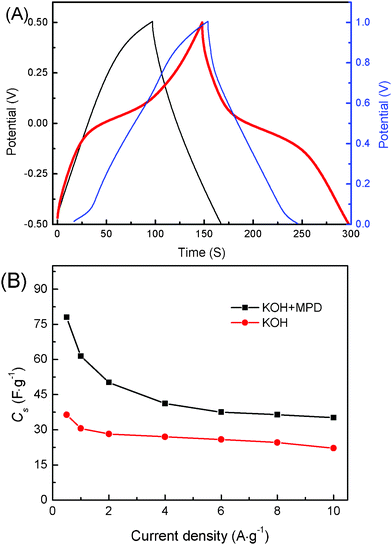 | ||
| Fig. 1 (a) GCD curves for the SCs based on KOH (−0.5∼0.5 V) and KOH+MPD (−0.5∼0.5 V and 0∼1 V) electrolytes, charge–discharge current density: 0.5 A g−1. (b) Cs of the SCs with KOH and KOH+MPD electrolytes on the current density of GCD testing from 0.5 to 10 A g−1. | ||
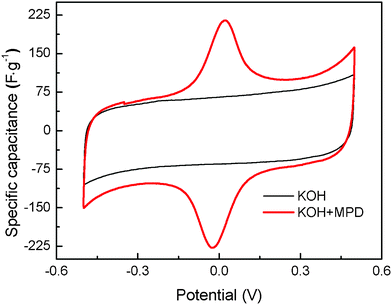 | ||
| Fig. 2 CV curves of the SCs employing KOH+MPD electrolyte and conventional electrolyte (KOH) at the same scan sweep of 5 mV s−1. | ||
The calculated ESR of supercapacitor based on KOH+MPD electrolyte (1.98 Ω cm2) is smaller than that of the supercapacitor using the KOH electrolyte (2.60 Ω cm2), implying a higher ionic conductivity of the KOH+MPD electrolyte system.
The specific capacitance of 78.01 F g−1 for the supercapacitor based on KOH+MPD electrolyte is reached at a current density of 0.5 A g−1, which is much higher than that one using KOH electrolyte (36.43 F g−1). Energy density (E, W h kg−1) and power density (W kg−1) of the supercapacitor are calculated according to the following equations:20
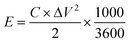 | (3) |
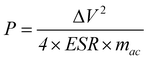 | (4) |
The energy density of the supercapacitor based on the KOH+MPD electrolyte is heightened by one time achieving 9.99 W h kg−1. At the same time, the supercapacitor employing KOH+MPD electrolyte can provide a large power density of 5.78 k W kg−1. The remarkable enhancement in the capacitance, energy storage and power density of supercapacitor based on KOH+MPD electrolyte can be attributed to the pseudocapacitive contributions brought by the redox reactions of redox mediator (MPD). The carbon-based supercapacitor employing the KOH+MPD electrolyte is a hybrid energy-storage application that contains two processes: the electric double-layer capacitance based on porous electrode material and pseudocapacitance from the quick redox reactions of the redox mediator.
Fig. 1B shows the GCD curves of supercapacitors employing KOH and KOH+MPD electrolytes at different current densities. With the increase of current density, the specific capacitance of supercapacitors slowly drops. However, a faster drop in capacitance with increasing the current density is found in the low current density region, which is ascribed to the contribution of pseudocapacitance from the redox processes in the system.25 Thus, the more significant drop in capacitance of supercapacitor based on KOH+MPD electrolyte implies more pseudocapacitive contributions for the system. This result supports the introduction of a redox mediator with stable and reversible redox process benefiting the supercapacitor applications.
The capacitive performances of carbon-based supercapacitors based on the KOH+MPD and KOH electrolyte were evaluated by CV measurements at scan rates of 5 mV s−-1, and the results are shown in Fig. 2. Theoretically, an ideal CV curve of an electrochemical capacitor would be a standard rectangular shape since the capacitance Cc would keep constant at a linear charging–discharging rate, and following eqn 5:26
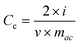 | (5) |
The supercapacitor with KOH reveals a quasi-rectangle shape without a redox peak, implying an ideal electric double layer capacitor with quick charge propagation.27 The CV curves of the supercapacitor based on the KOH+MPD electrolyte displays shapes with one pair of remarkable redox peaks (p1/p1′) appearing at about 0.022 V and −0.026 V, typical behavior of the combination of the electric double layer capacitance of porous carbon materials and pseudocapacitance from the redox reaction of redox mediator. The oxidation and reduction peak potentials in the CV graphs are denoted as EO and ER respectively. The smaller EO − ER (ΔE) value is a measure of better reversibility in the redox reaction.28 From Fig. 2, it is found that the supercapacitor with KOH+MPD shows a small ΔE (48 mV), which implies perfect reversibility of the redox reaction in the supercapacitors. On the basis of the CV curves we can see that the supercapacitor based on the KOH+MPD electrolyte exhibits better electrochemical properties than the one utilizing the KOH electrolyte, indicating MPD is available for the electrolyte systems.
The enhanced capacitive performances would result from the low resistance because the quick redox reaction affords a facile ion and charge transfer in the electrode materials, which was evident by the investigation of EIS. The Nyquist plots for the supercapacitors employing KOH and KOH+MPD electrolytes are shown in Fig. 3. It should be noted that the supercapacitor with the KOH+MPD electrolyte possesses a small interfacial charge-transfer resistance (Rct, which is calculated from the span of the single semi-circle along the x-axis from the high to low frequency region) and lower inner resistance (Ri, the intersecting point with the x-axis in the range of high frequency). This indicates that the higher ionic conductivity and more smooth charges (electrons) and ions transfer for new system. At the low-frequency region, imaginary parts of impedance plots are almost perpendicular to the real part, which verify good capacitance behaviors are of two systems.29
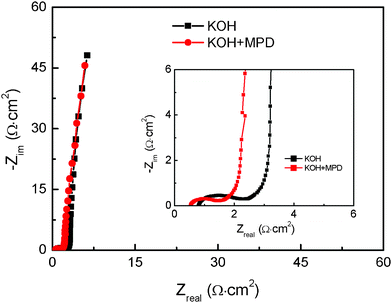 | ||
| Fig. 3 Nyquist plots of the SCs using KOH and KOH+MPD electrolytes. Inset: the close-up view of the left plot in the high frequency region. | ||
The relation of the imaginary part (C′′) of the capacitance vs. the frequency of supercapacitors employing KOH+MPD and KOH electrolytes is shown in Fig. 4. Both of the plots go through a maximum at a particular frequency f0. This frequency determines the dielectric relaxation time (τ), which can be deduced according to the following equation:30
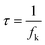 | (6) |
 | ||
| Fig. 4 Evolution of the real part of the capacitance vs. the frequency of the SCs based on KOH+MPD and KOH electrolytes. | ||
As we know, the smaller value represents a smaller time for the supercapacitor to reach half of the low frequency capacitance, implying the better power properties of the system. It can be seen that the frequency at which the peak maximum occurs shifts to higher frequency when the supercapacitor uses the new electrolyte. The τ of the KOH+MPD electrolyte system is 3.92 S, which implies that the redox-mediated alkaline electrolyte is suitable for the supercapacitor system.
Moreover, the cyclic behavior, i.e. the stability of the supercapacitors for a large number of charge–discharge cycles, is one of the most attractive aspects of the supercapacitors over rechargeable batteries. The cycling stability of the supercapacitor with the KOH+MPD electrolyte was performed at a constant charge–discharge current density of 2 A g−1 (Fig. 4). After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles, the supercapacitor maintains a high specific capacitance of 45.48 F g−1, and is about 90.68% of the initial capacitance (50.15 F g−1), indicating the excellent cycling ability of the supercapacitor. Therefore, we can draw the conclusion that the existence of stable and quick redox processes in this system not only improve the electrochemical properties, but also keep the long charge–discharge cycling ability of a conventional electrolyte.
000 charge–discharge cycles, the supercapacitor maintains a high specific capacitance of 45.48 F g−1, and is about 90.68% of the initial capacitance (50.15 F g−1), indicating the excellent cycling ability of the supercapacitor. Therefore, we can draw the conclusion that the existence of stable and quick redox processes in this system not only improve the electrochemical properties, but also keep the long charge–discharge cycling ability of a conventional electrolyte.
In summary, we proposed and successfully demonstrated a novel redox mediator for an alkaline redox-mediated electrolyte and applied it in carbon-based supercapacitors. The obtained experimental results (larger specific capacitances, higher energy densities, and long-term cyclic stability) demonstrated that this novel redox mediator was excellent. Furthermore, this method provides a facile and efficient approach to fabricate an excellent electrolyte for the high-energy supercapacitor devices. Fig. 5.
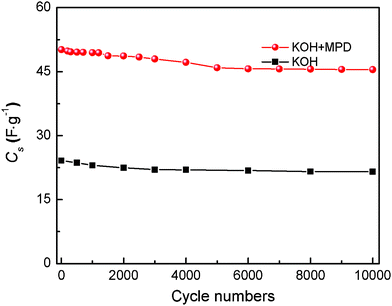 | ||
| Fig. 5 Capacitance retention of the SCs based on KOH+MPD and KOH electrolytes at a current density of 2 A g−1 in long-term cycles. | ||
Acknowledgements
This work was supported by the National High Technology Research and Development Program of China (No. 2009AA03Z217), the National Natural Science Foundation of China (No. 90922028 and 51002053), and the Key Project of Chinese Ministry of Education (No. 211204).References
- J. Huang, B. Sumpter and V. Meunier, Angew. Chem., Int. Ed., 2008, 47, 520 CrossRef CAS.
- B. Conway, Kluwer Academic/Plenum Publishers, New York, 2nd ed, 1999.
- R. Kotz and M. Carlen, Electrochim. Acta, 2000, 45, 2483 CrossRef CAS.
- Y. Zhu, S. Murali, M. Stoller, K. Ganesh, W. Cai, P. Ferreira, A. Pirkle, R. Wallace, K. Cychosz, M. Thommes, D. Su, E. Stach and R. Ruoff, Science, 2011, 332, 1537 CrossRef CAS.
- X. Lang, A. Hirata, T. Fujita and M. Chen, Nat. Nanotechnol., 2011, 6, 232 CrossRef CAS.
- J. L. Li, Q. Wang, F. Gao, W. S. Li, K. Z. Wu and X. D. Wang, New Carbon Mater., 2008, 23, 275 CrossRef.
- G. Lota, K. Fic and E. Frackowiak, Energy Environ. Sci., 2011, 4, 1592 CAS.
- G. Snook, P. Kao and A. Best, J. Power Sources, 2011, 196, 1 CrossRef CAS.
- C. Largeot, C. Portet, J. Chmiola, P. Taberna, Y. Gogotsi and P. Simon, J. Am. Chem. Soc., 2008, 130, 2730 CrossRef CAS.
- Q. Yang, Z. Y. Lu, Z. Chang, W. Zhu, J. Q. Sun, J. F. Liu, X. M. Sun and X. Duan, RSC Adv., 2012, 2, 1663 RSC.
- X. Zhao, C. Johnston, A. Crossley, S. Patrick and J. Grant, J. Mater. Chem., 2010, 20, 7637 RSC.
- W. T. Deng, X. B. Ji, Q. Y. Chen and C. E. Banks, RSC Adv., 2011, 1, 1171 RSC.
- P. Hall, M. Mirzaeian, S. Fletcher, F. Sillars, A. Rennie, G. Shitta-Bey, G. Wilson, A. Cruden and R. Carter, Energy Environ. Sci., 2010, 3, 1238 CAS.
- Q. Li, K. X. Li, C. Sun and Y. Li, J. Electroanal. Chem., 2007, 611, 43 CrossRef CAS.
- S. Roldan, Z. Gonzalez, C. Blanco, M. Granda, R. Menendez and R. Santamaria, Electrochim. Acta, 2011, 56, 3401 CrossRef CAS.
- G. Lota and E. Frackowiak, Electrochem. Commun., 2009, 11, 87 CrossRef CAS.
- S. Roldan, C. Blanco, M. Granda, R. Menéndez and R. Santamaría, Angew. Chem., Int. Ed., 2011, 50, 1699 CrossRef CAS.
- H. J. Yu, J. H. Wu, L. Q. Fan, Y. Z. Lin, K. Q. Xu, Z. Y. Tang, C. X. Cheng, S. Tang, J. M. Lin, M. L. Huang and Z. Lan, J. Power Sources, 2012, 198, 402 CrossRef CAS.
- L. H. Su, X. G. Zhang, C. H. Mi, B. Gao and Y. Liu, Phys. Chem. Chem. Phys., 2009, 11, 2195 RSC.
- H. J. Yu, J. H. Wu, L. Q. Fan, K. Q. Xu, X. Zhong, Y. Z. Lin and J. M. Lin, Electrochim. Acta, 2011, 56, 6881 CrossRef CAS.
- Z. Shao, I. Hsing, H. Zhang and B. Yi, Int. J. Energy Res., 2006, 30, 1216 CrossRef CAS.
- D. Carriazo, F. Pico, M. C. Gutierrez, F. Rubio, J. M. Rojo and F. D. Monte, J. Mater. Chem., 2010, 20, 773 RSC.
- S. Roldan, M. Granda, R. Menendez, R. Santamaria and C. Blanco, J. Phys. Chem. C, 2011, 115, 17606 CAS.
- L. Zhang and X. Zhao, Chem. Soc. Rev., 2009, 38, 2520 RSC.
- S. Yang, K. Chang, H. Tien, Y. Lee, S. Li, Y. Wang, J. Wang, C. Ma and C. Hu, J. Mater. Chem., 2011, 21, 2374 RSC.
- P. Ruch, M. Hahn, D. Cericola, A. Menzel, R. Kotz and A. Wokaun, Carbon, 2010, 48, 1880 CrossRef CAS.
- C. Vix-Guterl, S. Saadallah, K. Jurewicz, E. Frackowiak, M. Reda, J. Parmentier, J. Patarin and F. Beguin, Mater. Sci. Eng., B, 2004, 20, 148 CrossRef.
- S. Meher, P. Justin and G. Rao, Nanoscale, 2011, 3, 683 RSC.
- Y. Nian and H. Teng, J. Electrochem. Soc., 2002, 149, A1008 CrossRef CAS.
- H. J. Yu, Q. W. Tang, J. H. Wu, Y. Z. Lin, L. Q. Fan, M. L. Huang, J. M. Lin, Y. Li and F. D. Yu, J. Power Sources, 2012, 206, 463 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
