Palladium-catalysed direct regiospecific arylation at C5 of thiophenes bearing SO2R substituents at C3
Charles Beromeo
Bheeter
a,
Jitendra K.
Bera
b and
Henri
Doucet
*a
aInstitut Sciences Chimiques de Rennes, UMR 6226 CNRS-Université de Rennes “Catalyse et Organométalliques”, 35042 Rennes, France. Tel: +33 (0)2 23 23 63 84E-mail: henri.doucet@univ-rennes1.fr
bDepartment of Chemistry, Indian Institute of Technology Kanpur, 208016 Kanpur, India
First published on 13th June 2012
Abstract
The palladium catalysed direct arylation of thiophenes substituted at C3 by SO2R subtituents was found to be fully selective in favor of carbon C5. This reaction allows the synthesis of a wide variety of 5-aryl-3-sulfonic acid derivatives using as little as 0.5–1 mol% of Pd(OAc)2 as the catalyst in only one step.
The functionalisation of heteroaromatics has been extensively investigated in recent decades as they exhibit useful physical or biological properties. For example, some thiophene derivatives bearing SO2R as substituents have been described as non-steroidal anti-inflammatory drugs or anti-glaucoma agents (Fig. 1).
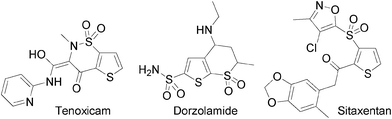 | ||
| Fig. 1 Examples of bioactive thiophenes bearing SO2R substituents. | ||
The palladium-catalysed direct arylation of several heteroaromatics via C–H bond activation using aryl halides has been successful in recent years.1,2 However, there are still limitations for these reactions in terms of regioselectivity and also heteroaromatics functional group tolerance.3–8 We have recently reported that thiophenes substituted at C2 by SO2R led regioselectively to the C5 arylated products.9 On the other hand, the regioselectivity of direct arylations of thiophene bearing SO2R at C3 has not been reported.10 The control of the regioselectivity for direct arylation remains an essential issue due to the presence of several C–H bonds with similar reactivity on most heterocycles. In the course of the palladium-catalysed arylation of 3-substituted thiophenes, in general, the most reactive position is carbon C2;11 although, arylation at C5 is also possible (Scheme 1).12
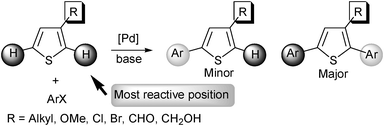 | ||
| Scheme 1 | ||
For example, in 2003 Sharp and co-workers reported conditions that allow the regioselective arylation of methyl 3-thiophene carboxylate.12a The use of Pd(PPh3)4 in toluene gave selectively the 2-arylated thiophene; whereas, Pd2(dba)3 in NMP gave a mixture of 2- and 5-arylated thiophenes in a 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51 ratio. In 1998, Lemaire and co-workers have reported the direct 2-arylation of 3-formyl-, 3-cyano and 3-nitrothiophene with aryl iodides.11a,b Bilodeau and co-workers have examined the regioselectivity of the arylation of 3-methylthiophene with bromobenzene using Pd[(P(t-Bu)3]2 as the catalyst. They obtained a mixture of the 2- and 5-phenylated thiophenes in a 3.3
51 ratio. In 1998, Lemaire and co-workers have reported the direct 2-arylation of 3-formyl-, 3-cyano and 3-nitrothiophene with aryl iodides.11a,b Bilodeau and co-workers have examined the regioselectivity of the arylation of 3-methylthiophene with bromobenzene using Pd[(P(t-Bu)3]2 as the catalyst. They obtained a mixture of the 2- and 5-phenylated thiophenes in a 3.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (30% yield of 2-phenylation and 9% yield of the 5-phenylated thiophene).12b Recently, Fagnou and co-workers have reported the direct arylation of 3-n-hexylthiophene with 4-bromonitrobenzene.12c A mixture of C2 and C5 arylation products was obtained in a 1.3
1 ratio (30% yield of 2-phenylation and 9% yield of the 5-phenylated thiophene).12b Recently, Fagnou and co-workers have reported the direct arylation of 3-n-hexylthiophene with 4-bromonitrobenzene.12c A mixture of C2 and C5 arylation products was obtained in a 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The direct arylation of 3-methoxythiophene has been explored by Borghese and co-workers.11c With this reactant, the 2-arylated thiophenes were regioselectively obtained in 28–60% yields. It should be noted that, with some very specific substrates, the C5 arylated thiophenes have been obtained in quite high regioselectivities. For example, a thiophene substituted at C3 by an acetal selectively led to the C5 arylated thiophenes.12e
1 ratio. The direct arylation of 3-methoxythiophene has been explored by Borghese and co-workers.11c With this reactant, the 2-arylated thiophenes were regioselectively obtained in 28–60% yields. It should be noted that, with some very specific substrates, the C5 arylated thiophenes have been obtained in quite high regioselectivities. For example, a thiophene substituted at C3 by an acetal selectively led to the C5 arylated thiophenes.12e
If the regioselectivity of the arylation can be controlled, such a reaction appears more attractive and useful than palladium catalysed Suzuki, Stille or Negishi cross-couplings for the synthesis of arylated thiophenes. This is because no previous preparation of an organometallic derivative and its transmetallation product using B(OR)3, XSnR3 or ZnX2 is required (Scheme 2).13,14
 | ||
| Scheme 2 | ||
Herein, we report on the palladium-catalysed direct regiospecific arylation at C5 of thiophenes bearing SO2R substituents at C3 with a variety of aryl bromides.
We initially examined the reactivity of both methyl 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate 1 and thiophene-3-sulfonic acid diethylamide 2 with 4-bromobenzonitrile using KOAc as the base, DMAc as the solvent in the presence of only 0.5 mol% Pd(OAc)2 as the catalyst (Scheme 3). From 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate 1, we expected the regioselective formation of the C5 arylated product, as we had previously observed that the use of esters as blocking groups at C2 position on a range of 3-substituted thiophenes allows control of the regioselectivity of the palladium-catalysed direct arylation at C5.4g Under these conditions, a regiospecific arylation at C5 was observed. However, in the course of this reaction, a complete decarboxylation of the thiophene derivative occurs to give 3 in 82% yield. The progress of this reaction was monitored by GC-MS and 1H NMR analysis. After 5 min, a ratio of 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 87 in 1
87 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was observed, and 3 was formed in 39% yield. A conversion of 92% of 4-bromobenzonitrile in favor of the formation of 3 was observed after 15 min. It should be noted that both at 5 and 15 min, no trace of non-decarboxylated coupling product could be detected.
2 was observed, and 3 was formed in 39% yield. A conversion of 92% of 4-bromobenzonitrile in favor of the formation of 3 was observed after 15 min. It should be noted that both at 5 and 15 min, no trace of non-decarboxylated coupling product could be detected.
 | ||
| Scheme 3 | ||
This in situ decarboxylation was found to be due to the poor thermal stability of 1. The heating of 1 at 130 °C in DMAc during 16 h in the presence of KOAc without palladium catalyst produces 2 in quantitative yield (Scheme 4). Even in the absence of base, 2 was produced in 54% yield.
 | ||
| Scheme 4 | ||
We then employed thiophene-3-sulfonic acid diethylamide 2 as the coupling partner. A mixture of C2 and C5 arylated thiophenes might have been produced. However, again, only the C5 arylated thiophene 3 was obtained. No trace of arylation at C2 was detected. The complete regioselectivity of this arylation is certainly due to the steric hindrance of the SO2NEt2 substituent. Because methyl 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate 1 is more affordable than thiophene-3-sulfonic acid diethylamide 2, it was employed as the reactant to study the scope of this reaction with other aryl bromides (Table 1).
| Entry | Aryl halide | Product | Yield (%)a |
|---|---|---|---|
| a Conditions: Pd(OAc)2 (0.005 mmol), methyl 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate (1.5 mmol), aryl halide (1 mmol), KOAc (2 mmol), DMAc, 130 °C, 16 h, isolated yields. b 4-chloropropiophenone as aryl halide. c 4-chloronitrobenzene as aryl halide. d 4-iodoanisole as aryl halide. | |||
| 1 |

|
 4
4
|
84 |
| 2 |

|
 5
5
|
77 |
| 3 |

|
 6
6
|
86 |
| 4 | 0b | ||
| 5 |
|
 7
7
|
83 |
| 6 | 26c | ||
| 7 |

|
 8
8
|
90 |
| 8 |
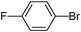
|
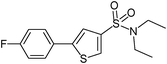 9
9
|
63 |
| 9 |

|
 10
10
|
78 |
| 10 |

|
 11
11
|
80 |
| 11 | 28d | ||
| 12 |

|
 12
12
|
77 |
| 13 |

|
 13
13
|
91 |
| 14 |

|
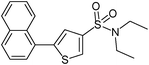 14
14
|
80 |
| 15 |

|
 15
15
|
89 |
| 16 |

|
 16
16
|
68 |
4-Bromoacetophenone, 4-bromobenzaldehyde, 4-bromopropiophenone, 4-bromonitrobenzene or 4-trifluoromethylbromobenzene reacted with methyl 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate 1 gave the products 4–8 in 77–90% yields (Scheme 5, Table 1, entries 1–3, 5 and 7). Again, in all cases, a regiospecific arylation at C5 and a complete decarboxylation was observed. A lower yield of 63% of 9 was obtained using 4-bromofluorobenzene, due to a partial conversion of this aryl bromide (Table 1, entry 8). It should be noted that even 4-chlorobromobenzene could be employed to give 10 in 78% yield (Table 1, entry 9). In the course of this reaction, no cleavage of the C–Cl bond was observed, allowing further transformations. The electron-rich aryl halides, 4-bromoanisole and 4-iodoanisole were then employed. In both cases, 11 was produced. However, a high yield of 80% was obtained from 4-bromoanisole; whereas, from 4-iodoanisole a large amount of this reactant was recovered unreacted (Table 1, entries 10 and 11).
 | ||
| Scheme 5 | ||
The meta-substituted aryl bromide, 3-bromoacetophenone gave product 12 in 77% yield (Table 1, entry 12). Even the ortho-substituted aryl bromides, 2-bromobenzonitrile and 1-bromonaphthalene gave the desired coupling products 13 and 14 in good yields (Table 1, entries 13 and 14). Pyridines are probably the most common heterocyclic motif found in pharmaceutically active compounds. We observed that 3- or 4-bromopyridines are also suitable reactants (Table 1, entries 15 and 16). It should be noted that regiospecific arylations at C5 of 1 with complete decarboxylation were observed in all cases. Two electron-deficient aryl chlorides were also employed as the coupling partners. From 4-chloropropiophenone, no formation of 6 was detected; whereas the use of 4-chloronitrobenzene allowed the formation of 7 in a low yield of 26% (Table 1, entries 4 and 6).
Methyl 3-(morpholinosulfamoyl)thiophene-2-carboxylate 17 reacted with 2- or 4-bromobenzonitriles or 1-bromonaphthalene also gave the 5-arylated thiophenes 18–20 in high yields with complete decarboxylation (Scheme 5, Table 2). From 5-bromopyridine, 21 was only obtained in moderate yield due to formation of side-products.
| Entry | Aryl bromide | Product | Yield (%)a |
|---|---|---|---|
| a Conditions: Pd(OAc)2 (0.005 mmol), methyl 3-(morpholinosulfamoyl)thiophene-2-carboxylate (2 mmol), aryl bromide (1 mmol), KOAc (2 mmol), DMAc, 150 °C, 16 h, isolated yields. | |||
| 1 |

|
 18
18
|
72 |
| 2 |

|
 19
19
|
77 |
| 3 |

|
 20
20
|
80 |
| 4 |
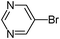
|
 21
21
|
45 |
The reaction is not limited to the use of sulfonic acid dialkylamides. Methyl 3-(N-butyl-N-phenylsulfamoyl)thiophene-2-carboxylate 22 was also successfully employed as a coupling partner (Scheme 5, Table 3). A similar reactivity to methyl 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate was observed. From 2- or 4-bromobenzonitriles, 3-bromonitrobenzene, 1-bromonaphthalene or 3-bromoisoquinoline 23 and 25–28 were obtained in 75–82% yields. Again, in all cases, a regiospecific arylation at C5 and a complete decarboxylation was observed.
On the other hand, thiophene-3-sulfonic acid benzylamide 29 was found to be unreactive. Using similar reaction conditions, no formation of the desired product 30 was detected (Scheme 6). The presence of a free NH on this reactant certainly poisons the catalyst.
 | ||
| Scheme 6 | ||
Finally, the reactivity of methyl 3-phenoxysulfonylthiophene-2-carboxylate was studied (Scheme 7, Table 4). In the presence of 4-bromobenzonitrile, product 31 was obtained in 80% yield with again a regiospecific 5-arylation with complete decarboxylation of the thiophene derivative (Table 4, entry 1). No formation of the C2 arylated product was detected. The use of other aryl bromides such as methyl 4-bromobenzoate, 3-chlorobromobenzene, 2-bromobenzonitrile or 3-bromopyridine gave 32–39 in 58–83% yields.
 | ||
| Scheme 7 | ||
| Entry | Aryl bromide | Product | Yield (%)a |
|---|---|---|---|
| a Conditions: Pd(OAc)2 (0.01 mmol), methyl 3-phenoxysulfonylthiophene-2-carboxylate (1.5 mmol), aryl bromide (1 mmol), KOAc (2 mmol), DMAc, 150 °C, 16 h, isolated yields. | |||
| 1 |

|
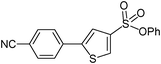 32
32
|
80 |
| 2 |

|
 33
33
|
84 |
| 3 |

|
 34
34
|
83 |
| 4 |

|
 35
35
|
72 |
| 5 |

|
 36
36
|
58 |
| 6 |

|
 37
37
|
78 |
| 7 |
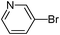
|
 38
38
|
77 |
| 8 |
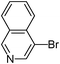
|
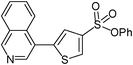 39
39
|
60 |
In summary, we have demonstrated that thiophenes substituted at C3 by SO2R functions can be coupled with a variety of aryl bromides, using only 0.5–1 mol% of the Pd(OAc)2 catalyst associated with KOAc as the base. In all cases, a regiospecific arylation at C5 was observed. From both methyl 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate 1 and thiophene-3-sulfonic acid diethylamide 2, the product 3 was obtained in similar yields due to a complete decarboxylation of 1. A similar regioselectivity was observed with other tertiary thiophene sulfonamides and also with a thiophene sulfonic acid phenyl ester. It should be noted that for these couplings a wide range of functional groups on the aryl bromide are tolerated, including formyl, acetyl, propionyl, ester, nitrile, nitro, ester, trifluoromethyl, fluoro or chloro. Such functional group tolerance should allow easy modification of structures for the preparation of important bioactive compounds. This procedure employs a relatively low loading of a commercially available air stable palladium catalyst. The major by-products are AcOH/KBr instead of metallic salts with classical coupling procedures. Moreover, no preparation of an organometallic derivative is required, reducing the number of steps in the preparation of these compounds. Despite their interest, the products prepared by this method are new, indicating a relatively limited access to such compounds using more traditional cross-coupling procedures.
Experimental
All reactions were run under argon in Schlenk tubes using vacuum lines. DMAc analytical grade was not distilled before use. Potassium acetate (99%) was used. Commercial aryl halides and methyl 3-(sulfamoylchloride)thiophene-2-carboxylate were used without purification. 1H and 13C spectrum were recorded with Bruker 300 or 400 MHz spectrometer solutions. Flash chromatography was performed on silica gel (230–400 mesh).Methyl 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate (1)15
Diethylamine (0.183 g, 2.5 mmol) and triethylamine (1.15 g, 8.0 mmol) were added to 5 mL of dry CH2Cl2 under an argon atmosphere at room temperature. Methyl 3-(sulfamoylchloride)thiophene-2-carboxylate (0.481 g, 2 mmol) was then added, and the reaction was stirred for 12 h. The product was extracted with CH2Cl2 (3 × 15 mL), washed successively with HCl 1N, water and dried over MgSO4. The solvent was evaporated under reduced pressure. Purification by flash chromatography (diethylether/pentane: 1/9) afforded 1 as a white solid in 86% (0.476 g) yield.1H NMR (300 MHz, DMSO-d6): δ: 7.77 (d, J = 5.2 Hz, 1H), 7.47 (d, J = 5.2 Hz, 1H), 3.91 (s, 3H), 3.45 (q, J = 7.8 Hz, 4H), 1.15 (t, J = 7.8 Hz, 6H).
Thiophene-3-sulfonic acid diethylamide (2)
Methyl 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate (0.692 g, 2.5 mmol) and KOAc (0.490 g, 5 mmol) was added in 5 mL of DMAc under an argon atmosphere at 150 °C. The reaction was stirred for 12 h. After evaporation of the solvent, the product was purified by flash chromatography (diethylether/pentane: 1/9) to afford 2 as a yellow solid in 81% (0.443 g) yield.1H NMR (300 MHz, DMSO-d6): δ: 7.89 (dd, J = 3.0, 1.2 Hz, 1H), 7.40 (dd, J = 5.2, 3.0 Hz, 1H), 7.28 (dd, J = 5.2, 1.2 Hz, 1H), 3.24 (q, J = 7.8 Hz, 4H), 1.14 (t, J = 7.8 Hz, 6H). 13C NMR (75 MHz, DMSO-d6): δ: 139.8, 131.2, 129.6, 125.8, 42.4, 14.6. Elemental analysis: calcd (%) for C8H13NO2S2 (219.33): C 43.81, H 5.97, found: C 43.78, H 5.87.
General procedure for coupling reactions
As a typical experiment, the reaction of the aryl bromide (1 mmol), thiophene derivative (1.5 mmol) and KOAc (0.196 g, 2 mmol) at 130 or 150 °C (see Tables) during 16 h in DMAc (4 mL) in the presence of Pd(OAc)2 0.5–1 mol% (see Tables) under argon affords the coupling product after evaporation of the solvent and purification on silica gel.5-(4-Cyanophenyl)-thiophene-3-sulfonic acid diethylamide (3)
4-Bromobenzonitrile (0.182 g, 1 mmol) and methyl 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate (0.416 g, 1.5 mmol) affords 3 in 82% (0.262 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.31 (d, J = 1.3 Hz, 1H), 7.99 (d, J = 1.3 Hz, 1H), 7.98 (d, J = 8.4 Hz, 2H), 7.88 (d, J = 8.4 Hz, 2H), 3.22 (q, J = 7.1 Hz, 4H), 1.08 (t, J = 7.1 Hz, 6H). 13C NMR (75 MHz, DMSO-d6): δ 144.2, 141.2, 137.1, 133.6, 132.0, 126.8, 123.9, 119.0, 111.2, 42.7, 14.7. Elemental analysis: calcd (%) for C15H16N2O2S2 (320.43): C 56.22, H 5.03, found: C 56.10, H 5.14.
5-(4-Acetylphenyl)-thiophene-3-sulfonic acid diethylamide (4)
4-Bromoacetophenone (0.199 g, 1 mmol) and methyl 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate (0.416 g, 1.5 mmol) affords 4 in 84% (0.283 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.27 (d, J = 1.3 Hz, 1H), 8.00 (d, J = 8.4 Hz, 2H), 7.91 (d, J = 1.3 Hz, 1H), 7.90 (d, J = 8.4 Hz, 2H), 3.22 (q, J = 7.1 Hz, 4H), 2.59 (s, 3H), 1.08 (t, J = 7.1 Hz, 6H). 13C NMR (75 MHz, DMSO-d6): δ 197.2, 144.5, 140.6, 136.4, 136.2, 130.9, 129.1, 125.7, 122.6, 42.1, 26.7, 14.2. Elemental analysis: calcd (%) for C16H19NO3S2 (337.46): C 56.95, H 5.68, found: C 56.87, H 5.79.
5-(4-Formylphenyl)-thiophene-3-sulfonic acid diethylamide (5)
4-Bromobenzaldehyde (0.185 g, 1 mmol) and methyl 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate (0.416 g, 1.5 mmol) affords 5 in 77% (0.249 g) yield.1H NMR (300 MHz, DMSO-d6): δ 10.01 (s, 1H), 8.31 (s, 1H), 8.00 (d, J = 8.4 Hz, 2H), 7.96 (d, J = 1.3 Hz, 1H), 7.95 (d, J = 8.4 Hz, 2H), 3.22 (q, J = 7.1 Hz, 4H), 1.08 (t, J = 7.1 Hz, 6H). 13C NMR (75 MHz, DMSO-d6): δ 192.4, 144.3, 140.6, 137.7, 135.6, 131.3, 130.4, 126.1, 123.1, 42.1, 14.2. Elemental analysis: calcd (%) for C15H17NO3S2 (323.43): C 55.70, H 5.30, found: C 55.62, H 5.18.
5-(4-propionylphenyl)-thiophene-3-sulfonic acid diethylamide (6)
4-Bromopropiophenone (0.213 g, 1 mmol) and methyl 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate (0.416 g, 1.5 mmol) affords 6 in 86% (0.302 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.28 (d, J = 1.4 Hz, 1H), 8.00 (d, J = 8.4 Hz, 2H), 7.92 (d, J = 1.4 Hz, 1H), 7.90 (d, J = 8.4 Hz, 2H), 3.22 (q, J = 7.1 Hz, 4H), 3.05 (q, J = 7.1 Hz, 2H), 1.08 (t, J = 7.1 Hz, 9H). 13C NMR (75 MHz, DMSO-d6): δ 199.6, 144.5, 140.6, 136.3, 136.0, 130.8, 128.8, 125.7, 122.6, 42.1, 31.2, 14.2, 8.0. Elemental analysis: calcd (%) for C17H21NO3S2 (351.49): C 58.09, H 6.02, found: C 58.01, H 6.14.
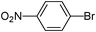
5-(4-Nitrophenyl)-thiophene-3-sulfonic acid diethylamide (7)
4-Bromonitrobenzene (0.202 g, 1 mmol) and methyl 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate (0.416 g, 1.5 mmol) affords 7 in 83% (0.282 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.37 (d, J = 1.4 Hz, 1H), 8.26 (d, J = 8.0 Hz, 2H), 8.06–8.04 (m, 3H), 3.22 (q, J = 7.1 Hz, 4H), 1.09 (t, J = 7.1 Hz, 6H). 13C NMR (75 MHz, DMSO-d6): δ 146.8, 143.1, 140.8, 138.5, 132.0, 126.6, 124.4, 124.0, 42.2, 14.2. Elemental analysis: calcd (%) for C14H16N2O4S2 (340.42): C 49.39, H 4.74, found C 49.28, H 4.87.
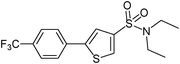
5-(4-Trifluoromethylphenyl)-thiophene-3-sulfonic acid diethylamide (8)
4-Trifluoromethylbromobenzene (0.225 g, 1 mmol) and methyl 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate (0.416 g, 1.5 mmol) affords 8 in 90% (0.327 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.31 (d, J = 1.4 Hz, 1H), 7.99 (d, J = 8.0 Hz, 2H), 7.96 (d, J = 1.4 Hz, 1H), 7.80 (d, J = 8.0 Hz, 2H), 3.22 (q, J = 7.1 Hz, 4H), 1.09 (t, J = 7.1 Hz, 6H). 13C NMR (75 MHz, DMSO-d6): δ 143.9, 140.6, 136.2, 131.0, 128.5 (q, J = 32.0 Hz), 126.4, 126.0 (q, J = 3.8 Hz), 123.0 (q, J = 272.0 Hz), 122.9, 42.1, 14.2. Elemental analysis: calcd (%) for C15H16F3NO2S2 (363.42): C 49.57, H 4.44, found C 49.67, H 4.58.
5-(4-Fluorophenyl)-thiophene-3-sulfonic acid diethylamide (9)
4-Bromofluorobenzene (0.175 g, 1 mmol) and methyl 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate (0.416 g, 1.5 mmol) affords 9 in 63% (0.197 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.18 (d, J = 1.3 Hz, 1H), 7.81 (dd, J = 8.8, 5.3 Hz, 2H), 7.73 (d, J = 1.3 Hz, 1H), 7.29 (t, J = 8.8 Hz, 2H), 3.20 (q, J = 7.1 Hz, 4H), 1.08 (t, J = 7.1 Hz, 6H). 13C NMR (75 MHz, DMSO-d6): δ 162.1 (d, J = 246.2 Hz), 144.7, 140.3, 129.6, 129.1 (d, J = 3.2 Hz), 127.9 (d, J = 8.4 Hz), 121.1, 116.1 (d, J = 21.9 Hz), 42.1, 14.2. Elemental analysis: calcd (%) for C14H16FNO2S2 (313.41): C 53.65, H 5.15, found C 53.57, H 5.21.
5-(4-Chlorophenyl)-thiophene-3-sulfonic acid diethylamide (10)
4-Bromochlorobenzene (0.191 g, 1 mmol) and methyl 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate (0.416 g, 1.5 mmol) affords 10 in 78% (0.257 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.21 (d, J = 1.3 Hz, 1H), 7.80 (d, J = 1.3 Hz, 1H), 7.78 (d, J = 8.5 Hz, 2H), 7.50 (d, J = 8.5 Hz, 2H), 3.20 (q, J = 7.1 Hz, 4H), 1.08 (t, J = 7.1 Hz, 6H). 13C NMR (75 MHz, DMSO-d6): δ 144.7, 140.6, 133.3, 131.5, 130.2, 129.4, 127.7, 121.9, 42.3, 14.4. Elemental analysis: calcd (%) for C14H16ClNO2S2 (329.87): C 50.98, H 4.89, found C 50.81, H 4.79.
5-(4-Methoxyphenyl)-thiophene-3-sulfonic acid diethylamide (11)
4-Bromoanisole (0.187 g, 1 mmol) and methyl 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate (0.416 g, 1.5 mmol) affords 11 in 80% (0.260 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.09 (d, J = 1.3 Hz, 1H), 7.67 (d, J = 8.5 Hz, 2H), 7.59 (d, J = 1.3 Hz, 1H), 7.00 (d, J = 8.5 Hz, 2H), 3.80 (s, 3H), 3.20 (q, J = 7.1 Hz, 4H), 1.08 (t, J = 7.1 Hz, 6H). 13C NMR (75 MHz, DMSO-d6): δ 159.6, 145.9, 140.1, 128.4, 127.1, 124.9, 119.6, 114.5, 55.2, 42.1, 14.2. Elemental analysis: calcd (%) for C15H19NO3S2 (325.45): C 55.36, H 5.88, found C 55.47, H 5.99.
5-(3-Acetylphenyl)-thiophene-3-sulfonic acid diethylamide (12)
3-Bromoacetophenone (0.199 g, 1 mmol) and methyl 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate (0.416 g, 1.5 mmol) affords 12 in 77% (0.260 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.24 (d, J = 1.3 Hz, 1H), 8.22 (s, 1H), 8.00 (d, J = 8.1 Hz, 1H), 7.94 (d, J = 8.1 Hz, 1H), 7.92 (d, J = 1.3 Hz, 1H), 7.60 (t, J = 8.1 Hz, 1H), 3.21 (q, J = 7.1 Hz, 4H), 2.65 (s, 3H), 1.09 (t, J = 7.1 Hz, 6H). 13C NMR (75 MHz, DMSO-d6): δ 198.2, 145.3, 140.9, 138.1, 133.3, 130.7, 130.6, 130.2, 128.6, 125.5, 122.5, 42.7, 27.4, 14.8. Elemental analysis: calcd (%) for C16H19NO3S2 (337.46): C 56.95, H 5.68, found: C 56.99, H 5.81.
5-(2-Cyanophenyl)-thiophene-3-sulfonic acid diethylamide (13)
2-Bromobenzonitrile (0.182 g, 1 mmol) and methyl 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate (0.416 g, 1.5 mmol) affords 13 in 91% (0.291 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.41 (d, J = 1.4 Hz, 1H), 7.99 (d, J = 7.9 Hz, 1H), 7.83–7.77 (m, 3H), 7.65–7.59 (m, 1H), 3.22 (q, J = 7.1 Hz, 4H), 1.09 (t, J = 7.1 Hz, 6H). 13C NMR (75 MHz, DMSO-d6): δ 141.2, 140.0, 135.1, 134.5, 133.9, 132.0, 129.9, 129.3, 125.2, 118.1, 109.5, 42.0, 14.1. Elemental analysis: calcd (%) for C15H16N2O2S2 (320.43): C 56.22, H 5.03, found: C 56.31, H 5.18.
5-Naphthalen-1-ylthiophene-3-sulfonic acid diethylamide (14)
1-Bromonaphthalene (0.207 g, 1 mmol) and methyl 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate (0.416 g, 1.5 mmol) affords 14 in 80% (0.276 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.36 (d, J = 1.5 Hz, 1H), 8.05–8.01 (m, 3H), 7.63–7.54 (m, 4H), 7.55 (d, J = 1.5 Hz, 1H), 3.24 (q, J = 7.1 Hz, 4H), 1.09 (t, J = 6.9 Hz, 6H). 13C NMR (75 MHz, DMSO-d6): δ 143.4, 139.7, 133.4, 130.8, 130.7, 130.0, 129.3, 128.6, 128.5, 127.2, 126.4, 125.4, 124.9, 124.4, 42.1, 14.1. Elemental analysis: calcd (%) for C18H19NO2S2 (345.48): C 62.58, H 5.54, found C 62.47, H 5.47.
5-Pyridin-3-ylthiophene-3-sulfonic acid diethylamide (15)
3-Bromopyridine (0.158 g, 1 mmol) and methyl 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate (0.416 g, 1.5 mmol) affords 15 in 89% (0.263 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.99 (s, 1H), 8.57 (d, J = 4.9 Hz, 1H), 8.28 (s, 1H), 8.16 (d, J = 7.9 Hz, 1H), 7.92 (s, 1H), 7.48 (dd, J = 7.9, 3.0 Hz, 1H), 3.20 (q, J = 7.1 Hz, 4H), 1.08 (t, J = 6.9 Hz, 6H). 13C NMR (75 MHz, DMSO-d6): δ 149.4, 146.4, 142.2, 140.5, 133.1, 130.6, 128.4, 124.0, 122.4, 42.2, 14.3. Elemental analysis: calcd (%) for C13H16N2O2S2 (296.41): C 52.68, H 5.44, found C 52.41, H 5.31.
5-Pyridin-4-ylthiophene-3-sulfonic acid diethylamide (16)
4-Bromopyridine hydrochloride (0.194 g, 1 mmol) and methyl 3-(N,N-diethylsulfamoyl)thiophene-2-carboxylate (0.416 g, 1.5 mmol) affords 16 in 68% (0.201 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.62 (bs, 2H), 8.36 (s, 1H), 8.08 (s, 1H), 7.77 (d, J = 4.3 Hz, 2H), 3.21 (q, J = 7.3 Hz, 4H), 1.08 (t, J = 7.1 Hz, 6H). 13C NMR (75 MHz, DMSO-d6): δ 150.4, 142.7, 140.6, 139.2, 131.7, 123.8, 119.7, 42.1, 14.2. Elemental analysis: calcd (%) for C13H16N2O2S2 (296.41): C 52.68, H 5.44, found C 52.72, H 5.24.
Methyl 3-(morpholinosulfamoyl)thiophene-2-carboxylate (17)
Morpholine (0.522 g, 6 mmol), and triethylamine (1.15 g, 8 mmol) were added to 5 mL of dry CH2Cl2 under argon atmosphere at room temperature. Methyl 3-(sulfamoylchloride)thiophene-2-carboxylate (0.481 g, 2 mmol) was then added, and the reaction was stirred for 12 h. The product was extracted with CH2Cl2 (3 × 15 mL), washed successively with HCl 1N, water and dried over MgSO4. The solvent was evaporated under reduced pressure. Purification by flash chromatography (diethylether/pentane: 3/7) afforded 17 as a white solid in 91% (0.530 g) yield.1H NMR (400 MHz, DMSO-d6): 8.02 (d, J = 5.2 Hz, 1H), 7.44 (d, J = 5.2 Hz, 1H), 3.84 (s, 3H), 3.65–3.60 (m, 4H), 3.20–3.10 (m, 4H).
13C NMR (100 MHz, DMSO-d6): δ: 160.0, 138.1, 134.3, 131.3, 130.1, 65.6, 53.1, 45.8. Elemental analysis: calcd (%) for C10H13NO5S2 (291.35): C 41.22, H 4.50, found: C 41.34, H 4.64.
4-[4-(Morpholine-4-sulfonyl)-thiophen-2-yl]-benzonitrile (18)
4-Bromobenzonitrile (0.182 g, 1 mmol) and methyl 3-(morpholinosulfamoyl)thiophene-2-carboxylate (0.436 g, 1.5 mmol) affords 18 in 72% (0.241 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.38 (d, J = 1.3 Hz, 1H), 7.99 (d, J = 8.4 Hz, 2H), 7.93 (d, J = 1.3 Hz, 1H), 7.91 (d, J = 8.4 Hz, 2H), 3.66 (t, J = 4.5 Hz, 4H), 2.96 (t, J = 4.5 Hz, 4H). 13C NMR (75 MHz, DMSO-d6): δ 143.8, 136.5, 135.7, 133.3, 133.1, 126.5, 123.8, 118.5, 110.8, 65.2, 45.8. Elemental analysis: calcd (%) for C15H14N2O3S2 (334.42): C 53.87, H 4.22, found: C 54.04, H 4.14.
2-[4-(Morpholine-4-sulfonyl)-thiophen-2-yl]-benzonitrile (19)
2-Bromobenzonitrile (0.182 g, 1 mmol) and methyl 3-(morpholinosulfamoyl)thiophene-2-carboxylate (0.436 g, 1.5 mmol) affords 19 in 77% (0.257 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.45 (d, J = 1.5 Hz, 1H), 8.00 (d, J = 6.9 Hz, 1H), 7.82–7.80 (m, 2H), 7.75 (d, J = 1.5 Hz, 1H), 7.66–7.60 (m, 1H), 3.66 (t, J = 4.5 Hz, 4H), 2.96 (t, J = 4.5 Hz, 4H). 13C NMR (75 MHz, DMSO-d6): δ 141.4, 135.0, 134.9, 134.4, 133.9, 133.8, 130.1, 129.4, 125.7, 118.2, 109.7, 65.2, 45.8. Elemental analysis: calcd (%) for C15H14N2O3S2 (334.42): C 53.87, H 4.22, found: C 54.01, H 4.30.
4-(5-Naphthalen-1-ylthiophene-3-sulfonyl)-morpholine (20)
1-Bromonaphthalene (0.207 g, 1 mmol) and methyl 3-(morpholinosulfamoyl)thiophene-2-carboxylate (0.436 g, 1.5 mmol) affords 20 in 80% (0.287 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.42 (d, J = 1.3 Hz, 1H), 8.09–8.00 (m, 3H), 7.65–7.53 (m, 4H), 7.49 (d, J = 1.3 Hz, 1H), 3.67 (t, J = 4.5 Hz, 4H), 3.00 (t, J = 4.5 Hz, 4H). 13C NMR (75 MHz, DMSO-d6): δ 143.5, 134.6, 133.3, 132.6, 130.6, 129.8, 129.4, 128.6, 128.5, 127.3, 126.4, 125.5, 125.4, 124.4, 65.2, 45.8. Elemental analysis: calcd (%) for C18H17NO3S2 (359.46): C 60.14, H 4.77, found: C 60.31, H 4.64.
4-(5-Pyrimidin-5-ylthiophene-3-sulfonyl)-morpholine (21)
5-Bromopyrimidine (0.158 g, 1 mmol) and methyl 3-(morpholinosulfamoyl)thiophene-2-carboxylate (0.436 g, 1.5 mmol) affords 21 in 45% (0.140 g) yield.1H NMR (300 MHz, DMSO-d6): δ 9.23 (s, 2H), 9.18 (s, 1H), 8.42 (d, J = 1.5 Hz, 1H), 8.00 (d, J = 1.5 Hz, 1H), 3.66 (t, J = 4.5 Hz, 4H), 2.96 (t, J = 4.5 Hz, 4H). 13C NMR (75 MHz, DMSO-d6): δ 157.8, 153.8, 138.7, 135.6, 133.5, 126.8, 124.2, 65.2, 45.8. Elemental analysis: calcd (%) for C12H13N3O3S2 (311.38): C 46.29, H 4.21, found: C 46.20, H 4.14.
Methyl 3-(N-butyl-N-phenylsulfamoyl)thiophene-2-carboxylate (22)
N-butylaniline (0.447 g, 3 mmol) and triethylamine (1.15 g, 8 mmol) were added to 5 mL of dry CH2Cl2 under argon atmosphere at room temperature. Methyl 3-(sulfamoylchloride)thiophene-2-carboxylate (0.481 g, 2 mmol) was then added, and the reaction was stirred for 12 h. The product was extracted with CH2Cl2 (3 × 15 mL), washed successively with HCl 1N, water and dried over MgSO4. The solvent was evaporated under reduced pressure. Purification by flash chromatography (diethylether/pentane: 4/6) afforded 22 as a white solid in 79% (0.558 g) yield.1H NMR (300 MHz, DMSO-d6): δ 7.87 (d, J = 5.2 Hz, 1H), 7.40–7.28 (m, 3H), 7.18 (d, J = 8.0 Hz, 2H), 7.10 (d, J = 5.2 Hz, 1H), 3.80–3.75 (m, 5H), 1.32–1.28 (m, 4H), 0.85–0.80 (m, 3H). 13C NMR (75 MHz, DMSO-d6): δ 160.0, 140.2, 138.2, 133.4, 130.9, 130.4, 129.1, 128.5, 127.8, 53.0, 50.5, 30.0, 18.9, 13.4. Elemental analysis: calcd (%) for C16H19NO4S2 (353.46): C 54.37, H 5.42, found: C 54.40, H 5.51.
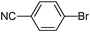
5-(4-Cyanophenyl)-thiophene-3-sulfonic acid nbutylphenyl-amide (23)
4-Bromobenzonitrile (0.182 g, 1 mmol) and methyl 3-(N-butyl-N-phenylsulfamoyl)thiophene-2-carboxylate (0.530 g, 1.5 mmol) affords 23 in 78% (0.309 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.11 (d, J = 0.9 Hz, 1H), 7.96 (d, J = 8.2 Hz, 2H), 7.90 (d, J = 8.2 Hz, 2H), 7.83 (d, J = 0.9 Hz, 1H), 7.40–7.31 (m, 3H), 7.13 (d, J = 7.3 Hz, 2H), 3.65 (t, J = 7.0 Hz, 2H), 1.32–1.27 (m, 4H), 0.83 (t, J = 7.0 Hz, 3H). 13C NMR (75 MHz, DMSO-d6): δ 143.6, 138.7, 138.5, 136.5, 133.1, 132.6, 129.1, 128.4, 127.9, 126.3, 123.4, 118.5, 110.7, 49.7, 29.7, 18.8, 13.3. Elemental analysis: calcd (%) for C21H20 N2O2S2 (396.53): C 63.61, H 5.08, found: C 63.54, H 5.20.
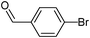
5-(4-Formylphenyl)-thiophene-3-sulfonic acid nbutylphenyl-amide (24)
4-Bromobenzaldehyde (0.185 g, 1 mmol) and methyl 3-(N-butyl-N-phenylsulfamoyl)thiophene-2-carboxylate (0.530 g, 1.5 mmol) affords 24 in 54% (0.215 g) yield.1H NMR (300 MHz, DMSO-d6): δ 10.03 (s, 1H), 8.11 (m, 1H), 7.99–7.96 (m, 4H), 7.80 (m, 1H), 7.41–7.33 (m, 3H), 7.14 (d, J = 7.2 Hz, 2H), 3.66 (t, J = 7.0 Hz, 2H), 1.32–1.30 (m, 4H), 0.83 (t, J = 7.0 Hz, 3H). 13C NMR (75 MHz, DMSO-d6): δ 192.4, 144.2, 138.7, 138.5, 137.6, 135.6, 131.4, 130.3, 129.1, 128.5, 127.9, 126.2, 123.1, 49.6, 29.7, 18.8, 13.3. Elemental analysis: calcd (%) for C21H21NO3S2 (399.53): C 63.13, H 5.30, found: C 63.23, H 5.11.
5-(3-Nitrophenyl)-thiophene-3-sulfonic acid nbutylphenyl-amide (25)
3-Bromonitrobenzene (0.202 g, 1 mmol) and methyl 3-(N-butyl-N-phenylsulfamoyl)thiophene-2-carboxylate (0.530 g, 1.5 mmol) affords 25 in 77% (0.320 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.51 (d, J = 1.7 Hz, 1H), 8.20 (td, J = 7.9, 1.7 Hz, 2H), 8.09 (d, J = 1.3 Hz, 1H), 7.90 (d, J = 1.3 Hz, 1H), 7.75 (t, J = 7.9 Hz, 1H), 7.44–7.32 (m, 3H), 7.14 (d, J = 7.9 Hz, 2H), 3.67 (t, J = 7.0 Hz, 2H), 1.35–1.28 (m, 4H), 0.83 (t, J = 7.0 Hz, 3H). 13C NMR (75 MHz, DMSO-d6): δ 148.4, 143.0, 138.6, 138.6, 133.8, 132.1, 132.1, 130.8, 129.1, 128.5, 127.9, 123.3, 123.0, 120.1, 49.6, 29.7, 18.8, 13.3. Elemental analysis: calcd (%) for C20H20N2O4S2 (416.52): C 57.67, H 4.84, found: C 57.60, H 4.81.
5-(2-Cyanophenyl)-thiophene-3-sulfonic acid nbutylphenyl-amide (26)
2-Bromobenzonitrile (0.182 g, 1 mmol) and methyl 3-(N-butyl-N-phenylsulfamoyl)thiophene-2-carboxylate (0.530 g, 1.5 mmol) affords 26 in 79% (0.313 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.19 (d, J = 1.5 Hz, 1H), 8.00 (d, J = 7.9 Hz, 1H), 7.83–7.78 (m, 2H), 7.70–7.61 (m, 2H), 7.41–7.30 (m, 3H), 7.15 (d, J = 7.7 Hz, 2H), 3.65 (t, J = 7.0 Hz, 2H), 1.31–1.29 (m, 4H), 0.82 (t, J = 7.0 Hz, 3H). 13C NMR (75 MHz, DMSO-d6): δ 141.2, 138.5, 138.0, 135.1, 134.3, 133.8, 133.3, 130.0, 129.3, 129.1, 128.5, 127.9, 125.1, 118.1, 109.6, 49.7, 29.7, 18.8, 13.3. Elemental analysis: calcd (%) for C21H20N2O2S2 (396.53): C 63.61, H 5.08, found: C 63.62, H 5.31.
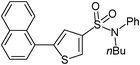
5-Naphthalen-1-yl-thiophene-3-sulfonic acid nbutylphenylamide (27)
1-Bromonaphthalene (0.207 g, 1 mmol) and methyl 3-(N-butyl-N-phenylsulfamoyl)thiophene-2-carboxylate (0.207 g, 1.5 mmol) affords 27 in 75% (0.316 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.29 (d, J = 0.9 Hz, 1H), 8.03–7.93 (m, 3H), 7.63–7.55 (m, 4H), 7.45–7.35 (m, 3H), 7.22 (d, J = 7.3 Hz, 2H), 7.12 (s, 1H), 3.66 (t, J = 7.0 Hz, 2H), 1.31–1.29 (m, 4H), 0.83 (t, J = 7.0 Hz, 3H). 13C NMR (75 MHz, DMSO-d6): δ 143.1, 138.6, 137.4, 133.3, 131.7, 130.5, 129.8, 129.3, 129.0, 128.5, 128.4, 127.8, 127.1, 126.4, 125.4, 125.2, 124.4, 49.4, 29.7, 18.9, 13.3. Elemental analysis: calcd (%) for C24H23NO2S2 (421.58): C 68.38, H 5.50, found: C 68.47, H 5.61.
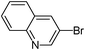
5-Quinolin-3-ylthiophene-3-sulfonic acid nbutylphenylamide (28)
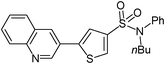
3-Bromoquinoline (0.208 g, 1 mmol) and methyl 3-(N-butyl-N-phenylsulfamoyl)thiophene-2-carboxylate (0.207 g, 1.5 mmol) affords 28 in 82% (0.346 g) yield.1H NMR (300 MHz, DMSO-d6): δ 9.30 (d, J = 2.2 Hz, 1H), 8.73 (d, J = 2.2 Hz, 1H), 8.12–8.03 (m, 3H), 7.90 (d, J = 1.5 Hz, 1H), 7.79 (td, J = 7.2, 1.5 Hz, 1H), 7.66 (t, J = 7.2 Hz, 1H), 7.42–7.31 (m, 3H), 7.16 (d, J = 6.7 Hz, 2H), 3.68 (t, J = 7.0 Hz, 2H), 1.33–1.31 (m, 4H), 0.82 (t, J = 7.0 Hz, 3H). 13C NMR (75 MHz, DMSO-d6): δ 147.9, 147.0, 142.4, 138.6, 138.5, 131.9, 131.8, 130.0, 129.1, 128.7, 128.5, 128.3, 127.9, 127.5, 127.3, 125.6, 122.7, 49.7, 29.7, 18.8, 13.4. Elemental analysis: calcd (%) for C23H22N2O2S2 (422.57): C 65.37, H 5.25, found: C 65.41, H 5.14.
Methyl 3-benzylsulfamoylthiophene-2-carboxylate (29)16
Benzylamine (0.321 g, 3 mmol) and pyridine (1.0 ml, 13 mmol) were added to 5 mL of dry CH2Cl2 under argon atmosphere at room temperature. Methyl 3-(sulfamoylchloride)thiophene-2-carboxylate (0.481 g, 2 mmol) was then added, and the reaction was stirred for 12 h. The product was extracted with CH2Cl2 (3 × 15 mL), washed successively with HCl 1N, water and dried over MgSO4. The solvent was evaporated under reduced pressure. Purification by flash chromatography (diethylether/pentane: 6/4) afforded 29 as a white solid in 70% (0.435 g) yield.1H NMR (300 MHz, DMSO-d6): δ 7.89 (d, J = 5.2 Hz, 1H), 7.75 (bs, 1H), 7.37 (d, J = 5.2 Hz, 1H), 7.20–7.15 (m, 5H), 4.15 (s, 2H), 3.84 (s, 3H).
Methyl 3-phenoxysulfonylthiophene-2-carboxylate (31)
Phenol (0.235 g, 2.5 mmol) and triethylamine (1.15 g, 8 mmol) were added to 5 mL of dry CH2Cl2 under an argon atmosphere at room temperature. Methyl 3-(sulfamoylchloride)thiophene-2-carboxylate (0.481 g, 2 mmol) was then added, and the reaction was stirred for 12 h. The product was extracted with CH2Cl2 (3 × 15 mL), washed successively with HCl 1N, water and dried over MgSO4. The solvent was evaporated under reduced pressure. Purification by flash chromatography (diethylether/pentane: 3/7) afforded 31 as a white solid in 89% (0.530 g) yield.1H NMR (300 MHz, DMSO-d6): δ: 8.04 (d, J = 5.2 Hz, 1H), 7.48–7.30 (m, 4H), 7.15 (d, J = 8.0 Hz, 2H), 3.90 (s, 3H). 13C NMR (75 MHz, DMSO-d6): δ: 159.0, 148.8, 135.9, 135.6, 132.7, 130.8, 127.6, 121.9, 53.3. Elemental analysis: calcd (%) for C12H10O5S2 (298.34): C 48.31, H 3.38, found: C 48.42, H 3.21.
5-(4-Cyanophenyl)-thiophene-3-sulfonic acid phenyl ester (32)
4-Bromobenzonitrile (0.182 g, 1 mmol) and methyl 3-phenoxysulfonylthiophene-2-carboxylate (0.446 g, 1.5 mmol) affords 32 in 80% (0.273 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.50 (d, J = 1.3 Hz, 1H), 8.17 (d, J = 1.3 Hz, 1H), 8.00 (d, J = 8.8 Hz, 2H), 7.91 (d, J = 8.8 Hz, 2H), 7.42 (t, J = 7.2 Hz, 2H), 7.33 (t, J = 7.2 Hz, 1H), 7.13 (d, J = 7.1 Hz, 2H). 13C NMR (75 MHz, DMSO-d6): δ 149.0, 144.7, 136.2, 136.0, 134.3, 133.1, 130.1, 127.6, 126.5, 123.6, 122.0, 118.4, 111.1. Elemental analysis: calcd (%) for C17H11NO3S2 (341.41): C 59.81, H 3.25, found: C 59.78, H 3.14.
4-(4-Phenoxysulfonylthiophen-2-yl)-benzoic acid methyl ester (33)
Methyl 4-bromobenzoate (0.215 g, 1 mmol) and methyl 3-phenoxysulfonylthiophene-2-carboxylate (0.446 g, 1.5 mmol) affords 33 in 84% (0.314 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.48 (d, J = 1.3 Hz, 1H), 8.11 (d, J = 1.3 Hz, 1H), 8.02 (d, J = 8.2 Hz, 2H), 7.95 (d, J = 8.2 Hz, 2H), 7.42 (t, J = 7.2 Hz, 2H), 7.33 (t, J = 7.2 Hz, 1H), 7.13 (d, J = 7.9 Hz, 2H), 3.88 (s, 3H). 13C NMR (75 MHz, DMSO-d6): δ 165.6, 149.0, 145.3, 136.0, 135.7, 134.2, 130.1, 130.0, 129.5, 127.6, 126.0, 122.9, 122.0, 52.2. Elemental analysis: calcd (%) for C18H14O5S2 (374.43): C 57.74, H 3.77, found: C 57.61, H 3.64.
5-(3-Chlorophenyl)-thiophene-3-sulfonic acid phenyl ester (34)
3-Bromochlorobenzene (0.191 g, 1 mmol) and methyl 3-phenoxysulfonylthiophene-2-carboxylate (0.446 g, 1.5 mmol) affords 34 in 83% (0.291 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.43 (d, J = 1.3 Hz, 1H), 8.08 (d, J = 1.3 Hz, 1H), 7.92 (s, 1H), 7.74–7.71 (m, 1H), 7.51–7.33 (m, 5H), 7.13 (d, J = 7.9 Hz, 2H). 13C NMR (75 MHz, DMSO-d6): δ 149.0, 145.0, 135.1, 134.1, 134.0, 133.8, 131.0, 130.0, 128.7, 127.5, 125.4, 124.5, 122.4, 122.0. Elemental analysis: calcd (%) for C16H11ClO3S2 (350.84): C 54.77, H 3.16, found: C 54.84, H 3.21.
5-(3,5-Bis-trifluoromethylphenyl)-thiophene-3-sulfonic acid phenyl ester (35)
3,5-Bis-trifluoromethylbromobenzene (0.293 g, 1 mmol) and methyl 3-phenoxysulfonylthiophene-2-carboxylate (0.446 g, 1.5 mmol) affords 35 in 72% (0.326 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.53 (d, J = 1.5 Hz, 1H), 8.48 (s, 2H), 8.46 (d, J = 1.5 Hz, 1H), 8.14 (s, 1H), 7.44 (t, J = 7.2 Hz, 2H), 7.34 (t, J = 7.2 Hz, 1H), 7.14 (d, J = 7.1 Hz, 2H). 13C NMR (75 MHz, DMSO-d6): δ 149.0, 143.2, 136.4, 134.5, 134.2, 131.2 (q, J = 32.0 Hz), 130.1, 127.6, 126.6 (m), 124.7, 123.0 (q, J = 273.3 Hz), 122.1. Elemental analysis: calcd (%) for C18H10F6O3S2 (452.39): C 47.79, H 2.23, found: C 47.87, H 2.20.
5-(2-Cyanophenyl)-thiophene-3-sulfonic acid phenyl ester (36)
2-Bromobenzonitrile (0.182 g, 1 mmol) and methyl 3-phenoxysulfonylthiophene-2-carboxylate (0.446 g, 1.5 mmol) affords 36 in 58% (0.198 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.57 (d, J = 1.5 Hz, 1H), 8.02 (dd, J = 5.6, 0.7 Hz, 1H), 7.95 (d, J = 1.5 Hz, 1H), 7.85–7.80 (m, 2H), 7.70–7.63 (m, 1H), 7.45–7.32 (m, 3H), 7.15 (d, J = 6.9 Hz, 2H). 13C NMR (75 MHz, DMSO-d6): δ 148.9, 142.2, 136.9, 134.6, 134.3, 133.9, 133.5, 130.2, 130.1 129.7, 127.6, 125.4, 122.0, 118.0, 109.8. Elemental analysis: calcd (%) for C17H11NO3S2 (341.41): C 59.81, H 3.25, found: C 59.87, H 3.34.
5-Naphthalen-1-yl-thiophene-3-sulfonic acid phenyl ester (37)
1-Bromonaphthalene (0.207 g, 1 mmol) and methyl 3-phenoxysulfonylthiophene-2-carboxylate (0.446 g, 1.5 mmol) affords 37 in 78% (0.286 g) yield.1H NMR (300 MHz, DMSO-d6): δ 8.60 (d, J = 1.5 Hz, 1H), 8.02 (d, J = 6.0 Hz, 2H), 7.83–7.77 (m, 1H), 7.63–7.55 (m, 4H), 7.45–7.39 (m, 4H), 7.21 (d, J = 6.9 Hz, 2H). 13C NMR (75 MHz, DMSO-d6): δ 149.1, 144.1, 135.5, 133.3, 133.0, 130.5, 130.1, 129.6, 129.2 128.5, 127.6, 127.3, 126.5, 125.4, 125.2, 124.1, 122.1. Elemental analysis: calcd (%) for C20H14O3S2 (366.46): C 65.55, H 3.85, found: C 56.22, H 5.03.
5-Pyridin-3-ylthiophene-3-sulfonic acid phenyl ester (38)
3-Bromopyridine (0.158 g, 1 mmol) and methyl 3-phenoxysulfonylthiophene-2-carboxylate (0.446 g, 1.5 mmol) affords 38 in 77% (0.244 g) yield.1H NMR (300 MHz, DMSO-d6): δ 9.01 (s, 1H), 8.59 (d, J = 4.8 Hz, 1H), 8.47 (d, J = 1.3 Hz, 1H), 8.20 (d, J = 6.6 Hz, 1H), 8.10 (d, J = 1.3 Hz, 1H), 7.48 (dd, J = 6.6, 4.8 Hz, 1H), 7.42 (t, J = 7.2 Hz, 2H), 7.36 (t, J = 7.2 Hz, 1H), 7.14 (d, J = 7.7 Hz, 2H). 13C NMR (75 MHz, DMSO-d6): δ 149.7, 149.0, 146.6, 143.3, 135.5, 134.1, 133.3, 130.1, 128.0, 127.6, 124.1, 122.6, 122.1. Elemental analysis: calcd (%) for C15H11NO3S2 (317.38): C 56.78, H 3.49, found: C 56.87, H 3.41.
5-Isoquinolin-4-ylthiophene-3-sulfonic acid phenyl ester (39)
4-bromoisoquinoline (0.208 g, 1 mmol) and methyl 3-phenoxysulfonylthiophene-2-carboxylate (0.446 g, 1.5 mmol) affords 39 in 60% (0.220 g) yield.1H NMR (300 MHz, DMSO-d6): δ 9.41 (s, 1H), 8.68 (d, J = 1.3 Hz, 1H), 8.61 (s, 1H), 8.26 (d, J = 7.9 Hz, 1H), 7.92–7.88 (m, 2H), 7.82–7.77 (m, 1H), 7.66 (d, J = 1.5 Hz, 1H), 7.50 (t, J = 7.2 Hz, 2H), 7.38 (t, J = 7.2 Hz, 1H), 7.21 (d, J = 7.7 Hz, 2H). 13C NMR (75 MHz, DMSO-d6): δ 153.6, 149.1, 143.6, 140.5, 136.4, 133.4, 132.8, 132.0, 130.0 128.4, 128.0, 127.8, 127.6, 126.2, 123.4, 123.2, 122.1. Elemental analysis: calcd (%) for C19H13NO3S2 (367.44): C 62.11, H 3.57, found: C 62.00, H 3.40.
Acknowledgements
This research was supported by a CEFIPRA fellowship. We thank the CNRS and “Rennes Metropole” for providing financial support.References
- (a) Y. Akita, A. Inoue, K. Yamamoto, A. Ohta, T. Kurihara and M. Shimizu, Heterocycles, 1985, 23, 2327 CrossRef CAS; (b) A. Ohta, Y. Akita, T. Ohkuwa, M. Chiba, R. Fukunaga, A. Miyafuji, T. Nakata, N. Tani and Y. Aoyagi, Heterocycles, 1990, 31, 1951 CrossRef CAS.
- (a) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174 CrossRef CAS; (b) T. Satoh and M. Miura, Chem. Lett., 2007, 36, 200 CrossRef CAS; (c) B.-J. Li, S.-D. Yang and Z.-J. Shi, Synlett, 2008, 949 CAS; (d) F. Bellina and R. Rossi, Tetrahedron, 2009, 65, 10269 CrossRef CAS; (e) L. Ackermann, R. Vincente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792 CrossRef CAS; (f) J. Roger, A. L. Gottumukkala and H. Doucet, ChemCatChem, 2010, 2, 20 CrossRef CAS; (g) C. Fischmeister and H. Doucet, Green Chem., 2011, 13, 741 RSC.
- (a) For recent examples of direct arylations of furans: M. Parisien, D. Valette and K. Fagnou, J. Org. Chem., 2005, 70, 7578 CrossRef CAS; (b) E. M. Beccalli, G. Broggini, M. Martinelli and S. Sottocornola, Synthesis, 2008, 136 CrossRef CAS; (c) B. Liégaut, D. Lapointe, L. Caron, A. Vlassova and K. Fagnou, J. Org. Chem., 2009, 74, 1826 CrossRef; (d) J. J. Dong, J. Roger, F. Požgan and H. Doucet, Green Chem., 2009, 11, 1832 RSC; (e) M. Ionita, J. Roger and H. Doucet, ChemSusChem, 2010, 3, 367 CrossRef CAS; (f) R. V. Smaliy, M. Beaupérin, H. Cattey, P. Meunier, J.-C. Hierso, J. Roger, H. Doucet and Y. Coppel, Organometallics, 2009, 28, 3152 CrossRef CAS; (g) J. Roger, F. Pozgan and H. Doucet, Adv. Synth. Catal., 2010, 352, 696 CrossRef CAS; (h) L. Franchi, M. Rinaldi, G. Vignaroli, A. Innitzer, M. Radi and M. Botta, Synthesis, 2010, 3927 CAS; (i) D. Lapointe, T. Markiewicz, C. J. Whipp, A. Toderian and K. Fagnou, J. Org. Chem., 2011, 76, 749 CrossRef CAS.
- (a) For recent examples of direct arylations of thiophenes: K. Masui, A. Mori, K. Okano, K. Takamura, M. Kinoshita and T. Ikeda, Org. Lett., 2004, 6, 2011 CrossRef CAS; (b) E. David, S. Pellet-Rostaing and M. Lemaire, Tetrahedron, 2007, 63, 8999 CrossRef CAS; (c) H. A. Chiong and O. Daugulis, Org. Lett., 2007, 9, 1449 CrossRef CAS; (d) P. Amaladass, J. A. Clement and A. K. Mohanakrishnan, Tetrahedron, 2007, 63, 10363 CrossRef CAS; (e) M. Nakano, H. Tsurugi, T. Satoh and M. Miura, Org. Lett., 2008, 10, 1851 CrossRef CAS; (f) J. J. Dong, J. Roger and H. Doucet, Tetrahedron Lett., 2009, 50, 2778 CrossRef CAS; (g) F. Derridj, J. Roger, S. Djebbar and H. Doucet, Org. Lett., 2010, 12, 4320 CrossRef CAS; (h) L. Chen, J. Roger, C. Bruneau, P. H. Dixneuf and H. Doucet, Chem. Commun., 2011, 47, 1872 RSC; (i) D. J. Schipper and K. Fagnou, Chem. Mater., 2011, 23, 1594 CAS.
- (a) For recent examples of direct arylations of pyrroles or indoles: F. Bellina, S. Cauteruccio and R. Rossi, Eur. J. Org. Chem., 2006, 1379 CrossRef CAS; (b) X. Wang, D. V. Gribkov and D. Sames, J. Org. Chem., 2007, 72, 1476 CrossRef CAS; (c) N. Lebrasseur and I. Larrosa, J. Am. Chem. Soc., 2008, 130, 2926 CrossRef CAS; (d) J. Roger and H. Doucet, Adv. Synth. Catal., 2009, 351, 1977 CrossRef CAS; (e) E. T. Nadres, A. Lazareva and O. Daugulis, J. Org. Chem., 2011, 76, 471 CrossRef CAS.
- (a) For recent examples of direct arylations of thiazoles:A. Mori, A. Sekiguchi, K. Masui, T. Shimada, M. Horie, K. Osakada, M. Kawamoto and T. Ikeda, J. Am. Chem. Soc., 2003, 125, 1700 CrossRef CAS; (b) A. Yokooji, T. Okazawa, T. Satoh, M. Miura and M. Nomura, Tetrahedron, 2003, 59, 5685 CrossRef CAS; (c) G. L. Turner, J. A. Morris and M. F. Greaney, Angew. Chem., Int. Ed., 2007, 46, 7996 CrossRef CAS; (d) L.-C. Campeau, M. Bertrand-Laperle, J.-P. Leclerc, E. Villemure, S. Gorelsky and K. Fagnou, J. Am. Chem. Soc., 2008, 130, 3276 CrossRef CAS; (e) F. Derridj, J. Roger, S. Djebbar and H. Doucet, J. Organomet. Chem., 2009, 694, 455 CrossRef CAS; (f) J. J. Dong, J. Roger, C. Verrier, T. Martin, R. Le Goff, C. Hoarau and H. Doucet, Green Chem., 2010, 12, 2053 RSC; (g) K. Beydoun and H. Doucet, ChemSusChem, 2011, 4, 526 CrossRef CAS.
- (a) For recent examples of direct arylations of imidazoles: F. Bellina, S. Cauteruccio, A. Di Flore and R. Rossi, Eur. J. Org. Chem., 2008, 5436 CrossRef CAS; (b) F. Bellina, S. Cauteruccio, A. Di Flore, C. Marchietti and R. Rossi, Tetrahedron, 2008, 64, 6060 CrossRef CAS; (c) J. Roger and H. Doucet, Tetrahedron, 2009, 65, 9772 CrossRef CAS.
- For recent examples of direct arylations of isoxazoles: Y. Fall, C. Reynaud, H. Doucet and M. Santelli, Eur. J. Org. Chem., 2009, 4041 CrossRef CAS.
- C. B. Bheeter, J. K. Bera and H. Doucet, J. Org. Chem., 2011, 76, 6407 CrossRef CAS.
- For a direct arylation of a furan substituted by a methylsulfide in the presence of an iridium catalyst: B. Join, T. Yamamoto and K. Itami, Angew. Chem., Int. Ed., 2009, 48, 3644 CrossRef CAS.
- (a) L. Lavenot, C. Gozzi, K. Ilg, I. Orlova, V. Penalva and M. Lemaire, J. Organomet. Chem., 1998, 567, 49 CrossRef CAS; (b) J. Fournier dit Chabert, B. Marquez, L. Neville, L. Joucla, S. Broussous, P. Bouhours, E. David, S. Pellet-Rostaing, B. Marquet, N. Moreau and M. Lemaire, Bioorg. Med. Chem., 2007, 15, 4482 CAS; (c) A. Borghese, G. Geldhof and L. Antoine, Tetrahedron Lett., 2006, 47, 9249 CrossRef CAS; (d) O. Rene and K. Fagnou, Org. Lett., 2010, 12, 2116 CrossRef CAS.
- (a) B. Glover, K. A. Harvey, B. Liu, M. J. Sharp and M. F. Tymoschenko, Org. Lett., 2003, 5, 301 CrossRef CAS; (b) P. Forgione, M.-C. Brochu, M. St-Onge, K. H. Thesen, M. D. Bailey and F. Bilodeau, J. Am. Chem. Soc., 2006, 128, 11350 CrossRef CAS; (c) B. Liégault, I. Petrov, S. I. Gorlesky and K. Fagnou, J. Org. Chem., 2010, 75, 1047 CrossRef; (d) J. J. Dong, D. Roy, R. Jacob Roy, M. Ionita and H. Doucet, Synthesis, 2011, 3530 CAS; (e) J. J. Dong and H. Doucet, Eur. J. Org. Chem., 2010, 611 CrossRef CAS; (f) S. Tanaka, S. Tamba, D. Tanaka, A. Sugie and A. Mori, J. Am. Chem. Soc., 2011, 133, 16734 CrossRef CAS.
- J. Hassan, M. Sévignon, C. Gozzi, E. Schulz and M. Lemaire, Chem. Rev., 2002, 102, 1359 CrossRef CAS.
- (a) J. J. Li, G. W. Gribble, Palladium in Heterocyclic Chemistry, Pergamon: Amsterdam, 2000. Search PubMed; (b) H. Doucet and M. Santelli, Synlett, 2006, 2001 CrossRef CAS.
- J. Bastide, R. Badon, J. P. Cambon and D. Vega, Pestic. Sci., 1994, 40, 293 CrossRef CAS.
- D. Tondi, F. Morandi, R. Bonnet, M. P. Costi and B. K. Shoichet, J. Am. Chem. Soc., 2005, 127, 4632 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |

 23
23
 24
24

 25
25

 26
26

 27
27
 28
28