Hydrogen fermentation using lactate as the sole carbon source: Solution for ‘blind spots’ in biofuel production
Akihiro
Ohnishi
*,
Yuji
Hasegawa
,
Shinko
Abe
,
Yukiko
Bando
,
Naoshi
Fujimoto
and
Masaharu
Suzuki
Department of Fermentation Science, Faculty of Applied Bio-Science, Tokyo University of Agriculture, 1-1 Sakuragaoka 1-chome, Setagaya-ku, Tokyo 156-8502, Japan
First published on 13th July 2012
Abstract
Lactate is not believed to be a suitable substrate for hydrogen fermentation, although glucose and starch are. To eliminate this ‘blind spot’ in hydrogen fermentation, we showed that it is feasible to produce hydrogen with lactate as the sole carbon source by using mixed microflora as well as a single culture. Microflora were isolated from a methane fermentation system, and their hydrogen productivity, fermentation properties, and diversity were analyzed. Since previous studies used inoculum pretreatment to improve efficiency, we used both pretreated and non-pretreated samples. The hydrogen yield of the non-pretreated microflora sample was 0.43 mol/mol lactate, which is approximately 49 times the values reported previously. Megasphaera elsdenii was found to be the main lactate utilizing-hydrogen producing bacteria (LU-HPB) in this sample, but it is not heat-shock tolerant. Thus, heat-shock pretreatment reduces microbial diversity, including lactic acid bacteria (LAB) and LU-HPB, in the inoculum. This is another ‘blind spot’ of hydrogen fermentation from lactate. The hydrogen yield of M. elsdenii was approximately 0.4 mol/mol lactate. Considering the balance of metabolites in the reaction, even if the substrate was completely consumed for lactic acid fermentation, 0.8 mol hydrogen per mol of glucose may be recovered via homo-type lactic acid fermentation. Thus, LU-HPB do not require inoculum and/or substrate pretreatment and may improve the utilization of recalcitrant substrates such as xylose by cooperating with LAB. To our knowledge, this is the first study to identify and characterise a microbial hydrogen fermenter that can use lactate as the sole carbon source.
1. Introduction
The global requirement for sustainably produced biofuels is increasing. Since hydrogen will be the ultimate fuel in the future, biohydrogen is a hot topic of research within the field of biofuels.1–7 Biohydrogen is commonly produced by anaerobic fermentation processes involving anaerobic photosynthetic bacteria (primarily microalgae such as cyanobacteria) or nonphotosynthetic bacteria.8 Of the processes currently in use, anaerobic dark (nonphotosynthetic) fermentation shows the greatest capacity for hydrogen generation.9 Many researchers are actively studying hydrogen fermentation to postulate various technical theories, because understanding of the current status of the available technologies is limited, and the process has numerous constraints.4,10,11 However, because the experiments in this field are based on hypothetical preconditions, academic and industrial researchers may face a ‘blind spot’—a fixed idea that is a misconception due to misinterpreted information. We believe that the most important features of hydrogen fermentation that are misinterpreted are lactate, lactic acid bacteria (LAB), and pretreatment. These features are vital to the stability of the hydrogen fermentation reaction. Previously, it was believed that LAB should not be used in this reaction and that lactate production should be avoided; further, pretreatment methods such as heat shock were used. However, the benefits of these manipulations are outweighed by the fact that a high energy investment is required to carry them out. Thus, if these hypothetical preconditions are ignored, i.e., if it is assumed that LAB and lactate are not harmful and that heat-shock pretreatment is not essential for hydrogen fermentation, some of the disadvantages of these manipulations can be prevented.Several previous studies have shown that while various substrates including glucose and starch are suitable for hydrogen fermentation, lactate is not.12,13 For example, Baghchehsaraee et al.14 reported that no significant hydrogen production was observed when lactate was used as the sole carbon source. Therefore, to date, lactate has been omitted from analytical studies on hydrogen fermentation substrates. In other words, a hydrogen fermentation system that can produce hydrogen from lactate as the main carbon source does not currently exist.
LAB produce lactate from sugars such as glucose via the following reactions:
| Homofermenters: C6H12O6 → 2CH3CHOHCOOH | (1) |
| Heterofermenters: C6H12O6 → CH3CHOHCOOH + C2H5OH + CO2 | (2) |
Similarly, hydrogen-producing bacteria (HPB) such as those of the genus Clostridium produce hydrogen from sugars via the following reactions:
| C6H12O6 + 2H2O → 2CH3COOH + 2CO2 + 4H2 | (3) |
| C6H12O6 → CH3CH2CH2COOH + 2CO2 + 2H2 | (4) |
LAB, which are present in various biomasses and comprise environmental microflora, have been reported to inhibit hydrogen production by the genus Clostridium; in reactions with this mix of substrates and microorganisms, a reduced hydrogen yield is associated with a simultaneous increase in the lactate levels.15,16 Thus, LAB have been implicated as substrate competitors and hydrogen fermentation inhibitors.
With these issues in mind, pretreatment methods such as heat shocking the inoculum or substrate have been used to make hydrogen fermentation systems more effective.17 The heat shock method is based on the fact that LAB are mostly nonsporogenic and their heat tolerance is lower than that of the genus Clostridium. Many studies have reported various pretreatment methods for mixed inocula or substrates to ensure increased hydrogen production.18,19 In particular, heat shock as a pretreatment method has been shown to be ideal in terms of the hydrogen yield. In other words, while lactate and LAB are considered to negatively affect hydrogen fermentation, pretreatment methods including heat shock are considered essential for this process. However, pretreatment processes are not economically viable for sustained hydrogen fermentation, because they require a substantially large energy input, a significant problem faced by hydrogen fermentation systems in practice.
Thus, the notions that most researchers have in mind are that (1) hydrogen fermentation systems using lactate are not viable, (2) pretreatment methods such as heat shock are essential for hydrogen fermentation, and (3) LAB are harmful for hydrogen fermentation and must be removed from fermentation systems. The purpose of the present study is to identify ‘blind spots’ in these notions and provide solutions for them. To this end, we first assessed the ability of various environmental microflora with and without heat-shock pretreatment to enable mesophilic hydrogen fermentation using lactate as the sole carbon source. Significant hydrogen fermentative microflora were then detected from anaerobic digestion systems, and their hydrogen fermentation stability was evaluated in a subsequent batch experiment. Further, the fermentation characteristics of hydrogen production were investigated on the basis of hydrogen productivity, the volatile fatty acids (VFAs) produced, the microflora involved, and the characteristics of the main hydrogen producer. Lastly, the main hydrogen producer was isolated as a lactate-utilizing hydrogen fermenter and its hydrogen productivity, metabolites, and heat-shock tolerance were determined. To our knowledge, this is the first report on microbial hydrogen production via fermentation using lactate as the sole carbon source.
2. Results
2.1 Selection of hydrogen fermentation microflora using lactate
In the first stage of the first batch test for the selection of microflora, hydrogen production over 500 ml l−1 was not observed.In the first stage of the second batch test, pretreated inocula did not produce a large amount of hydrogen. In contrast, all of the non-pretreated inocula, except topsoil from a backyard, produced a considerable amount of hydrogen, particularly the non-pretreated inocula of acid slurry (1342 ml l−1, Table 1), acid sludge (1291 ml l−1), and effluent (1095 ml l−1). In all cases, the generated gas had 41%–57% hydrogen, and no methane was detected. The greatest hydrogen production was observed in the case of the non-pretreated acid slurry. Surprisingly, its pretreated counterpart did not produce any hydrogen.
Thus, the non-pretreated inoculum from acid slurry was used for hydrogen fermentation in the second stage of the sequential batch test.
| Batches | |||
|---|---|---|---|
| Inoculum | Pre-treatment | 1 | 2 |
| a Pretreatment involved heat shock at 90 °C for 10 min. | |||
| Acid slurry | Heat shocka | 0 | 0 |
| No | 22 | 1342 | |
| Acid sludge | Heat shocka | 0 | 0 |
| No | 3 | 1291 | |
| Effluent | Heat shocka | 0 | 0 |
| No | 111 | 1095 | |
| Garbage compost | Heat shocka | 94 | 32 |
| No | 190 | 318 | |
| Topsoil of courtyard | Heat shocka | 4 | 24 |
| No | 157 | 401 | |
| Topsoil of backyard | Heat shocka | 109 | 25 |
| No | 21 | 18 | |
2.2 Evaluation of hydrogen fermentation stability and fermentation properties
For the second stage of the batch tests, acid slurry was obtained from the methane fermentation system on 3 different days. Each sample was used as pretreated and non-pretreated inocula.Fig. 1 shows the results of the second stage. No clear hydrogen production was observed with the pretreated inoculum in any of the sequential batch tests. However, significant hydrogen production was observed with the non-pretreated inoculum. Although hydrogen production in the first batch test was only 155 ml l−1, it increased substantially in the second to fifth batches; the average hydrogen production in these batches was 1400 ml l−1. The composition of the gas was approximately 50% hydrogen and carbon dioxide in all cases, and no methane was detected. In the fifth batch, the hydrogen production reached approximately 1600 ml l−1.
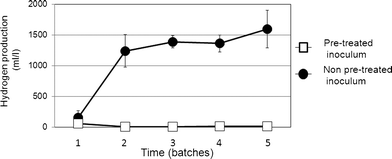 | ||
| Fig. 1 Variations in hydrogen productivity during the second stage of the sequential batch test. | ||
2.3 Variation in the VFA types and levels in the sequential batch test
The relationships among the endproducts of hydrogen fermentation must be better understood in order to better understand the complexities of hydrogen fermentation. Therefore, we analyzed the levels of VFAs during the second stage of the sequential batch test using acid slurry as the inoculum.The results of this analysis are shown in Fig. 2. Lactate, which was the substrate in the first batch test, (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 mg l−1) was the only VFA in the fermentation products when the non-pretreated inoculum was used. In contrast, acetate (3777 ± 119 mg l−1), propionate (3680 ± 571 mg l−1), butyrate (1978 ± 571 mg l−1), and valerate (1260 ± 260 mg l−1) were obtained in the fermentation products during the second to fifth batch tests. Formate (338 ± 106 mg l−1) and iso-butyrate (259 ± 42 mg l−1) were also observed in trace amounts in the fermentation products during the second to fifth batch tests. A small amount of lactate (642 mg l−1) was detected in the second batch test, but none was detected in the third to fifth tests. Other VFAs such as malate and citrate were produced at very low or non-detectable levels (data not shown). When the pretreated inoculum was used, the VFAs in the fermentation products (Fig. 2b) were predominantly lactate (14
440 mg l−1) was the only VFA in the fermentation products when the non-pretreated inoculum was used. In contrast, acetate (3777 ± 119 mg l−1), propionate (3680 ± 571 mg l−1), butyrate (1978 ± 571 mg l−1), and valerate (1260 ± 260 mg l−1) were obtained in the fermentation products during the second to fifth batch tests. Formate (338 ± 106 mg l−1) and iso-butyrate (259 ± 42 mg l−1) were also observed in trace amounts in the fermentation products during the second to fifth batch tests. A small amount of lactate (642 mg l−1) was detected in the second batch test, but none was detected in the third to fifth tests. Other VFAs such as malate and citrate were produced at very low or non-detectable levels (data not shown). When the pretreated inoculum was used, the VFAs in the fermentation products (Fig. 2b) were predominantly lactate (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 960 ± 896 mg l−1) and small amounts of acetate (965 ± 215 mg l−1), propionate (166 ± 52 mg l−1), and iso-butyrate (259 ± 42 mg l−1) throughout the sequential batch test. A trace amount of butyrate (189 mg l−1) was also detected but only in the second batch test.
960 ± 896 mg l−1) and small amounts of acetate (965 ± 215 mg l−1), propionate (166 ± 52 mg l−1), and iso-butyrate (259 ± 42 mg l−1) throughout the sequential batch test. A trace amount of butyrate (189 mg l−1) was also detected but only in the second batch test.
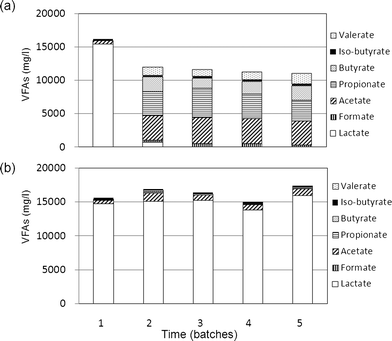 | ||
| Fig. 2 Variations in the types and levels of VFAs detected during the second stage of the sequential batch test with acid slurry as the inoculum. (a) Pretreated and (b) non-pretreated inocula. | ||
2.4 Analysis of microflora in the sequential batch test
For microbial analysis by PCR-DGGE, the fermentation supernatant was sampled during each batch of the second stage of the sequential batch test. Preliminary experiments employing PCR amplification with universal primers for small-subunit rDNA indicated that archaebacteria and fungi were absent from the microbial communities sampled (data not shown). Detailed results regarding the changes in the bacterial community structure as assessed by DGGE band profiling of the 16S rDNA are shown in Fig. 3.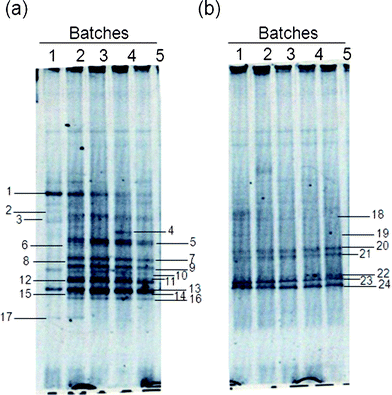 | ||
| Fig. 3 PCR-DGGE band profiles during hydrogen fermentation from lactate by selected microflora obtained from an acid slurry. (a) Non-pretreated and (b) pretreated. | ||
When the non-pretreated inoculum was used, clear changes in the band profile were detected between the first batch test and the subsequent ones (Fig. 3a). A band of high fluorescence intensity was clearly detected after the first batch test, while the intensity of the bands decreased in the second test and remained stable up to the fifth batch test. When the pretreated inoculum was used, no changes in the fluorescence intensity were observed through the sequential batch test.
The 24 bands resolved by DGGE were excised and purified for sequence determination. Table 2 lists the detected microflora. Comparison of the 16S rDNA sequences of the cultivated strains with those in the GenBank database showed that all 24 bands had high sequence similarities of over 97% with known strains. When the non-pretreated inoculum was used, the phylogenetic analysis related the detected bands to 9 species from 5 genera: Lactobacillus fermentum (band 1), Lactobacillus amylolyticus (band 2), Lactobacillus perolens (band 3), Clostridium subterminale (band 4), Megasphaera elsdenii (bands 5, 8–13, and 16), Pectinatus cerevisiiphilus (bands 6 and 7), Clostridium sporogenes (band 14), Clostridium lundense (band 15), and Propionibacterium freudenreichii (band 17). When the pretreated inoculum was used, the results related the detected bands to 2 species from 2 genera: Clostridium magnum (bands 18 and 22–24) and Paenibacillus azoreducens (bands 19–21). Thus, compared to the non-pretreated inoculum, the pretreated inoculum showed a lower band number and variation.
| Non-pretreated inoculum | Pretreated inoculum | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Band no. | Closely related | Accession no. | Similarity (%) | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 |
| a +: PCR-DGGE band detected. | |||||||||||||
| 1 | Lactobacillus fermentum | HQ697664 | 100 | +a | + | + | + | + | |||||
| 2 | Lactobacillus amylolyticus | Y17361 | 100 | + | |||||||||
| 3 | Lactobacillus perolens | Y19168 | 99 | + | + | + | + | + | |||||
| 4 | Clostridium subterminale | AF241843 | 100 | + | |||||||||
| 5 | Megasphaera elsdenii | AY038996 | 98 | + | + | + | + | ||||||
| 6 | Pectinatus cerevisiiphilus | AY659947 | 98 | + | |||||||||
| 7 | Pectinatus cerevisiiphilus | AY659947 | 97 | + | + | + | + | ||||||
| 8 | Megasphaera elsdenii | AY038996 | 98 | + | + | + | + | ||||||
| 9 | Megasphaera elsdenii | AB609705 | 98 | + | + | + | + | ||||||
| 10 | Megasphaera elsdenii | AY038996 | 97 | + | + | + | + | ||||||
| 11 | Megasphaera elsdenii | AY038996 | 98 | + | + | + | + | ||||||
| 12 | Megasphaera elsdenii | AY038996 | 98 | + | + | + | + | ||||||
| 13 | Megasphaera elsdenii | AY038996 | 97 | + | + | + | + | ||||||
| 14 | Clostridium sporogenes | X68189 | 98 | + | + | + | + | ||||||
| 15 | Clostridium lundense | AY858804 | 99 | + | + | + | + | ||||||
| 16 | Megasphaera elsdenii | AY038996 | 97 | + | + | + | + | ||||||
| 17 | Propionibacterium freudenreichii | Y10819 | 99 | + | + | ||||||||
| 18 | Clostridium magnum | X77835 | 100 | + | + | + | + | + | |||||
| 19 | Paenibacillus azoreducens | AJ27224 | 98 | + | + | + | + | + | |||||
| 20 | Paenibacillus azoreducens | AJ27224 | 99 | + | + | + | + | + | |||||
| 21 | Paenibacillus azoreducens | AJ27224 | 100 | + | + | + | + | + | |||||
| 22 | Clostridium magnum | X77835 | 100 | + | + | + | + | + | |||||
| 23 | Clostridium magnum | X77835 | 100 | + | + | + | + | + | |||||
| 24 | Clostridium magnum | X77835 | 99 | + | + | + | + | + | |||||
2.5 Distribution of different bacteria in the hydrogen fermentation samples
The dominant occurrence of Megasphaera in sample solutions of the fourth and fifth batches in the sequential batch test using non-pretreated inocula were confirmed at the single-cell level using FISH with the probes Mega-X (Cy3 labelled) and EUB338 (Alexa488 labelled). In both samples, bacterial cells detectable by FISH with EUB338 accounted for all of the DAPI-stained cells. The occurrence of the genus Megasphaera (detected using Mega-X) in the fourth and fifth batches was 52.7% ± 6.8% and 70.8% ± 1.8%, respectively (Fig. 4).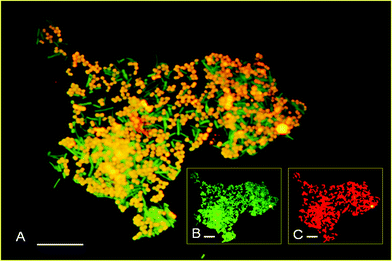 | ||
| Fig. 4 Micrographs of samples from the fifth batch with non-pretreated inoculum. (A) Megasphaera cells that hybridized with the FISH probes Mega-X (Cy3 labelled) and EUB338 (Alexa488 labelled) are shown in yellow; other bacterial cells that reacted with EUB338 (Alexa488 labelled) are shown in green. (B) all bacterial cells that reacted with EUB338 (Alexa488 labelled) are shown in green. (C) Megasphaera cells that reacted with Mega-X (Cy3 labelled) are shown in red. Bar, 10 μm. | ||
2.6 Isolation of anaerobes and evaluation of hydrogen fermentation potential
The bacteria among the hydrogen fermentation microflora in the acid slurry inoculum with and without pretreatment were isolated, and their hydrogen productivity was evaluated.Five anaerobic isolates, namely, AXX1, AXX2, AMM1, A15, and A27, were isolated from the supernatant of the fifth batch using non-pretreated microflora obtained from acid slurry. The characteristics of the isolates are shown in Table 3 and Fig. 4. Phylogenetic analysis based on the 16S rDNA sequence was performed for these isolates. AXX1 and AXX2 were found to be M. elsdenii, showing 96% and 97% similarity with this species, respectively. AMM1 and A27 were found to be C. sporogenes, showing 98% and 99% similarity, respectively. Lastly, A15 was found to be Pect. cerevisiiphilus, showing 97% similarity.
| Hydrogen productivity (ml l−1) | |||||
|---|---|---|---|---|---|
| Isolate | Closely related | Accession no. | Similarity (%) | pretreateda | non pretreated |
| a Pretreatment at 90 °C for 10 min. | |||||
| AXX1 | Megasphaera elsdenii | AY038996 | 96 | 0 | 1468 ± 2 |
| AXX2 | Megasphaera elsdenii | AY038996 | 97 | 0 | 1404 ± 114 |
| AMM1 | Clostridium sporogenes | X68189 | 98 | 0 | 346 ± 10 |
| A27 | Clostridium sporogenes | X68189 | 99 | 0 | 409 ± 27 |
| A15 | Pectinatus cerevisiiphilus | AY659947 | 97 | 0 | 0 |
The hydrogen productivity and VFA types and levels for all isolates used as pretreated and non-pretreated inoculum were analyzed in triplicate. When the pretreated inoculum was used, no hydrogen production was detected for any isolate. When the non-pretreated inoculum was used, the hydrogen productivity of AXX1, AXX2, AMM1, A27, and A15 was 1468 ± 2, 1404 ± 114, 346 ± 10)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 409 ± 27, and 0 ml l−1, respectively. When the non-pretreated inoculum was used, the main VFAs in the fermentation products of each isolate were as follows: AXX1 and AXX2 produced acetate (approximately 3200 mg l−1), propionate (approximately 3700 mg l−1), valerate (approximately 1200 mg l−1), butyrate (approximately 1000 mg l−1), and formate (approximately 500 mg l−1); AMM1 and A27 produced lactate (approximately 15
409 ± 27, and 0 ml l−1, respectively. When the non-pretreated inoculum was used, the main VFAs in the fermentation products of each isolate were as follows: AXX1 and AXX2 produced acetate (approximately 3200 mg l−1), propionate (approximately 3700 mg l−1), valerate (approximately 1200 mg l−1), butyrate (approximately 1000 mg l−1), and formate (approximately 500 mg l−1); AMM1 and A27 produced lactate (approximately 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg l−1) and acetate (approximately 1600 mg l−1); and A15 produced only lactate (approximately 15
000 mg l−1) and acetate (approximately 1600 mg l−1); and A15 produced only lactate (approximately 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg l−1).
000 mg l−1).
3. Discussion
In this paper, we present novel findings regarding hydrogen fermentation and its characteristics with lactate as the sole carbon source. Mixed microflora culture and monoculture were used to obtain experimental evidence. A better understanding of the characteristics of hydrogen fermentation will help to establish this process on a field scale and in the design of new research to optimise hydrogen fermentation from low-grade biomass for future applications.3.1 Hydrogen fermentation microflora using lactate
In the first stage of the batch test, several hydrogen fermentation microflora utilizing lactate as the sole carbon source were obtained from the non-pretreated environmental microflora samples (Table 1). Since the maximum hydrogen productivity of 1342 ml l−1 was obtained with environmental microflora in the acid slurry, these microflora were used in the second stage of the sequential batch test. When the non-pretreated inoculum was used, stable hydrogen production and increased lactate consumption (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg l−1) were observed after the second batch, and the maximum hydrogen production eventually reached approximately 1600 ml l−1 (Fig. 1) in the fifth batch test. These microflora, which produced a maximum hydrogen yield of 0.43 mol/mol lactate, were isolated. The main VFAs produced were found to be acetate (3777 mg l−1) and propionate (3680 mg l−1). Further, the average hydrogen yield during the second to fifth batches was 0.36 mol/mol lactate.
000 mg l−1) were observed after the second batch, and the maximum hydrogen production eventually reached approximately 1600 ml l−1 (Fig. 1) in the fifth batch test. These microflora, which produced a maximum hydrogen yield of 0.43 mol/mol lactate, were isolated. The main VFAs produced were found to be acetate (3777 mg l−1) and propionate (3680 mg l−1). Further, the average hydrogen yield during the second to fifth batches was 0.36 mol/mol lactate.
The results obtained here are vastly different from those of several previous studies. The hydrogen yield using lactate as the substrate was previously found to be only 2.2 ml H2/g lactate, which is a hydrogen yield of approximately 0.008 mol/mol lactate14 and a substrate conversion efficiency of 0.5%.24 The maximum hydrogen yield in this study was about 49-fold greater than this previously reported value. Other interesting points about our results are the rapid lactate consumption and the effective hydrogen production at a high lactate concentration (approximately 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg l−1). Kim et al.12 reported that a high lactate concentration (16
000 mg l−1). Kim et al.12 reported that a high lactate concentration (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg l−1) inhibited hydrogen production and decreased HPB activity. Therefore, we believe that the microflora composition of the sample in our study is ideal.
000 mg l−1) inhibited hydrogen production and decreased HPB activity. Therefore, we believe that the microflora composition of the sample in our study is ideal.
The microflora samples that produced hydrogen by fermenting lactate were analyzed by PCR-DGGE, and the main bacteria that were consistently detected whenever the hydrogen production was high were C. lundense, C. sporogenes, L. fermentum, L. perolens, M. elsdenii, and Pect. cerevisiiphilus (Fig. 3a and Table 2). The previously reported hydrogen fermentation or lactate consumption ability of these bacterium is as follows: the genus Lactobacillus, a typical LAB, was not found to consume lactate or produce hydrogen;25 there are no related reports about the genus Pectinatus; the genera Clostridium and Megasphaera were found to be useful hydrogen producers that use glucose as the main substrate from various biomasses, and their hydrogen yield was 2.4 mol/mol hexose and 2.2 mol/mol hexose, respectively.23,26 However, thus far, none of the detected bacteria was previously reported to use lactate as the sole carbon source for hydrogen fermentation. Thus, in this study, we identified very useful hydrogen fermentation microflora that can use lactate as the sole carbon source.
3.2 Lactate-utilizing HPB and hydrogen production mechanism
The main bacteria involved in hydrogen fermentation from the fermented supernatant of the microflora samples by cultivation, phylogenetic analysis was carried out on the basis of 16S rDNA, and the hydrogen productivity and metabolites produced were evaluated via the batch test using monoculture.The anaerobic isolates AXX1 and AXX2, which were found to be M. elsdenii, showed significant hydrogen production (1468 ± 2 ml l−1 and 1404 ± 114 ml l−1, respectively) (Table 3), and all the lactate added as the substrate was consumed by 48 h of cultivation (Fig. 5). Low hydrogen production was observed for isolates AMM1 (346 ± 10 ml l−1) and A27 (409 ± 27 ml l−1), which were identified to be C. sporogenes, but no lactate was consumed as a substrate. It is known that Clostridium decomposes several amino acids, which leads to the evolution of H2 and CO2. Woods et al.27 reported that C. tetanomorphum produces approximately 0.3 mol hydrogen per mol of substrate from amino acids such as glutamate. It is likely that the hydrogen produced by C. sporogenes was from the decomposition of amino acids in the artificial medium. Isolate A15, which was Pect. cerevisiiphilus, was also not a HPB utilizing lactate. Thus, we concluded that the only useful lactate utilizing-hydrogen producing bacteria (LU-HPB) is M. elsdenii. The hydrogen yield of M. elsdenii in monoculture was approximately 0.40 mol/mol lactate, and the main VFAs produced as metabolites were acetate (approximately 3200 mg l−1) and propionate (approximately 3700 mg l−1).
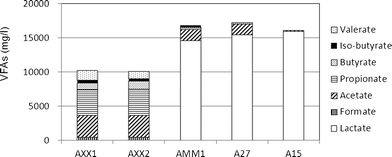 | ||
| Fig. 5 Differences in VFAs as fermentation products produced by the different isolates. | ||
Many of our results proved that M. elsdenii is the main hydrogen producer among hydrogen fermentation microflora. The hydrogen yield was very similar between the microflora (0.43 mol/mol lactate) and the isolated M. elsdenii (AXX1 and AXX2) (0.40 mol/mol lactate). Similarly, the main VFAs produced were acetate and propionate, at similar proportions in both cases. Moreover, the FISH analysis showed that the predominance of Megasphaera cells in the sample was observed simultaneously with the increase in hydrogen production in the fourth and fifth batches of the sequential batch test. Therefore, it is very likely that the hydrogen production increased because of the predominance of Megasphaera. PCR-DGGE and phylogenetic analyses also revealed that the organism of the genus Megasphaera in the hydrogen fermentation microflora was M. elsdenii (Fig. 3 and Table 2). Therefore, we concluded that M. elsdenii was the main LU-HPB among the hydrogen fermentation microflora.
Lactate is fermented to acetate and propionate by various bacteria via the methylmalonyl-CoA or acrylyl-CoA pathway.28Propionibacterium spp. ferment lactate via the following reaction:
| 3CH3CHOHCOOH → CH3COOH + 2CH3CH2COOH + CO2 + H2O | (5) |
This reaction may be similar in the case of other lactate utilizing bacterium, but the hydrogen productivity differs. The hydrogen production equation in the case of M. elsdenii was calculated by simple mass balancing of the main metabolites. On the basis of this balancing, the following equation was proposed to show the reaction through which M. elsdenii produces hydrogen:
| 2CH3CHOHCOOH → CH3COOH + CH3CH2COOH + CO2 + H2 | (6) |
Several anaerobic bacteria belonging to the phylum Firmicutes have the unique ability to generate energy via lactate fermentation. Metabolic pathways utilizing lactate have been reported in several anaerobes.29–31 On the basis of previous reports, a schematic representation of a putative metabolic pathway has been generated (Fig. 6). On the basis of this hypothesis, the theoretical yield of hydrogen production from lactate metabolised by M. elsdenii is 0.5 mol/mol lactate. The hydrogen yield of 0.4 mol/mol lactate observed in the present study, which is 80% of this theoretically maximum hydrogen yield, is thus a plausible value.
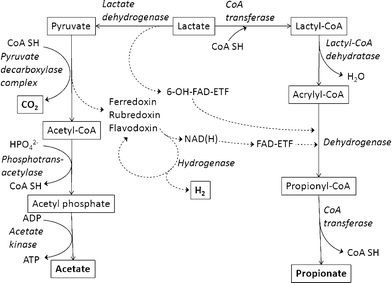 | ||
| Fig. 6 Schematic representation of a putative metabolic pathway of hydrogen fermentation using lactate; 6-OH-FAD-ETF, 6-hydroxy-7,8-dimethyl-10-(ribityl-5′-ADP)-isoalloxazine; ETF, electron-transferring flavoprotein; solid arrows, reaction pathways; dashed-line arrows, transfer of reducing equivalents; boxed structures, stating materials and products; bold text, final products; italics, enzyme. | ||
Another interesting aspect of biohydrogen production is the conversion of biomass and water to hydrogen via a chemical catalyst. Marquecvich et al.32 reported a high hydrogen yield using acetate as the substrate. Assuming that the reaction has reached completion, the steam reforming of lactate can be expressed as follows:
| CH3CHOHCOOH + 3H2O → 3CO2 + 6H2 | (7) |
A maximum of 6 hydrogen molecules can be obtained from a mole of lactate and H2O. However, it is inevitable that all catalysts undergo decay because of deactivation sue to poisoning and/or fouling. Nevertheless, bioconversion of lactate to hydrogen is a low-cost feasible technique. In addition, LU-HPB produce large amounts of VFAs, mainly acetate (approximately 3200 mg L−1) and propionate (approximately 3700 mg l−1), which can then be used as substrates for further biofuel production, for example, for methane (CH4) production by methanogens or hydrogen production by photosynthetic bacteria. Some photosynthetic bacteria such as Rhodopseudomonas spp. can produce hydrogen using acetate and propionate as electron donors. Theoretically, these substrates can be utilized to produce hydrogen according to the following reaction:
| Acetate: CH3COOH + 2H2O → 4H2 + 2CO2 | (8) |
| Propionate: CH3CH2COOH + 4H2O → 7H2 + 3CO2 | (9) |
Shi et al.33 reported a good hydrogen yield of 65% and 28% using acetate and propionate, respectively, with Rhodopseudomonas capsulate. Using LU-HPB for these processes will be effective for additional energy recovery.
3.3 Effect of pretreatment via heat shock
The effect of inoculum pretreatment via heat shock (at 90 °C for 10 min) was also evaluated in the batch test. The various environmental microflora used as inocula were either pretreated or non-pretreated. However, the results obtained were contradictory to those of the general hydrogen fermentation theory. In the first stage of the batch test, no hydrogen was produced by any pretreated inoculum (Table 1). In contrast, clear hydrogen production was observed when non-pretreated inocula from the methane fermentation system and garbage compost were used. Since acid slurry yielded the maximum hydrogen production of 1342 ml ml−1, this environmental microflora sample was used in the second stage of the analysis.In the 5 sequential batches of the second stage, no hydrogen production or lactate consumption were observed when pretreated inocula were used (Fig. 1 and 2). In contrast, stable and high hydrogen production and lactate consumption were observed in all batches when the non-pretreated inocula were used. This finding indicates that hydrogen productivity from lactate was lost by heat-shock pretreatment.
These microflora samples were analyzed by PCR-DGGE, and the difference in the non-pretreated and pretreated inocula could be attributed to the microflora composition of the samples (Fig. 3 and 4 and Table 2). The hydrogen fermentation microflora in the non-pretreated acid slurry comprised a wide variety of organisms including non-spore-forming bacteria (Lactobacillus, Megasphaera, and Pectinatus) and spore-forming bacteria (genus Clostridium). The predominant LU-HPB was M. elsdenii (Fig. 4). In contrast, the microflora in the pretreated acid slurry comprised a few organisms including the spore-forming C. magnum and Paeni. azoreducens. Luo et al.34 reported that C. magnum was the main bacterium in the hydrogen fermentation process in which glucose was used as the substrate and the inoculum was pretreated (105 °C, 2 h) topsoil, but it seems that C. magnum cannot ferment lactate to produce hydrogen. In the present study, we found that heat-shock pretreatment clearly reduced the microbial diversity in the inoculum, and we consider it reasonable that pretreatment tends to bias the inoculum toward spore-forming species, such as the heat-resistant Clostridium spp. (Table 2). It is well known that Lactobacillus spp. are easily eliminated by pretreatment.17 Thus, only the non-pretreated inocula had the main LU-HPB M. elsdenii and all species of the genus Lactobacillus as LAB.
To understand heat-shock tolerance better, the hydrogen productivity of pretreated isolates was evaluated. The hydrogen productivity of M. elsdenii (LU-HPB) and C. sporogenes was found to be hampered by heat shock (Table 3). Because M. elsdenii lack heat-shock tolerance, they are easily eliminated from pretreated inocula. Thus, although spore-forming bacteria such as Clostridium spp., which produce hydrogen from simple sugars,17 are enriched by heat-shock pretreatment of the inoculum, this pretreatment causes a reduction in the microbial diversity of the inoculum, by eliminating LAB and LU-HPB such as M. elsdenii. This decrease in the microbial diversity is also undesirable for the degradation of recalcitrant substrates such as cellulose or xylose that are present in various biomasses, since the number of metabolic degradation pathways possible is lowered by the reduced diversity.
Thus, the elimination of LAB and also LU-HPB by heat-shock pretreatment is a ‘blind spot’ in hydrogen fermentation. All studies that have reported the details of the effect of lactate on hydrogen fermentation12–14 used heat-shock pretreatment for the inoculum and therefore misinterpreted the findings to indicate that hydrogen cannot be produced from lactate, which is not true.
3.4 Evaluation of the recovery of hydrogen productivity from glucose by a complex system comprising LAB and LU-HPB
The total hydrogen yield from glucose via lactic acid fermentation (by LAB) and hydrogen fermentation (by LU-HPB) was estimated on the basis of the hydrogen fermentation data from the present experiment and data from previous studies. The typical hydrogen fermentation reactions are given above in eqn (3) and (4). General HPB such as Clostridium or Megasphaera were found to produce 2–3 mol of hydrogen from 1 mol of glucose under optimal conditions. It is well known that homo- or heterofermentative LAB ferment glucose to lactate following eqn (1) and (2) given above. Generally, the experimental results correspond with the theoretical values.35 Thus, in the case of hydrogen fermentation microflora including LAB, glucose consumption by LAB is a result of a decrease in or termination of hydrogen production as an indication of substrate competition. In most hydrogen fermentation systems, heat-shock pretreatment is carried out to avoid this situation. However, the main problems are the depleted microbial diversity and the decrease in the energy balance.23LU-HPB, the new tool discovered in this study, may solve both these problems simultaneously. Homo- or heterofermentative LAB produce 2 or 1 mol of lactate from 1 mol of glucose, respectively. M. elsdenii produced 0.40 mol of hydrogen from 1 mol of lactate in this study. Therefore, even if the substrate was consumed because of lactic acid fermentation, 0.4 (with hetero type LAB) to 0.8 (with homo type LAB) hydrogen mol/mol glucose would be recovered. Thus, the energy efficiency in field-scale hydrogen fermentation systems could be improved on the basis of this theory.
It has been reported that some LAB produce lactate from pentoses such as xylose, which does generate hydrogen. Xylose is a saccharification product of cellulosic biomasses like wood, paper, and agricultural byproducts.36,37 Yokoyama et al.38 reported a hydrogen yield of 0.56 mol/mol xylose using anaerobic microflora, and Li et al.39 reported a hydrogen yield of 0.96 mol/mol xylose using a recombinant Thermoanaerobacterium strain. On the other hand, Abdel-Rahman et al.40 reported that Enterococcus mundtii QU 25 produced 490 mM lactate from 334 mM xylose, showing a lactate yield of 1.51 mol/mol xylose. Even if M. elsdenii cannot directly use xylose for hydrogen fermentation, wild-type organisms can produce a hydrogen yield of 0.61 mol/mol xylose via lactic acid fermentation (E. mundtii as LAB) and hydrogen fermentation (M. elsdenii as LU-HPB). Coupling lactic acid fermentation as a pivot with hydrogen fermentation may improve substrate utilization and the efficiency of hydrogen fermentation from complex waste biomass.
4. Experimental
4.1 Collection of environmental microflora and preparation of inocula
Samples, including environmental microflora, were collected from soil, compost, and methane fermentation systems, and they were packed in ice for transport to the laboratory and maintained at 4 °C until experimentation.Soils were obtained from a courtyard and backyard (approximately 100–200 mm depth) at the Tokyo University of Agriculture (TUA). Garbage compost was obtained from the primary composting stage of a field-scale composter that treats kitchen garbage in Yorii, Saitama Prefecture. Further, samples were obtained from a field-scale biogas plant (treats 100 kg of garbage per day), TUA system (35° 64′ N, 139° 63′ E; floor area, 17 m2), used for the treatment of garbage from the student mess and a restaurant. This is a two-phase methane fermentation system equipped with a raw garbage resolution system. The acid slurry, acid sludge, and effluent were sampled from this system.
Approximately 10 g of each sample was placed in a 500 ml separating funnel with 90 ml sterile water to produce a suspension of living microflora. The suspension was homogenised by shaking at 230 rpm for 5 min. The homogenate was halved and placed in two centrifugal tubes to be used as the pre- and non-pretreated inoculum. Heat-shock pretreatment at 90 °C for 10 min was performed using a heat block for one of these aliquots.
4.2 Batch test of hydrogen fermentation using lactate as the sole carbon source
Sequential batch tests at 2 separate stages were performed at 37 °C in a 125 ml top-sealed flask with a working volume of 50 ml. The first stage was carried out to select microflora suitable for hydrogen fermentation from lactate. The second stage was carried out to evaluate the hydrogen production stability and fermentation properties of the selected environmental microflora. Fifty millilitres of artificial medium (5 g l−1 of tryptone, 3 g l−1 of yeast extract, 18 g l−1 of 70% sodium lactate, pH 7.0) was added to the flask, and the gaseous phase in the flask was replaced with anaerobic N2 gas. The flask was then sealed with an aluminium seal and a rubber stopper and autoclaved at 121 °C and 1.2 atm.Non-pretreated and pretreated inocula (0.5 ml) were inoculated into a top-sealed flask containing 50 ml of artificial medium. The inoculated flask was incubated with shaking on a shaking incubator (BR-3000LF; Taitec). After the first incubation for 24 h at 37 °C and 90 rpm, the evolved biogas was collected via a syringe. After that, 0.5 ml of the incubated suspension was retrieved from the flask via a syringe and inoculated into another flask. For suppression of hydrogenotrophic methanogens, the incubation time was controlled at 24 h in first batch test and at 48 h for the subsequent tests.20
In the first stage, 2 batches of the successive inoculation-and-cultivation process were set up using 12 inocula from 6 environmental microflora (detailed in Table 1). In the second stage, 5 batches of the process were set up using a specific microflora sample as the inoculum, and this assay was performed in triplicate.
4.3 Analytical methods
The H2, CH4, N2, and CO2 contents were measured using gas chromatography (model GC323; GL Science) according to the manufacturer's instructions. The chromatograph was equipped with a thermal conductivity detector (TCD) and a molecular sieve 5A column (60/80 mm). The temperatures of the injection, detector, and column were maintained at 160 °C. Argon was used as the carrier gas at a flow rate of 30 ml min−1. The hydrogen volume was calculated from the hydrogen concentration and the volume of biogas, corrected to standard temperature and pressure (0 °C, 1 atm). The hydrogen yield was also calculated on the basis of the amount of lactate consumed.The types and levels of VFAs was measured using high-performance liquid chromatography (HPLC) (PU-2080 Intelligent pump; JASCO) with a Gelpack column (GL-C610H-S, 7.8 × 300 mm; Hitachi Chemical), post-column reactor (RU-2080-51 reaction coil unit; JASCO), Intelligent UV/VIS detector (UV-2070; JASCO), and data processor (Chromatocorder 21; SIC). The mobile phase used was 3 mM HClO4 (flow rate, 0.5 ml min−1). The individual VFAs were detected using a post-labelling method with 0.2 mM bromothymol blue and 15 mM Na2HPO4 (flow rate, 1.5 ml min−1). The column temperature was 60 °C, and the detection wavelength was 445 nm. All samples were analyzed after filtration through a glass fibre filter (Whatman GF/C).
The microbial compositions of the samples were determined using culture-independent methods (fluorescence in situ hybridization [FISH] and polymerase chain reaction-denaturing gradient gel electrophoresis [PCR-DGGE]) and a culture-dependent method.
4.4 FISH analysis
Cell fixation, cell wall permeabilisation, FISH, and DAPI staining were performed in accordance with a previous report.21 The following 2 FISH probes were used (all numbers correspond to the complementary positions in Escherichia coli 16S rRNA): EUB338 (GCTGCCTCCCGTAGGAGT, 338–355) with Alexa488 labelling for domain bacteria-specific oligonucleotides and Mega-X (GACTCTGTTTTTGGGGTTT, 1297–1315) with Cy3 labelling for Megasphaera-specific oligonucleotides.21,22 The results were observed with a confocal laser scanning microscope (FV-500 Fluoview; Olympus) and a fluorescence microscope (Biozero BZ-8000; Keyence) equipped with OP-66834 (for DAPI), OP-66835 (for Alexa488), and OP-66837 (for Cy3).4.5 DNA extraction
DNA was extracted from each sample using the bead beating method. Each sample (1 ml) was suspended in 2.5 ml of 10 mM phosphate buffer (pH 8.0) and then mixed with 1 ml of extraction reagent (0.1 M NaCl, 0.5 M Tris-HCl, 10% SDS, pH 8.0). The bacterial cells were crushed using 10 cycles (3000 rpm, 30 s) of bead beating, and the crude DNA was purified by phenol-chloroform extraction.234.6 PCR-DGGE analysis
The V3 region of the 16S rRNA gene (rDNA) was amplified by PCR, and the PCR product was then subjected to DGGE.234.7 Bacterial isolation and hydrogen fermentation test
Through a procedure outlined previously,23 anaerobic bacteria including HPB were isolated after culture on artificial agar plates (containing 2.0% of agar) and purified, and their 16S rDNA gene sequence was determined.Hydrogen fermentation was tested using the batch test with artificial medium containing lactate as the sole carbon source. The precultivated strain was suspended in sterile saline and inoculated into the flask (final OD660 = 0.1) via a syringe. After incubation for 48 h, the evolved biogas was collected via a syringe and the gas composition analyzed.
5. Conclusion
In the present study, we effectively identified complex microflora that can produce hydrogen using lactate as the sole carbon source. The maximum hydrogen yield was 0.43 mol/mol lactate. The microflora sample was obtained from an anaerobic methane fermentation system, and the main hydrogen producer was found to be M. elsdenii. A batch test showed that isolated M. elsdenii was able to produce approximately 0.4 mol hydrogen/mol lactate. Further, since this bacterium has no heat tolerance, heat-shock pretreatment eliminates it from the microflora inoculum, and so lactate-utilizing hydrogen production is not possible with this inoculum. M. elsdenii may contribute to improving the energy balance and cooperate with LAB in hydrogen fermentation systems to improve the efficiency of hydrogen production.References
- R. Luque, L. Herrero-Davila, J. M. Campelo, J. H. Clark, J. M. Hidalgo, D. Luna, J. M. Marinas and A. A. Romero, Energy Environ. Sci., 2008, 1, 542–564 CAS.
- W. D. Huang and Y. H. P. Zhang, Energy Environ. Sci., 2011, 4, 784–792 CAS.
- J. A. Choi, J. H. Hwang, B. A. Dempsey, R. A. I. Abou-Shanab, B. Min, H. Song, D. S. Lee, J. R. Kim, Y. Cho and S. Hong, Energy Environ. Sci., 2011, 4, 3513 CAS.
- J. Lü, C. Sheahan and P. Fu, Energy Environ. Sci., 2011, 4, 2451–2466 Search PubMed.
- H. Reardon, J. Hanlon, R. Hughes, A. Godula-Jopek, T. Mandal and D. Gregory, Energy Environ. Sci., 2012, 5, 5951–5979 CAS.
- D. P. Serrano, J. Dufour and D. Iribarren, Energy Environ. Sci., 2012, 5, 6126–6135 CAS.
- B. Vogel, T. Feck, J. U. Grooß and M. Riese, Energy Environ. Sci., 2012, in press Search PubMed.
- A. Daday, G. R. Lambert and G. D. Smith, Biochem. J., 1979, 177, 139 CAS.
- S. J. Schropp, M. I. Scranton and J. R. Schwarz, Limnol. Oceanogr., 1987, 32, 396–402 CrossRef CAS.
- V. Fourmond, S. Canaguier, B. Golly, M. J. Field, M. Fontecave and V. Artero, Energy Environ. Sci., 2011, 4, 2417–2427 CAS.
- P. P. Liebgott, S. Dementin, C. Léger and M. Rousset, Energy Environ. Sci., 2011, 4, 33–41 CAS.
- T. H. Kim, Y. Lee, K. H. Chang and S. J. Hwang, Bioresour. Technol., 2012, 103, 136–141 CrossRef CAS.
- M. Matsumoto and Y. Nishimura, J. Biosci. Bioeng., 2007, 103, 236–241 CrossRef CAS.
- B. Baghchehsaraee, G. Nakhla, D. Karamanev and A. Margaritis, Int. J. Hydrogen Energy, 2009, 34, 2573–2579 CrossRef CAS.
- T. Noike, H. Takabatake, O. Mizuno and M. Ohba, Int. J. Hydrogen Energy, 2002, 27, 1367–1371 CrossRef CAS.
- J. Jo, C. Jeon, D. Lee and J. Park, J. Biotechnol., 2007, 131, 300–308 CrossRef CAS.
- C. Li and H. H. P. Fang, Crit. Rev. Environ. Sci. Technol., 2007, 37, 1–39 CrossRef CAS.
- Y. Mu, H. Q. Yu and G. Wang, Enzyme Microb. Technol., 2007, 40, 947–953 CrossRef CAS.
- J. Wang and W. Wan, Int. J. Hydrogen Energy, 2008, 33, 2934–2941 CrossRef CAS.
- O. Pakarinen, P. Kaparaju and J. Rintala, Bioresour. Technol., 2011, 102, 8952–8957 CrossRef CAS.
- A. Ohnishi, S. Abe, Y. Bando, N. Fujimoto and M. Suzuki, Int. J. Hydrogen Energy, 2012, 37, 2239–2247 CrossRef CAS.
- A. Ohnishi, S. Abe, S. Nashirozawa, S. Shimada, N. Fujimoto and M. Suzuki, Appl. Environ. Microbiol., 2011, 77, 5533–5535 CrossRef CAS.
- A. Ohnishi, Y. Bando, N. Fujimoto and M. Suzuki, Int. J. Hydrogen Energy, 2010, 35, 8544–8553 CrossRef CAS.
- B. E. Logan, S. E. Oh, I. S. Kim and S. Van Ginkel, Environ. Sci. Technol., 2002, 36, 2530–2535 CrossRef CAS.
- P. Vos, G. Garrity, D. Jones, N. Krieg, W. Ludwig, F. RaineyvK. Schleifer and W. Whitman, 2009.
- I. K. Kapdan and F. Kargi, Enzyme Microb. Technol., 2006, 38, 569–582 CrossRef CAS.
- D. D. Woods and C. E. Clifton, Biochem. J., 1937, 31, 1774 CAS.
- S. Seeliger, P. H. Janssen and B. Schink, FEMS Microbiol. Lett., 2002, 211, 65–70 CrossRef CAS.
- G. Counotte, R. Prins, R. Janssen and M. DeBie, Appl. Environ. Microbiol., 1981, 42, 649 CAS.
- H. Danner, M. Ürmös, M. Gartner and R. Braun, Appl. Biochem. Biotechnol., 1998, 70–72, 887–894 CrossRef CAS.
- J. Ladd and D. Walker, Biochem. J., 1959, 71, 364 CAS.
- M. Marquevich, S. Czernik, E. Chornet and D. Montané, Energy Fuels, 1999, 13, 1160–1166 CrossRef CAS.
- X. Shi and H. Yu, Int. Biodeterior. Biodegrad., 2006, 58, 82–88 CrossRef CAS.
- Y. Luo, H. Zhang, M. Salerno, B. E. Logan and M. A. Bruns, Int. J. Hydrogen Energy, 2008, 33, 6566–6576 CrossRef CAS.
- J. M. Neill, Massachusetts Agricultural College, 1922 Search PubMed.
- B. Ahring, K. Jensen, P. Nielsen, A. Bjerre and A. Schmidt, Bioresour. Technol., 1996, 58, 107–113 CrossRef CAS.
- B. Lavarack, G. Griffin and D. Rodman, Biomass Bioenergy, 2002, 23, 367–380 CrossRef CAS.
- H. Yokoyama, N. Moriya, H. Ohmori, M. Waki, A. Ogino and Y. Tanaka, Appl. Microbiol. Biotechnol., 2007, 77, 213–222 CrossRef CAS.
- S. Li, C. Lai, Y. Cai, X. Yang, S. Yang, M. Zhu, J. Wang and X. Wang, Bioresour. Technol., 2010, 101, 8718–8724 CrossRef CAS.
- M. A. Abdel-Rahman, Y. Tashiro, T. Zendo, K. Hanada, K. Shibata and K. Sonomoto, Appl. Environ. Microbiol., 2011, 77, 1892–1895 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
