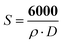Highly transparent nanocomposite films from water-based poly(2-ethyl-2-oxazoline)/TiO2 dispersions
Annalisa
Colombo
a,
Francesco
Tassone
b,
Michele
Mauri
a,
Domenico
Salerno
c,
John K.
Delaney
d,
Michael R.
Palmer
d,
René De La
Rie
d and
Roberto
Simonutti
*a
aDepartment of Materials Science, University of Milano-Bicocca, via R. Cozzi 53, 20125, Milano, Italy. E-mail: roberto.simonutti@unimib.it; Fax: 02 6448 5440; Tel: 02 6448 5132
bCentre for Nano-Science and Technology, IIT@POLIMI, via G. Pascoli 70/3, 20133, Milano, Italy
cDepartment of Experimental Medicine, University of Milano-Bicocca, via Cadore 48, 20052, Monza (MB), Italy
dScientific Research Department, National Gallery of Art, Washington, D.C. 20565, USA
First published on 25th June 2012
Abstract
Aqueous dispersions containing TiO2 nanoparticles and hydrophilic poly(2-ethyl-2-oxazoline) allow the preparation of highly transparent nanocomposites containing up to 44 wt% of TiO2. Transmission Electron Microscopy (TEM) images indicate that the nanoparticles are homogeneously distributed in the polymeric matrix with no significant agglomeration. Nanocomposite films remain highly transparent to visible light, absorb the ultraviolet (UV) radiation, and their refractive indices can be regulated in the range from 1.52 to 1.65 by the content of TiO2 nanoparticles. While specular gloss measurements reveal an increase of the root-mean-square (RMS) surface roughness with TiO2 content, distinctness-of-image gloss measurements demonstrate that it does not negatively affect the image information carried by the underlying layer.
1. Introduction
Polymeric nanocomposites, namely polymer matrices containing nanometric particles, are extensively studied for their improved properties compared to the pure polymer.1,2 The presence of a nanometric filler can enhance mechanical properties,3,4 thermal stability5 and gas barrier properties.6 In the last few years nanocomposites with optimized optical properties have also been described. Due to a unique combination of optical and mechanical properties, nanocomposites have a wide range of applications in the optoelectronics and coatings industries. The research has been focused mainly on the following goals: luminescence,7,8 change (increase) of the refractive index (RI),9 and UV absorption.10 In particular, high RI polymer nanocomposites can be obtained by incorporation of inorganic building blocks with high RI (such as InP, PbS, TiO2, ZrO2, or ZnS) into organic matrices at the nanoscale. Although InP and PbS have high RIs (>4.0 at 500 nm),11 they exhibit significant absorption coefficients in the visible region and cannot be used for applications where transparency in the visible region is required. At present, the most frequently employed inorganic materials in the literature, concerning high RI and transparent hybrid nanocomposites, are TiO2 (n ≈ 2.5 at 500 nm in its anatase form),11 ZrO2 (n ≈ 2.2 at 589 nm)12 and ZnO (n ≈ 2.0 at 550 nm).13,14 These materials are used as nanoscale building blocks because of their high transparency in the visible and near-infrared (NIR) regions. Negligible absorption in the visible region is a necessary but not sufficient condition for producing optically transparent nanocomposites; in fact intense light scattering of inorganic domains, due to the large inorganic domain size and RI mismatch between the inorganic phase and the matrix, could determine an opaque appearance of the material.15 The amount of scattered light can be evaluated by using the Rayleigh scattering formula; as a rule of thumb scattering can be neglected when the average diameter of the particles is less than 50–60 nm. Much effort is aimed at increasing the functional inorganic fraction, and several methods have been developed in order to avoid aggregation16,17 which usually has a negative impact on the properties of the composite.Considering polymers used as an embedding matrix for the inorganic nanoparticles, poly(meth)acrylates represent the most studied class since they feature intrinsic high optical clarity, especially in the case of polymethylmethacrylate (PMMA),18–20 and the possibility to modulate the physical and chemical properties of the polymer by simply varying the nature of the side chains. Unfortunately in the preparation of PMMA based composites the use of organic solvents is needed. The fabrication of a nanocomposite with tailorable optical properties from aqueous dispersions should then be considered as a relevant target in the perspective of a green chemistry. Two approaches are possible, one exploits the use of polymer latexes, that actually are colloidal dispersions of hydrophobic polymers in aqueous solutions,21 the other exploits directly water soluble polymers. We chose to follow the latter route since it can provide systems with higher transparency.
Considering nanocomposites based on water soluble polymers, only a few examples are reported in the literature, regarding gelatin,22 poly(ethylene-oxide),23 and poly(vinylalcohol).24 In this case, the polarity of the macromolecular chains can prevent agglomeration and flocculation of the nanoparticles that present hydrophilic surfaces, for example oxides, because of the interface tension reduction. In fact one of the main efforts in the fabrication of the nanocomposite is the surface modification of the nanoparticles in order to make them compatible with the polymer; this complex and often expensive procedure is not necessary for hydrophilic polymers and the nanoparticles can be simply dispersed in water without any treatment. A water soluble polymer with increasing applications in recent years is poly(2-ethyl-2-oxazoline). This polymer is biodegradable, non-toxic and highly stable (the amide is tertiary and, as a consequence, it is difficult to hydrolyze). It features several recognized applications as an adhesive and as a coating.25,26 In the conservation of cultural heritage, for example, it is currently used as pigment binder, a consolidant of flaking painted layers, and a barrier film for canvas and amber.27 Furthermore, polyoxazolines are an interesting class of polymers due to their ability to form functional materials and nanostructures with applications also as stimuli-responsive systems.28,29
In this paper we report for the first time the preparation and optical characterization of poly(2-ethyl-2-oxazoline) films containing well dispersed TiO2 nanoparticles. Nanocomposite films with very high concentrations of TiO2 nanoparticles (40 nm average diameter) in the polymeric matrix (16 wt%, 28 wt% and 44 wt%) were obtained and refractive indices, UV absorption and transparency were tested using UV-visible spectroscopy. The surface of nanocomposites was studied by Atomic Force Microscopy (AFM) and water contact angle (WCA) measurements. The optical quality of the films was further investigated by specular gloss and distinctness-of-image gloss (GDoI) measurements.
2. Experimental section
2.1 Synthesis of TiO2 nanoparticles
TiO2 nanoparticles were synthesized according to the method reported by Niederberger et al.,30 in which 4 mL of TiCl4 (99.9%, Sigma Aldrich) were slowly added to 80 mL of anhydrous benzyl alcohol (≥ 99.0%, Sigma Aldrich) under stirring at room temperature. Benzyl alcohol was made anhydrous using MgSO4 and treatment in vacuum at 323 K. Nanoparticles with the desired size were obtained in after 24 h at 353 K. The resulting white suspension was centrifuged and washed three times, twice with ethanol and once with tetrahydrofuran. The nanoparticles were dried under environmental atmosphere at room temperature overnight and then ground into a powder.2.2 Characterization of TiO2 nanoparticles
Powder X-ray diffraction (XRD) patterns were obtained using a D8 Discover (Bruker) diffractometer. Nanoparticle size and morphology were studied using a transmission electron microscope JEM-1011 (JEOL) operated at 200 kV. TEM samples of the nanoparticle water dispersions were prepared by leaving 1–2 drops of the dispersion onto a Lacey Carbon Copper Grid. In order to measure the surface area of nanoparticles, nitrogen adsorption–desorption isotherms were measured at liquid nitrogen temperature using an ASAP 2010 analyzer (Micrometrics). The powder was outgassed for 12 h at 473 K. The surface area of the nanoparticles was calculated using the Brunauer, Emmett, and Teller model (BET model).31 The nanoparticle size in water dispersions was analyzed with a Zetasizer Dynamic Light Scattering (Malvern Instrument Ltd.) at room temperature using a λ = 632.8 nm He-Ne laser. 1H Magic Angle Spinning NMR spectra were recorded on an Avance 300 spectrometer (Bruker) (standard spinning rate of 12 kHz) with a recycle delay of 4 s; 128 scans were acquired. Thermogravimetry measurements were carried out with a TA Q500 analyzer (TA Instruments), heating to 1073 K at 10 K min−1 in air.2.3 Films preparation
Poly(2-ethyl-2-oxazoline) (Aquazol 200®, Polymer Chemistry Innovation,![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w = 200
w = 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000)20 film (A0) was prepared and used as a reference. A solution of 5 wt% polymer in distilled water was cast on Mylar® (Du Pont) sheets and microscope slides; films were obtained by evaporation of the solvent at room temperature and environmental pressure. Nanocomposite films were obtained by mixing different ratios of the nanoparticles dispersions in the aqueous polymeric solution. TiO2 dispersions were prepared by adding the desired amount of dried nanoparticles powder to water and by ultrasonicating the mixture (ultrasonic processor Vibra-Cell 750 Sonics, 21s pulse, 7s pause, 350 W) for 15 min, in order to break bigger agglomerates. In fact, before ultrasonication, nanoparticles precipitated at the bottom of the beaker in a few hours. Dispersions had a positive zeta potential (+ 45 mV) and a pH of about 3.0. Films based on an increasing concentration of nanoparticles in the polymeric matrix: 16 wt%, 28 wt% and 44 wt%, called A16, A28, A44 respectively, were prepared by brushing the dispersion on a Mylar® (Du Pont) sheet and allowing water evaporation at room temperature and environmental pressure. TEM samples of the nanocomposite films have been prepared leaving 1–2 drops of the polymeric dispersion in water containing dispersed nanoparticles onto a Lacey Carbon Copper Grid, and waiting for evaporation of the solvent.
000)20 film (A0) was prepared and used as a reference. A solution of 5 wt% polymer in distilled water was cast on Mylar® (Du Pont) sheets and microscope slides; films were obtained by evaporation of the solvent at room temperature and environmental pressure. Nanocomposite films were obtained by mixing different ratios of the nanoparticles dispersions in the aqueous polymeric solution. TiO2 dispersions were prepared by adding the desired amount of dried nanoparticles powder to water and by ultrasonicating the mixture (ultrasonic processor Vibra-Cell 750 Sonics, 21s pulse, 7s pause, 350 W) for 15 min, in order to break bigger agglomerates. In fact, before ultrasonication, nanoparticles precipitated at the bottom of the beaker in a few hours. Dispersions had a positive zeta potential (+ 45 mV) and a pH of about 3.0. Films based on an increasing concentration of nanoparticles in the polymeric matrix: 16 wt%, 28 wt% and 44 wt%, called A16, A28, A44 respectively, were prepared by brushing the dispersion on a Mylar® (Du Pont) sheet and allowing water evaporation at room temperature and environmental pressure. TEM samples of the nanocomposite films have been prepared leaving 1–2 drops of the polymeric dispersion in water containing dispersed nanoparticles onto a Lacey Carbon Copper Grid, and waiting for evaporation of the solvent.
2.4 Films characterization
Nanocomposite films were optically characterized with a Lambda 1050 (Perkin Elmer) spectrophotometer with air as the reference spectrum. AFM images were acquired with a Nanowizard II (JPK Instruments, Berlin) instrument. Measurements were performed in tapping mode in air using stiff silicon cantilevers (RTSP Veeco, resonant frequencies of ≈300 kHz, and a spring constant of ≈40 N m−1). Images were acquired at 1 Hz scan rate and 512 × 512 pixel resolution. All data in this study were verified by sampling a wide range of areas over the sample surfaces. Water Contact Angle measurements were performed with a PGX pocket goniometer (FIBRO systems) using 2 μL droplets, with at least 5 repetitions for each sample. Specular glosses at 20°, 60° and 85° of the films were determined according to ASTM D523 using a micro-Tri-gloss apparatus (BYK Garderner).32 Each result was an average of six measurements (repeated three times) acquired in different points. The relative error of these results is less than 1%. In order to perform specular gloss measurements, films were cast on microscope slides with the uncoated side spray-painted black. Distinctness-of-image gloss measurements were performed on the same samples according to ASTM E430 using a Dorigon II abridged goniophotometer (Hunter Lab).33 Each result is an average of six measurements. The relative error of these results is less than 1%.3. Results and discussion
3.1 Characterization of the nanoparticles, their colloidal dispersions and nanocomposite films
The powder XRD pattern of TiO2 nanoparticles synthesized by the non-aqueous route is shown in Fig. 1. The sample is highly crystalline anatase without indication of other TiO2 phases. The line width of the (101) peak corresponds to a crystallite size of 5.6 nm by the Scherrer formula using 0.9 as the shape factor. The surface area of the TiO2 nanoparticles has been calculated by BET method which gives 249 m2 g−1. By using the following relation based on simple geometrical considerations:where S (m2 g−1) is the surface area, ρ (g cm−3) is the anatase density and D (nm) is the diameter of the particles,30 the primary particle size can be evaluated to be about 6.2 nm. The two measured primary particle size values are in good agreement,34 even if BET provides a slightly larger value probably because a small amount of surface is lost in the primary particles aggregation. In fact investigation with TEM revealed that the primary nanoparticles of about 5–6 nm diameter, with an aspect ratio close to 1, are aggregated in elongated objects whose the longest axis measures about 40 nm, such as reported in the inset of Fig. 1a.
 | ||
| Fig. 1 a) XRD pattern of synthesized TiO2 nanoparticles and the corresponding TEM image in the inset. b) DLS measurement of particle size of the as synthesized TiO2 dispersed in distilled water at room temperature; the solid line corresponds to the fitting of experimental data by a Gaussian distribution. | ||
The possibility to prepare a stable dispersion of titania nanoparticles in water is investigated by DLS. DLS measurements reveal that once dispersed in water, titania nanoparticles present an hydrodynamic diameter of 40 ± 20 nm, in reasonable agreement with TEM measurements (Fig. 1b).
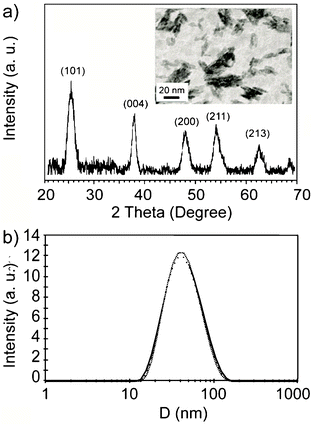
Since benzyl alcohol has an active role in the synthesis of titania, as stated in several papers by the authors that proposed this synthetic route,35 the presence of benzyl alcohol residues on the titania surface has been evaluated by TGA and 1H MAS NMR. Thermogravimetric trace (Fig. 2a) shows an overall weight loss of about 19% from about 310 K to 900 K. The weight loss can be divided in three regions. The first one from 310 K to 450 K (maximum of the first derivative at 369 K) can be attributed to the release of physically adsorbed water. The second one from 480 K to 620 K is characterized by a smooth decrease of the sample weight with the increasing temperature (broad peak in the first derivative with maximum at 546 K); the third weight loss instead is quite abrupt and takes place in less than 50 K around 693 K. The broad phenomenon is characteristic of a desorption process. In fact, physi-adsorbed molecules on a surface are released in a wide range of temperatures especially when the surface is highly defective as in nanoparticles. Taking into account the synthetic method used and that the boiling point of benzyl alcohol is about 478 K, the weight loss between 480 K to 620 K can be attributed prevalently to the desorption of benzyl alcohol from the titania surface.24 However the significant shift to higher temperature of the evaporation indicates a strong interaction between the benzyl alcohol and the titania surface.
 | ||
| Fig. 2 a) Thermogravimetric trace of TiO2 nanoparticles and its first derivative. b) 1H MAS NMR of TiO2 nanoparticles recorded at 300 MHz; in the inset a possible interaction between benzyl alcohol and titania is depicted. | ||
The weight loss occurring at the highest temperature can be related to the sintering of the nanoparticles once the organic molecules covering the surface are completely removed. The sintering process advances through the condensation of hydroxyl groups and elimination of water.36
1H Magic Angle Spinning (MAS) NMR is a valuable tool for characterizing adsorbed molecules on solid surfaces.37 Magic angle spinning is effective in reducing but not completely cancelling the proton–proton dipolar couplings. Therefore, in a solid material, residual dipolar couplings dominate the appearance of 1H MAS NMR spectra. Molecules endowed with restricted mobility are characterized by strong dipolar couplings leading to broad spectra spanning tens of ppm, in which different chemical species cannot be recognized. Fast motions, instead, can average residual dipolar couplings to zero allowing the detection of well resolved spectra. In the case of TiO2 nanoparticles two sharp resonances at 7.4 ppm and 4.9 ppm dominate the 1H MAS NMR spectrum (Fig. 2b), underneath these resonances a quite broad peak ranging from 15 to 0 ppm is also recognizable. The chemical shifts of the sharp peaks are quite close to those measured for benzyl alcohol in solution. This indicates that a relevant part of the benzyl groups anchored on the titania surface exhibit a liquid-like mobility. However, the occurrence of a broad peak points out the presence of rigid protons having restricted mobility. They may be associated to hydrogen bonded OH groups of the titania, in agreement with the high temperature weight loss measured by TGA, or to some benzyl groups which are interacting more strongly with the anatase surface.
Overall, TGA and 1H MAS NMR provide a picture of nanoparticles with an heterogeneous surface characterized by the presence of highly mobile organic molecules, hydrogen bonded OH groups and possibly organic molecules fixed on the surface. This heterogeneity explains the ability of the anatase nanoparticles to form stable dispersions in water.
Nanocomposite films were prepared by dissolving PEOX in water and subsequent addition of titania. The mixture was ultrasonicated in order to disrupt loose aggregates of titania nanoparticles; once prepared the dispersion is stable for several months and it can be used without any further treatment. Films for microscopical and optical characterization were prepared by brushing the dispersion on Mylar® sheets; evaporation of water led to the formation of the nanocomposite. Films were transparent, did not present cracks and were easily removed from the supporting sheets. Transparent films can be also obtained by brushing the dispersion on several different supports including cotton fabrics and painted surfaces.
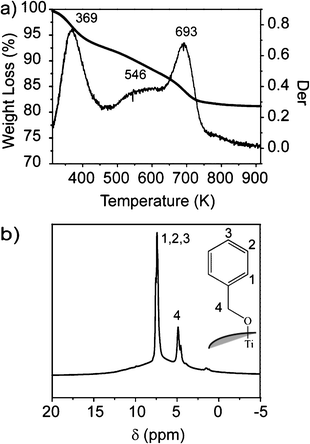
In Fig. 3, TEM micrographs of A16 and A44 nanocomposites are reported. The images confirm that the nanoparticles are well dispersed in the polymer matrix as aggregates of the primary crystallites of about 5–6 nm; statistical analysis of the images provides an average dimension of the aggregates of about 40 nm, comparable with the value measured in solution by DLS. Moreover, despite the small inter-aggregate distance due to high concentration of anatase nanoparticles, the aggregates are homogeneously distributed in the polymeric matrix. This result demonstrates that the fabrication procedure of the nanocomposite film does not induce further agglomeration of the aggregates.
 | ||
| Fig. 3 TEM images of titania PEOX nanocomposite films: a) A16 and b) A44. Bar scale: 50 nm. | ||
AFM was used to examine the surface of the films. Fig. 4 shows AFM “height trace” and “phase lag” images of the pristine polymer film and the nanocomposite films. The pure polymer surface (Fig. 4a) appears to be smooth and characterized by an average peak-to-valley height of about 1 nm; the nanocomposite surface roughness increases with nanoparticle content as clearly shown from the increased scale of the height surface profile (Fig. 4d). In order to quantitatively characterize the surface alteration due to the presence of the nanoparticles we have computed the bidimensional autocorrelation of the profile image and we have extracted the correlation length by fitting the obtained autocorrelation function of the image with a 2D Gaussian profile. We chose to identify as correlation length the Half Width at Half Maximum (HWHM) of the function. Calculated values are reported in Table 1. It should be noted that in the A0 sample the correlation length is equal to the AFM tip radius which is the lower threshold of the instrument sensitivity. On the contrary, the presence of nanoparticles results into a sizeable increase of the HWHM of the correlation function to a value which is comparable with the nanoparticles diameter.
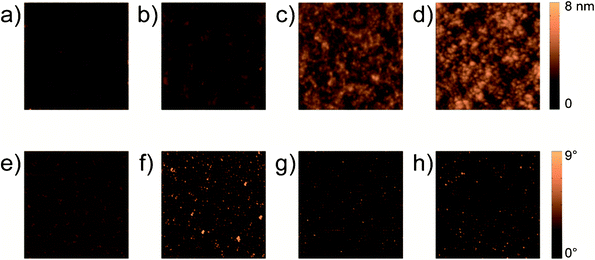 | ||
| Fig. 4 AFM images of the samples in “height trace” mode: a) film A0; b) film A16; c) film A28, d) film A44. AFM images of the samples in “phase lag” mode: e) film A0; f) film A16; g) film A28; h) film A44. Image size is 2 μm × 2 μm. The colour scale bar corresponds to 8 nm for the images in “height trace” mode and 9° for the images in “phase lag.” | ||
| Film | Correlation length (HWHM) (nm) | WCA (°) |
|---|---|---|
| A0 | <13 | 53 ± 3 |
| A16 | 33 | 67 ± 5 |
| A28 | 30 | 82 ± 3 |
| A44 | 40 | 88 ± 4 |
Phase lag images (Fig. 4e–h) show that most of the surface in all films presents similar dynamics of the vibrating AFM cantilever. In nanocomposites small spots of the phase-lag image show a large deviation from the average value. A significant change in the dynamics is expected when the tip interacts with different types of surfaces: the soft organic phase or the hard inorganic one. Therefore the presence of small spots showing large deviation of the phase-lag suggests that some nanoparticles protrude out of the polymer surface. Actually, the fact itself that the particles are not completely encased in the polymer is an indication of reduced compatibility, although films appear homogenous and without cracks.
WCA measurements also reveal a change in the nanocomposite surface with increasing TiO2 content (Fig. 5). Table 1 reports average values and deviations for the studied systems. The water contact angle goes from 55° (Fig. 5a) for the pure polymer up to 91° (Fig. 5d) for the most concentrated nanocomposite film.
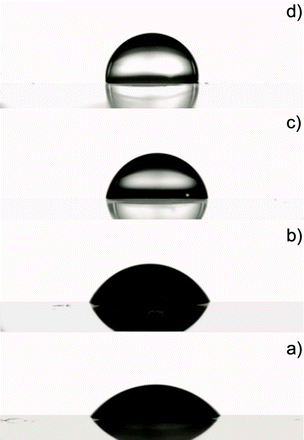 | ||
| Fig. 5 Water contact angle measurements of the pristine polymer: a) A0: 55°; and of the nanocomposite films: b) A16: 64°; c) A28: 82°; d) A44: 91°. | ||
The change of wetting behaviour can be attributed to the increased roughness of the nanocomposite and to the presence of benzyl groups on the titania surface. It is well established that the wettability of a solid surface is governed both by surface geometrical microstructures and by the chemical composition, as pointed out in the case of nanograss and similar systems.38–40
The observed reduced compatibility of the titania nanoparticles on the nanocomposite surface is in apparent contrast to the good stability of their dispersion in the polymeric matrix. However, we must consider that both PEOX and the titania surface present a complex structure which may be responsible for the observed behaviour of the nanocomposite. In particular, PEOX presents both an hydrophobic main chain and polar side chains, resulting into a peculiar balance between solubility in water41 and aggregation above a lower critical solution temperature (LCST). In the nanocomposite, both hydrogen bonds between PEOX and residual hydroxyls42 on the titania surface and CH–π interactions between the phenyl rings covering the titania surface and the hydrophobic main chain of PEOX43 might help to stabilize the dispersion of nanoparticles. Additionally, phenyl rings tend to repel each other, possibly contributing to stabilize the nanoparticle dispersion both in water and in PEOX. Instead, at the solid–air interface, the aggregation driving force in the polymer is possibly stronger than the PEOX–nanoparticle interaction, thus PEOX might not be anymore able to wet the nanoparticle surfaces, which remain exposed to air. As a first conclusion, we obtained materials whose surface properties, such as the roughness and hydrophobicity, can be tuned by the nanoparticles content in the polymeric matrix.
3.2 Optical properties of nanocomposite films
Pure poly(2-ethyl-2-oxazoline) (A0) and nanocomposite films fabricated with increasing concentrations of nanoparticles are optically transparent for the most part of the UV-visible range. Their UV-visible spectra are reported in Fig. 6. Below 400 nm it is possible to observe the pronounced nanocomposite absorption edge, linked to the nanoparticle band gap. Therefore, unlike the pristine polymer, the nanocomposite films provide complete UV filtering up to the proximity of the visible region.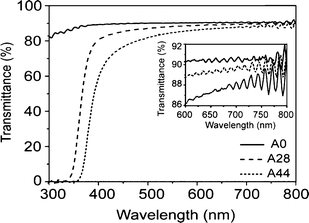 | ||
| Fig. 6 UV-visible spectra of the pure polymer (A0) and nanocomposite films A28 and A44. In the inset the near IR spectra in the region comprised between 600–800 nm is shown. | ||
Because of the high concentration of the nanoparticles in the polymeric matrix, the refractive index of the nanocomposites is expected to be larger than the value for the pure polymer. The effective nanocomposite refractive index is predicted by the following equation:
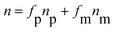 | (1) |
The inset of Fig. 6 shows the near IR portion of the spectra of the samples at an enlarged scale. Assuming that surface and bulk scattering are negligible at long wavelengths, the average transmittance in this range is given by T0 = (1 − R)2, with R = (n − 1)2/(n + 1)2 the interface reflectance.45 With these assumptions, the 91.5% transmittance of the A0 film at 694 nm correspond to n = 1.52, matching literature data. Good agreement with the index predicted by the last equation is also found for nanocomposites: indeed the 89.8% transmittance at 694 nm for the film A28 corresponds to a refractive index of 1.60, while the 88.4% of transmittance for A44 corresponds to a refractive index of 1.65. All measured values are slightly lower compared to the expected values calculated. A reason for this minor deviation could be linked to an overestimation of the particles refractive index because of the small size of the crystallites. Moreover, as discussed above, a fraction of water and benzyl alcohol molecules are present on the surface of the nanoparticles. Thus, the effective index of the nanoparticles is possibly lower compared to the refractive index of bulk anatase. Finally, the conformation of the polymer chains in close proximity to the nanoparticles surface is possibly modified by the different environment. This may lead to a lower density of the polymer, which becomes significant for the large volume fractions of nanoparticles present in our samples.46,9
The film thicknesses, x, were calculated by measuring the wavelength separation between adjacent transmission peaks Δλ in the region of interference, using the Fabry–Pérot law,47 according to:
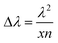 | (2) |
The weak change of transmittance in the visible range is not due to the TiO2 band gap, thus Rayleigh scattering can be hypothesized.48–50 Below the near IR range, and into the visible region, the slopes of the nanocomposite transmission spectra T(λ) between 400 nm and 650 nm were evaluated using the Rayleigh law,51,52 which applies well for spherical scatterers having diameters below λ/10:

| (3) |
Fig. 7 shows the logarithm of nanocomposites bulk absorbance α(λ)x = −In(T(λ)/TA0(λ)), normalized to the pristine polymer absorbance, versus the logarithm of the wavelength λ, along with a linear fitting with slope −4. The good fit with such a slope shows that the transmittance losses indeed can be attributed to Rayleigh scattering in the region between 400 and 650 nm. Using nm = 1.52, np = 2.49, the thicknesses measured from the near IR transmission, and the measured concentrations fp, the nanoparticle diameter could be estimated to be around 30 nm. This small diameter confirms that no further agglomeration occurs during the formation of the films. The larger value of the hydrodynamic diameter of the agglomerate measured by DLS (around 40 nm) is reasonable in view of their highly irregular shape and internal structure shown in the TEM micrographs. Both factors certainly increase the real drag force on the agglomerate compared to a compact body, thus resulting into a larger radius estimate when using a simple Stokes law.
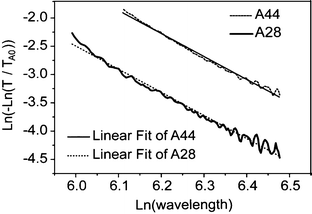 | ||
| Fig. 7 Logarithm of nanocomposite bulk absorbance for A28 and A44 films, normalized to the pristine polymer absorbance, versus the logarithm of the wavelength in the visible range. The curves were fitted with two straight lines with slope −4 distinctive of Rayleigh scattering. | ||
In conclusion, UV-visible spectroscopy shows that the nanocomposite films are essentially transparent, with a weak bulk scattering mainly linked to the high concentration of the nanoparticles in the films. Moreover, the measurements showed that the films absorb the UV radiation up to the proximity of the visible and have refractive indices higher than the pure polymer growing with the concentration of the nanoparticles in the polymeric matrix.
The optical quality of the films was assessed through optical measurements of reflectivity and surface scattering. Measurements of specular gloss Gs(Θ) at 20°, 60° and 85° and distinctness of image gloss (GDoI) were performed allowing an assessment of the impact of surface scattering on the visual appearance of the film. Specular gloss is a measurement of the ratio of the luminous flux reflected from the sample at an angle Θ, relative to the luminous flux reflected from a standard smooth glass surface with a refractive index of 1.567. Increase in the bulk RI will increase specular gloss but surface roughness reduces the specular gloss through scattering. An estimate of the RMS surface roughness σ can be obtained by fitting the measured specular gloss using the Fresnel Equation Gs(Θ, n)53 times a term that accounts for the loss from scattering, in this case from the Total Integrated Scattering model (TIS):54
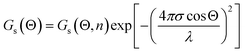 | (4) |
Fig. 8 shows the predicted gloss Gs(Θ, n), assuming no loss from surface scattering, and the measured specular gloss Gs(Θ) at 20° and at a wavelength λ of 550 nm versus the measured refractive indices of the samples. The x-error bars represent a relative error below 1% on the measured refractive indices of films, resulting into about 3% of relative error on the predicted gloss. Films of pristine polymer and with the lowest concentration of nanoparticles in the polymeric matrix (A16) seem to produce quite a smooth surface on the polished glass, while some relevant deviations from the values expected for a perfectly smooth surface appear for the more concentrated nanocomposites A28 and A44. The mismatch is attributed to the increased surface roughness σ, which is evaluated from last equation, and summarized in Table 2.
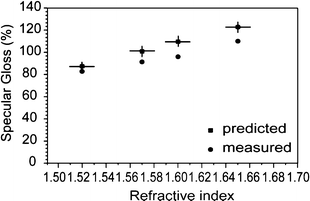 | ||
| Fig. 8 Measured (dots) specular gloss values Gs(Θ), and predicted (squares) values Gs(Θ, n) versus film refractive indices at Θ = 20° incident angle. | ||
| Film | TiO2 (% w/w) | R.I. | σ (nm) | GDoI |
| A0 | 0 | 1.52 | 11 ± 3 | 98.5 ± 0.5 |
| A16 | 16 | 1.57 | 15 ± 2 | 98.0 ± 0.5 |
| A28 | 28 | 1.60 | 17 ± 2 | 95 ± 1 |
| A44 | 44 | 1.65 | 16 ± 2 | 96 ± 1 |
We remark that σ grows with the TiO2 content in the nanocomposite, in agreement with the AFM height image results presented above.
In the perspective of using this material as a protective coating, we evaluate distinctness-of-image gloss (GDoI) which evaluates the effectiveness of the coating in preserving the information of an image covered by the film. This measurement compares the amount of scattered light at ± 0.3° from the specular angle Θ = 30°, I30°![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ±
±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3°, to light reflected in the specular direction, I30°, according to the formula GDoI = 100 (I30° − I30°
0.3°, to light reflected in the specular direction, I30°, according to the formula GDoI = 100 (I30° − I30°![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ±
±![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3°)/I30°. Therefore, a value of 100% indicates that no light is diffused off-axis, while lower values indicate that a fraction of light is scattered slightly off-axis, blurring image formation in a far optical system such as the eye. Such an off-axis scattering is mainly related to surface roughness,55 as the contribution of Rayleigh bulk scattering is instead quite diffuse in angle, and is therefore negligible into the very low aperture used in DoI measurements (below 0.3°). Results for GDoI are also shown in Table 2. GDoI is always very close to 100%, i.e. the fraction of light diffused slightly off-axis is relatively small for all samples. Indeed, also specular gloss measurements point out to a relatively weak total surface scattering. The slight decrease of GDoI with increasing nanoparticle content indicates an increase in surface roughness, also in agreement with specular gloss measurements. However, we remark that the RMS surface roughness is only slightly larger in A28 and A44 compared to A16, an increase which is not sufficient to explain the more significant reduction of GDoI. Here, a change in the structure of surface roughness over length scales much beyond those considered in AFM measurements is probably involved. Detailed investigation of these aspects of the nanocomposite surface will be the subject of further study.
0.3°)/I30°. Therefore, a value of 100% indicates that no light is diffused off-axis, while lower values indicate that a fraction of light is scattered slightly off-axis, blurring image formation in a far optical system such as the eye. Such an off-axis scattering is mainly related to surface roughness,55 as the contribution of Rayleigh bulk scattering is instead quite diffuse in angle, and is therefore negligible into the very low aperture used in DoI measurements (below 0.3°). Results for GDoI are also shown in Table 2. GDoI is always very close to 100%, i.e. the fraction of light diffused slightly off-axis is relatively small for all samples. Indeed, also specular gloss measurements point out to a relatively weak total surface scattering. The slight decrease of GDoI with increasing nanoparticle content indicates an increase in surface roughness, also in agreement with specular gloss measurements. However, we remark that the RMS surface roughness is only slightly larger in A28 and A44 compared to A16, an increase which is not sufficient to explain the more significant reduction of GDoI. Here, a change in the structure of surface roughness over length scales much beyond those considered in AFM measurements is probably involved. Detailed investigation of these aspects of the nanocomposite surface will be the subject of further study.
Conclusions
In this work, we have shown a simple and environmentally benign method for the preparation of nanoparticle–polymer composite with high nanoparticle content. By simple mixing in water of titania nanoparticles and hydrophilic poly(2-ethyl-2-oxazoline) it is possible to obtain a dispersion stable up to several months; the dispersion, as conventional paint formulations, can be brushed on different type of surfaces and smooth transparent films can be obtained by evaporation of water at ambient conditions. TEM images showed that nanoparticles are clustered in elongated aggregates of about 40 nm confirming the DLS measurements of the aqueous dispersion, and that they are homogeneously distributed in the polymeric matrix. On the other hand, regardless of the TiO2 content, AFM images showed that some nanoparticles protruded out of the film surface, modifying the hydrophilic behavior of the pristine polyoxazoline. In particular, a growth of the water contact angle up to just more than 90° has been observed. This phenomenon has been attributed to the partial coverage of the TiO2 surface with the benzyl alcohol originating from the nanoparticles synthesis. Moreover, films were characterized by an increase of the surface roughness with the nanoparticles concentration in the polymeric matrix, according to AFM height trace and specular gloss measurements. From this point of view, nanocomposite films were characterized by tunable surface properties, such as roughness and hydrophobicity.Optical properties were also investigated and refractive index and UV filtering can be tailored by the increasing concentration of nanoparticles in the polymeric matrix. In particular, films characterized by high refractive index up to about 1.65 and UV absorption up to the proximity of the visible range were obtained. Moreover, nanocomposite films were optically transparent, albeit showing weak bulk scattering that may result in a slight coloring of the film (having an impact when used as a functional coating), and weak surface scattering resulting in a slight reduction of gloss. Thus, several optical properties of these nanocomposites, and their visual appearance when used as coatings, may be tailored by varying the concentration and nature of the nanoparticles in the polymeric matrix. The good chemical and physical properties of these materials could allow their application in the paints and coatings industry. In particular, these films were tested on matte painted surfaces made with acrylic colours cast on different fabrics. Unlike the pristine polymer, that completely modified the optical appearance of the painted surfaces, nanocomposite films respected the gloss and the colour of the pristine layers. It could be an interesting application in the field of the cultural heritage conservation in the treatment of matte painted surfaces, such as frescos, temperas, watercolours and so on. In fact, in this case, the maintenance of the pristine painted surface appearance after a restoration treatment is important for conservation aims.
Acknowledgements
We thank the Cariplo Foundation for the financial support of part of this work and Dr Kaloian Koynov for helpful discussions. Funding for MM was provided by a “Dote Ricercatore” FSE project of Regione Lombardia.References
- M. Alexandre and P. Dubois, Mater. Sci. Eng., R, 2000, 28, 1–63 CrossRef.
- A. C. Balazs, T. Emrick and T. P. Russell, Science, 2006, 314, 1107–1110 CrossRef CAS.
- A. Usuki, Y. Kojima, M. Kawasumi, A. Okada, Y. Fukushima, T. Kurauchi and O. Kamigaito, J. Mater. Res., 1993, 8, 1179–1184 CrossRef CAS.
- Y. Kojima, A. Usuki, M. Kawasumi, A. Okada, Y. Fukushima, T. Kurauchi and O. Kamigaito, J. Mater. Res., 1993, 8, 1185–1189 CrossRef CAS.
- P. Kiliaris and C. D. Papaspyrides, Prog. Polym. Sci., 2010, 35, 902–958 CrossRef CAS.
- M. A. Osman, V. Mittal, M. Morbidelli and U. W. Suter, Macromolecules, 2004, 37, 7250–7257 CrossRef CAS.
- H. Song and S. Lee, Nanotechnology, 2007, 18, 055402 CrossRef.
- M. C. Neves, M. A. Martins, P. C. R. Soares-Santos, P. Rauwel, R. A. S. Ferreira, T. Monteiro, L. D. Carlos and T. Trindade, Nanotechnology, 2008, 19, 155601 CrossRef.
- R. J. Nussbaumer, W. R. Caseri, P. Smith and T. Tervoort, Macromol. Mater. Eng., 2003, 288, 44–49 CrossRef CAS.
- H. Althues, R. Poetschke, G. M. Kim and S. Kaskel, J. Nanosci. Nanotechnol., 2009, 9, 2739–2745 CrossRef CAS.
- E. D. Palik and G. Ghosh, Handbook of optical constants of solids, Academic Press, San Diego, 1998 Search PubMed.
- R. H. French, S. J. Glass, F. S. Ohuchi, Y. N. Xu and W. Y. Ching, Phys. Rev. B: Condens. Matter, 1994, 49, 5133–5141 CrossRef CAS.
- T. Tsuzuki, Macromol. Mater. Eng., 2008, 293, 109–113 CrossRef CAS.
- M. M. Demir, M. Memesa, P. Castignolles and G. Wegner, Macromol. Rapid Commun., 2006, 27, 763–770 CrossRef CAS.
- Y. Q. Li, S. Y. Fu, Y. Yang and Y. W. Mai, Chem. Mater., 2008, 20, 2637–2643 CrossRef CAS.
- H. Althues, J. Henle and S. Kaskel, Chem. Soc. Rev., 2007, 36, 1454–1465 RSC.
- H. Goesmann and C. Feldmann, Angew. Chem., Int. Ed., 2010, 49, 1362–1395 CrossRef CAS.
- D. Sun, N. Miyatake and H.-J. Sue, Nanotechnology, 2007, 18, 215606 CrossRef.
- C. Lin, M. T. Berry, R. Anderson, S. Smith and P. S. May, Chem. Mater., 2009, 21, 3406–3413 CrossRef CAS.
- M. M. Demir, K. Koynov, U. Akbey, C. Bubeck, I. Park, I. Lieberwirth and G. Wegner, Macromolecules, 2007, 40, 1089–1100 CrossRef CAS.
- J. Oberdisse, Soft Matter, 2006, 2, 29–36 RSC.
- F. Papadimitrakopoulos, P. Wisniecki and D. E. Bhagwagar, Chem. Mater., 1997, 9, 2928–2933 CrossRef CAS.
- M. Weibel, W. Caseri, U. W. Suter, H. Kiess and E. Wehrli, Polym. Adv. Technol., 1991, 2, 75–80 CrossRef CAS.
- K. Lee and S. Lee, J. Appl. Polym. Sci., 2012, 124, 4038–4046 CrossRef CAS.
- J. E. Glass, Water-soluble polymers: beauty with performance, American Chemical Society, Washington, DC, 1986 Search PubMed.
- V. Dorge and F. C. Howlett, Painted wood: history & conservation, Getty Conservation Institute, Los Angeles, 1998 Search PubMed.
- J. Arslanoglu, Western Association for Art Conservation (WAAC), 2004, 26, 10–15 Search PubMed.
- R. Hoogenboom and H. Schlaad, Polymers, 2011, 3 Search PubMed.
- N. Adams and U. S. Schubert, Adv. Drug Delivery Rev., 2007, 59, 1504–1520 CrossRef CAS.
- M. Niederberger, M. H. Bartl and G. D. Stucky, Chem. Mater., 2002, 14, 4364–4370 CrossRef CAS.
- S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60, 309–319 CrossRef CAS.
- ASTM, in Standard Test Method for Specular Gloss, Philadelphia, 2000, pp. D523–589 Search PubMed.
- ASTM, in Standard Test Method for Measurement of Gloss of High-Gloss Surfaces by GoniophotometryPhiladelphia, 2000, pp. E430–497 Search PubMed.
- H. Borchert, E. V. Shevehenko, A. Robert, I. Mekis, A. Kornowski, G. Grubel and H. Weller, Langmuir, 2005, 21, 1931–1936 CrossRef CAS.
- M. Niederberger, M. H. Bard and G. D. Stucky, J. Am. Chem. Soc., 2002, 124, 13642–13643 CrossRef CAS.
- R. Mueller, H. K. Kammler, K. Wegner and S. E. Pratsinis, Langmuir, 2003, 19, 160–165 CrossRef CAS.
- R. Brindle, M. Pursch and K. Albert, Solid State Nucl. Magn. Reson., 1996, 6, 251–266 CrossRef CAS.
- C. Dorrer and J. Ruehe, Adv. Mater., 2008, 20, 159–163 CrossRef CAS.
- G. Caputo, B. Cortese, C. Nobile, M. Salerno, R. Cingolani, G. Gigli, P. D. Cozzoli and A. Athanassiou, Adv. Funct. Mater., 2009, 19, 1149–1157 CrossRef CAS.
- P. Pareo, G. L. De Gregorio, M. Manca, M. S. Pianesi, L. De Marco, F. Cavallaro, M. Mari, S. Pappada, G. Ciccarella and G. Gigli, J. Colloid Interface Sci., 2011, 363, 668–675 CrossRef CAS.
- F. P. Chen, A. E. Ames and L. D. Taylor, Macromolecules, 1990, 23, 4688–4695 CrossRef CAS.
- P. Sozzani, A. Comotti, S. Bracco and R. Simonutti, Chem. Commun., 2004, 768–769 RSC.
- C. H. Chen, J. E. Wilson, R. M. Davis, W. Chen and J. S. Riffle, Macromolecules, 1994, 27, 6376–6382 CrossRef CAS.
- C. L. Lu and B. Yang, J. Mater. Chem., 2009, 19, 2884–2901 RSC.
- E. Hecht, Optics, 4th edn, Addison-Wesley, Reading, Mass., 2002 Search PubMed.
- F. W. Mont, J. K. Kim, M. F. Schubert, E. F. Schubert and R. W. Siegel, J. Appl. Phys., 2008, 103, 083120 CrossRef.
- K. Nassau, The physics and chemistry of color: the fifteen causes of color, 2nd edn, Wiley, New York, 2001 Search PubMed.
- H. I. Elim, B. Cai, Y. Kurata, O. Sugihara, T. Kaino, T. Adschiri, A. L. Chu and N. Kambe, J. Phys. Chem. B, 2009, 113, 10143–10148 CrossRef CAS.
- Y. F. Liu, C. L. Lu, M. J. Li, L. Zhang and B. Yang, Colloids Surf., A, 2008, 328, 67–72 CrossRef CAS.
- P. Tao, Y. Li, A. Rungta, A. Viswanath, J. N. Gao, B. C. Benicewicz, R. W. Siegel and L. S. Schadler, J. Mater. Chem., 2011, 21, 18623–18629 RSC.
- W. Caseri, Chem. Eng. Commun., 2009, 196, 549–572 CrossRef CAS.
- M. C. Tan, S. D. Patil and R. E. Riman, ACS Appl. Mater. Interfaces, 2010, 2, 1884–1891 CAS.
- G. H. Meeten, Optical properties of polymers, ElsevierLondon; New York, 1986 Search PubMed.
- H. E. Bennet, J. Opt. Soc. Am., 1963, 53, 1389 CrossRef.
- J. K. Delaney, E. R. de la Rie, M. Elias, L. P. Sung and K. M. Morales, Stud. Conserv, 2008, 53, 170–186 CAS.
| This journal is © The Royal Society of Chemistry 2012 |