Bromodimethylsulfonium bromide as a potential candidate for photocatalytic selective oxidation of benzylic alcohols using oxygen and visible light†
Sarifuddin
Gazi
and
Rajakumar
Ananthakrishnan
*
Department of Chemistry, Indian Institute of Technology, Kharagpur, Kharagpur 721 302, India. E-mail: raja.iitchem@yahoo.com; Fax: (+91) 3222-255303; Tel: (+91) 3222-282322
First published on 26th June 2012
Abstract
Selective photooxidation of different benzylic alcohols has been carried out in the presence of molecular oxygen and a catalytic amount of bromodimethylsulfonium bromide (BDMS) under visible light irradiation. The above method was found to be efficient for the oxidation of primary and secondary benzylic alcohols into their corresponding aldehydes and ketones with excellent product yield. Unlike other studies, the advantages of this study are the metal-free green protocol, utilization of a household compact fluorescent lamp (CFL) lamp as the visible light source, and the high selectivity of the reaction. A mechanistic study infers that the carbonyl compound obtained by the process possesses an oxygen atom which comes from molecular oxygen. From the study, we propose that the conversion of the benzylic alcohol to its corresponding aldehyde could be following a peroxy radical intermediate. The first-hand results revealed the photocatalytic potential of BDMS in general and its capability for selective alcohol oxidation, in particular.
Introduction
Carbonyl compounds (aldehyde or ketones) are some of the most important building blocks used in organic synthesis. Hence, selective oxidation of alcohols to achieve carbonyl compounds is a necessary and widely used organic reaction both on the laboratory scale as well as in large-scale synthesis in industry.1 Many organic named reactions like the Hantzsch dihydropyridine synthesis, the Biginelli reaction, the Aldol reaction and other transformations found in the literature, involve carbonyl compounds as one of the essential substrates.2 Thus, carbonyl compounds are vital to organic chemists. Among various processes known to give carbonyl compounds, the most well known method is selective oxidation of alcohols to carbonyl compounds.3 Generally, the oxidation of alcohols has been achieved by employing stoichiometric amounts of inorganic oxidizing agents traditionally chromates or permanganates.4 However, the problem associated with these kinds of oxidants are their expense and production of noxious heavy-metal waste in large quantities. Hence, much attention has been paid to the selective oxidation of alcohols by catalytic routes.For instance, Fujita et al. used a Cp*Ir complex for the dehydrogenative-oxidation of alcohols.5 But the use of toxic metal complexes as oxidants for selective oxidation of alcohols is not acceptable from a green chemistry point of view. Therefore, in recent years many chemists have tried to develop alternate methods for the selective oxidation of alcohols to aldehydes or ketones by green and environmentally acceptable processes.6 As an alternative to the inorganic oxidizing agent, oxygen (or even better air) is among the cheaper and less polluting oxidants, since it produces water as the sole by-product.7 The implementation of a transition metal-based catalyst (metal complexes or nanoparticles) in combination with oxygen represents an emerging alternative to the traditional methods. For example; Murahashi et al. used a ruthenium-cobalt bimetallic catalyst for aerobic oxidation of alcohol, and a few others have also reported the oxidation of alcohols utilizing molecular oxygen and metal-based catalysts.8 Recently, some other groups have shown that the selective oxidation of alcohols can be done by different metal nanoparticles (as photocatalysts).9 Though metal containing catalysts are effective for alcohol oxidation, they are all expensive (Au, Pt, Ru, Nb, etc.) and nanomaterial catalysts could possibly be harmful to the environment due to their size and activity (nanoparticles of CdS, Mn-based, CeO2, etc.). Hence, a search for a metal-free catalyst is advantageous. Karimi et al. have developed a TEMPO based organocatalyst for the selective oxidation of alcohols.10 In the recent years, visible light mediated organic synthesis has become very popular, where different metal (Ru, Ir) complexes or various dyes, like Eosin Y, 9-mesityl-10-methylacridinium perchlorate, etc., are used as the photocatalyst.11
In our laboratory, we are trying to find different metal complexes or dyes, which can be used as the visible light responsive photocatalyst for organic transformations or water decontamination. Recently, we found that resin supported Eosin Y is an effective photocatalyst which utilizes visible light and helps in the photoreduction of 4-nitrophenol to 4-aminophenol. Very recently, we have also shown that the synthesis of 2-arylpyridine can easily be achieved by a photocatalytic route using Ru(bpy)3]2+ as a photoredox catalyst.12 Methodologies for basic reactions like selective oxidation by green process are highly demanding.13 Hence, reports on this are frequently found in the literature. There are several methods for aerobic oxidations of alcohols in the literature which involve a bromine-catalyzed route in the presence of acid additives and/or light sources.14 However, in most of these cases the effective oxidation needs a large amount of bromine. Itoh and co-workers showed that primary alcohols can be converted to carboxylic acids in the presence of inorganic bromo additives under UV irradiation.15 Later, the same group reported that when MgBr2·Et2O was used as the bromo additive, the oxidation of primary alcohols to carboxylic acids occurred under visible light irradiation.16 They proposed that the oxidation had occurred through an aldehyde intermediate.
To our surprise, unlike Itoh's work, when photooxidation of 4-chlorobenzyl alcohol (reaction conditions: 10 mol% BDMS, acetonitrile, oxygen atmosphere, and visible light irradiation) was carried out, it yielded the corresponding aldehyde as the exclusive product (Scheme 1) after 4 h of irradiation. Even after prolonging the irradiation for 12 h, we could not identify any carboxylic acid as the product of the present photoreaction. This initial result indicated that BDMS can provide better selectivity in the photooxidation reaction with respect to other inorganic bromo-reagents. According to Itoh and co-workers, the latter could help to stop the reaction with carboxylic acid as the final product in the photooxidation process. Hence, the BDMS system offers better control in the oxidation step, where the oxidation can be frozen in the aldehyde stage.
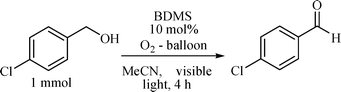 | ||
| Scheme 1 Oxidation of 4-chlorobenzyl alcohol to 4-chlorobenzaldehyde under the visible irradiation (45 W CFL). | ||
Moreover, photocatalytic activity of BDMS is not established in the literature. The application of BDMS for the visible light assisted photocatalytic oxidation of organic compounds is also a novel green proposal. From a green chemistry point of view, photocatalytic oxidation of organic compounds under metal-free conditions, with the utilization of molecular oxygen and visible light, is a growing area. Hence, in this present study, our focus is directed on visible light assisted photocatalytic selective oxidation of benzylic alcohols to carbonyl compounds under an oxygen atmosphere and metal-free conditions.
Results and discussion
In organic synthesis, BDMS has been used as a potential reagent as well as an effective pre-catalyst.17 For example, BDMS has been employed as a brominating agent in the α-bromination of β-ketoesters and 1,3-diketones,18 it has also been used as a catalyst in the aza-Michael reaction19 and Mannich type reactions.20 Furukawa et al. have shown that alcohols are converted to corresponding bromides when they are heated at 80 °C in the presence of BDMS.21 Hence, when 1 mmol of 4-chlorobenzyl alcohol was heated with 1 mmol of BDMS in acetonitrile for 4 h, 4-chlorobenzyl bromide was found to be as the exclusive product (Scheme 2) in a high yield (90%).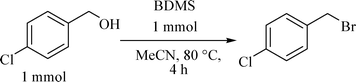 | ||
| Scheme 2 Conversion of 4-chlorobenzyl alcohol to 4-chlorobenzyl bromide by BDMS. | ||
When the same reaction was carried out with 10 mol% of BDMS, a small amount of the bromo-product (15%) was obtained and no oxidative product (aldehyde or carboxylic acid) was isolated. Here, besides the product (benzylic bromide), the starting material (unreacted alcohol) is also recovered. This BDMS mediated bromination reaction goes via a nucleophilic substitution reaction as mentioned by Furukawa et al. (Scheme 3).21
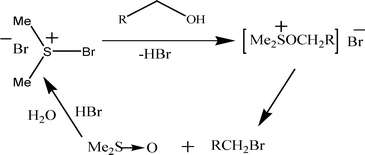 | ||
| Scheme 3 Mechanistic pathway of the bromination reaction by BDMS. | ||
Inspired by our initial result (Scheme 1), we wanted to generalize the methodology for achieving a variety of carbonyl compounds from different benzylic alcohols (primary and secondary).
Generally, solvent plays a crucial role in any chemical reaction, hence, the photocatalytic oxidation of 4-chlorobenzyl alcohol in presence of BDMS (10 mol%) was carried out with different solvents. The result reveals that acetonitrile is found to be superior to other solvents such as ethanol, tBuOH, dichloromethane, and toluene in terms of both the reaction time as well as the yields of the reaction. Table 1 represents the screening of reaction conditions to achieve the optimum condition. Here, for the photocatalytic oxidation, the conversion of 4-chlorobenzyl alcohol to 4-chlorobenzaldehyde has been taken as a representative example.
|
|
||||
|---|---|---|---|---|
| Entry | BDMS/mol% | Solvent | Time/h | Yield (%)a |
| a Isolated yield. b Before adding the substrate alcohol, the catalyst was irradiated in the solvent for 15 min the under oxygen atmosphere. c Reaction was carried out under dark conditions. d The reaction was carried out under an argon atmosphere. | ||||
| 1 | — | MeCN | 12 | 0 |
| 2 | 1 | MeCN | 4 | trace |
| 3 | 5 | MeCN | 4 | 40 |
| 4 | 10 | MeCN | 4 | 90 |
| 5b | 10 | MeCN | 4 | 98 |
| 6c | 10 | MeCN | 12 | 0 |
| 7d | 10 | MeCN | 12 | 0 |
| 8 | 10 | CH2Cl2 | 4 | <10 |
| 9 | 10 | EtOH | 4 | 30 |
| 10 | 10 | t BuOH | 4 | 20 |
| 11 | 10 | Toluene | 4 | <10 |
From the optimization study on the reaction conditions, we found that in the absence of the catalyst (BDMS), the formation of the oxidized product, the corresponding aldehyde, was not observed (entry 1, Table 1). Similar results are also noticed when the reaction was performed under dark conditions (entry 6) or under an inert argon atmosphere (entry 7). Hence, this screening process reveals that here, the catalyst, visible light, and molecular oxygen are all equally essential for facilitating the oxidation reaction.
To generalize the present methodology, various benzylic alcohols were subjected to photooxidation under the optimized reaction conditions. Table 2 shows the results for photooxidation of several primary benzylic alcohols under the present reaction conditions. The primary alcohols are converted to the corresponding aldehydes (entries 1–7). Some secondary alcohols were also subjected to photooxidation and were found to be transformed to the corresponding ketones (Table 2, entries 8–12). The yields of the product of this photooxidation are good to excellent and this implies a high selectivity of the present protocol. When 1,2-diphenylethane-1,2-diol was taken as the benzylic alcohol, we found 70% benzoin and 20% benzil after 6 h of irradiation (entry 12).
| Entry | Substrate | Product | Time/h | Yield (%) |
|---|---|---|---|---|
| a A typical procedure follows: initially, a solution of Me2SBr2 (0.1 mmol) in acetonitrile (10 mL) in a Pyrex tube was irradiated for 15 min with a fluorescent lamp (45 W) under an oxygen atmosphere with stirring. Then to this reaction mixture, 1 mmol of substrate alcohol was added and allowed to be irradiated. The progress of the reaction was monitored by TLC. All the products were isolated and obtained as pure. | ||||
| 1 |
|
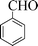
|
6 | 95 |
| 2 |
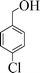
|
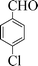
|
4 | 98 |
| 3 |
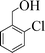
|
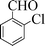
|
6 | 97 |
| 4 |
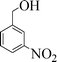
|
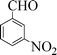
|
7 | 93 |
| 5 |
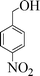
|
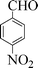
|
10 | 95 |
| 6 |
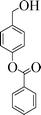
|
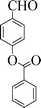
|
6 | 98 |
| 7 |
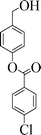
|
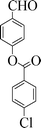
|
6 | 98 |
| 8 |
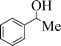
|
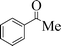
|
6 | 88 |
| 9 |
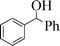
|
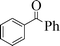
|
6 | 90 |
| 10 |
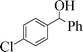
|
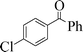
|
6 | 93 |
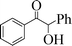
| 11 |
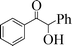
|
|
6 | 95 |
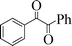
| 12 |
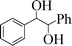
|
|
6 | 70 |
Mechanistic investigation
The initial screening study reveals that molecular oxygen plays a crucial role in the present oxidative transformation. To verify the involvement of molecular oxygen in the current process, we performed the photocatalytic oxidation of benzylic C–H, which does not have any oxygen atom. The initial experiment was conducted with toluene instead of benzylic alcohol (Scheme 4).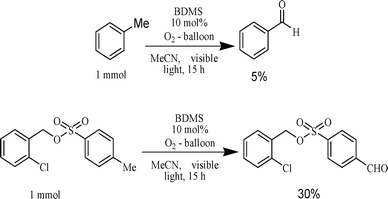 | ||
| Scheme 4 Photocatalytic oxidation of methyl group with domestic fluorescent lamp. | ||
We obtained benzaldehyde as the sole oxidation product (5%, after 15 h of irradiation). Again when 2-chlorobenzyl-4-methylbenzenesulfonate was subjected to visible light aided photolysis in the presence of 10 mol% BDMS under an oxygen atmosphere, we obtained the corresponding aldehyde with a better yield (30% in 15 h irradiation). These results infer that the visible light aided photocatalytic aerobic oxidation of benzylic C–H with the catalyst BDMS occurs by the incorporation of an oxygen atom available from molecular oxygen. Similar findings were also well established by Zhong et al. in their report about photocatalytic oxidation of benzylic alcohol with metal bromide.22
The experimental results obtained in this present study suggest that the selective oxidation of benzylic CH3 group to aldehyde involves the incorporation of the oxygen atom originated from molecular oxygen. In the case of benzylic alcohol oxidation, it is also expected that the oxygen atom of the carbonyl compounds come from the molecular oxygen. Here, there is an exchange of alcoholic oxygen by the molecular oxygen is anticipated.22 Probably, a peroxy radical intermediate is formed from benzylic alcohol under oxygen atmosphere and visible irradiation in the presence of BDMS, where selective cleavage of the C–O bond in the benzylic alcohol occurs and consequently, a new C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond formation takes place to yield carbonyl compound.
O bond formation takes place to yield carbonyl compound.
When the substrate alcohol (4-chlorobenzylic alcohol) and catalyst (BDMS, 10 mol%) were added together into the solvent and stirred at room temperature under an oxygen atmosphere without irradiation, it was observed that some amount of bromination product (8%) was formed (Case I, Scheme 5). Here the formation of the oxidation product (aldehyde) did not occur. But, when the same reaction was carried out under visible light irradiation, it was found that the corresponding aldehyde was formed as the major oxidation product (90%) and that the bromination product (5%) was formed as the minor one (Case II, Scheme 5). Again, when the substrate (alcohol) was added to the pre-irradiated mixture (15 min irradiation) containing catalyst in the solvent under an oxygen atmosphere, the bromination reaction was found to be suppressed (Scheme 5, Case III). These experimental observations suggest that in the former case (Case II), probably, there was a small amount of bromide ion (originated from BDMS, like Case I) in the reaction mixture which acted as a nucleophile to generate some amount of bromination product by a substitution reaction. In the latter case (Case III), bromine radicals were generated from BDMS in the presence of molecular oxygen under the visible light irradiation. As a result of which, there was a lack of a bromide nucleophile for the substitution reaction to occur. Instead, the bromine radical helped the production of a benzylic radical which further undergoes oxidation in the presence of molecular oxygen. From the experimental results and previous literature, a plausible reaction mechanism has been proposed for the present photocatalytic oxidation of alcohol by BDMS in the Scheme 6.
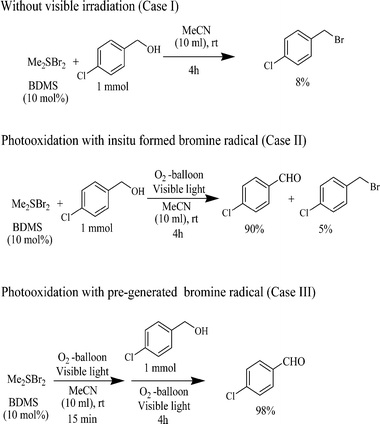 | ||
| Scheme 5 Photocatalytic oxidation of 4-chlorobenzyl alcohol under controlled reaction conditions. | ||
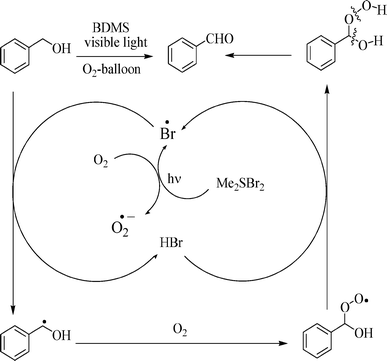 | ||
| Scheme 6 Proposed mechanism for the BDMS aided photocatalytic oxidation of benzylic alcohols with visible irradiation under oxygen atmosphere. | ||
In this photocatalytic process, i) initially, the bromine radicals are formed from BDMS in the presence of oxygen and visible light irradiation; ii) these bromine radicals facilitate the formation of benzylic radicals which further get converted into peroxy radical intermediates; iii) then the peroxy radicals are capable of generating a bromine radical from in situ HBr and transformed to the corresponding aldehyde via a hypothetical intermediate.22 It is assumed that the oxidation goes through a bromine radical, but the further oxidation process is found to be restricted. This fact suggests that the catalyst BDMS provides a smart pathway which is yet to be explored. Interestingly, we observed a higher quantum yield value (43) for the formation of benzaldehyde from benzyl alcohol (Entry 1 in Table 2). Such high quantum yields are typically known for the product formation via photochemical chain reactions. Hence, the present reaction is also expected to involve a chain reaction via bromine radical step. Further, the quantum yield study can also be used as a support for the bromo-radical production and regeneration steps, presumed in the proposed mechanism (Scheme 6). To obtain further insight into the mechanism a detailed study is needed, which is beyond the objective of the present study.
Conclusions
In summary, we have demonstrated a simple photocatalytic route for selective oxidation of various benzylic alcohols to corresponding carbonyl compounds. The methodology developed is found to be highly efficient to yield the desired product with high yield (>90%). The methodology discussed here is environmentally benign as it involves an inexpensive and easy to handle reagent BDMS, a metal-free reagent. It also uses molecular oxygen as the oxidant and is promoted by visible light. Here, we made some understanding about the photocatalytic potential of the BDMS as well. We believe that in the near future, the work discussed in this study will find its suitability for a variety of applications in the field of photocatalysis and synthetic organic chemistry.Experimental section
General procedure for the bromodimethylsulfonium bromide (BDMS) mediated preparation of 4-chlorobenzyl bromide from 4-chlorobenzyl alcohol
In a 50 ml round-bottomed flask, 4-chlorobenzyl alcohol (1 mmol) and BDMS (1 mmol) were added into the solvent (25 ml of acetonitrile), and the reaction mixture was refluxed. The progress of the reaction was monitored by TLC. After 4 h the alcohol was found to be consumed, and the reaction was stopped. The organic solvent was evaporated by rotary evaporator. The crude mixture thus obtained was treated with saturated aqueous solution of NaHCO3; the organic part was extracted with dichloromethane, dried over anhydrous Na2SO4 and then filtered. The product was obtained after the evaporation of the filtrates by rotary evaporator. The bromo-product thus obtained, was found to be pure enough and characterized by 1H NMR and 13C NMR.General procedure for photocatalytic oxidation of benzylic alcohol under the visible irradiation
In a 30 ml pyrex glass tube 10 ml of acetonitrile was taken, and the tube was kept 5 cm away from a 45 W household white lamp (Philips CFL). The catalyst, BDMS (0.1 mmol) was added to the reaction vessel, and the solution was allowed to be irradiated and stirred under an oxygen atmosphere (oxygen balloon). After 15 min of irradiation, 1 mmol of benzylic alcohol was added to the reaction mixture, and the reaction mixture was irradiated under an oxygen atmosphere with stirring. The progress of the reaction was monitored by TLC. The irradiation was prolonged until the complete consumption of alcohol in the reaction mixture (6–10 h irradiation). The organic solvent was evaporated from the irradiated sample by rotary evaporator. The crude thus obtained was treated with saturated aqueous solution of NaHCO3; the organic part was extracted with dichloromethane, dried over anhydrous Na2SO4 and then filtered. The product was obtained after the evaporation of the filtrates by rotary evaporator. The oxidized product, the corresponding carbonyl compound thus obtained, was found to be pure enough and characterized by 1H NMR and 13C NMR. Intensity of the lamp (45 W CFL) used in this study was 2.98 × 1016 Einstein s−1, which was determined by standard actinometry.12,231H and 13C NMR spectral data
Acknowledgements
We thank the DST, New Delhi for NMR facility and our Department of Chemistry, Indian Institute of Technology Kharagpur for research support and instrumental facilities.References
- (a) A. Antinolo, F. C. Hermosilla, V. Cadierno, J. Alvarez and A. Otero, ChemCatChem, 2012, 4, 123–128 CrossRef CAS; (b) B. Singaram, M. V. Rangaishenvi and H. C. Brown, J. Org. Chem., 1991, 56, 1543–1549 CrossRef CAS; (c) A. Abad, C. Almela, A. Corma and H. Garcia, Tetrahedron, 2006, 62, 6666–6672 CrossRef CAS.
- (a) B. Loev and K. M. Snader, J. Org. Chem., 1965, 30, 1914–1916 CrossRef CAS; (b) G. C. Nandi, S. Samai and M. S. Singh, J. Org. Chem., 2010, 75, 7785–7795 CrossRef CAS; (c) M. Iwata, R. Yazaki, I. H. Chen, D. Sureshkumar, N. Kumagai and M. Shibasaki, J. Am. Chem. Soc., 2011, 133, 5554–5560 CrossRef CAS; (d) A. B. Northrup and D. W. C. MacMillan, J. Am. Chem. Soc., 2002, 124, 6798–6799 CrossRef CAS.
- (a) A. Dhakshinamoorthy, M. Alvaro and H. Garcia, ACS Catal., 2011, 1, 48–53 CrossRef CAS; (b) T. Balcha, J. R. Strobl, C. Fowler, P. Dash and R. W. J. Scott, ACS Catal., 2011, 1, 425–436 CrossRef CAS.
- (a) D. G. Lee and U. A. Spitzer, J. Org. Chem., 1970, 35, 3589–3590 CrossRef CAS; (b) M. Hudlicky, Oxidation in Organic Chemistry (ACS Monograph Series), American Chemical Society, Washington DC, 1990 Search PubMed; (c) S. V. Ley, A. Madfin, Comprehensive Organic Synthesis, Vol. 7 (ed.: B. M. Trost, I. Fleming, S. V. Ley), Pergamon, Oxford, 1991, pp. 251–289 Search PubMed; (d) P. V. Prabhakaran, S. Venkatachalam and K. N. Ninan, Eur. Polym. J., 1999, 35, 1743–1746 CrossRef CAS.
- K. Fujita, T. Yoshida, Y. Imori and R. Yamaguchi, Org. Lett., 2011, 13, 2278–2281 CrossRef CAS.
- R. A. Sheldon, I. W. C. E. Arends, G. J. Brink and A. Dijksman, Acc. Chem. Res., 2002, 35, 774–781 CrossRef CAS.
- (a) Y. Xie, W. Mo, D. Xu, Z. Shen, N. Sun, B. Hu and X. Hu, J. Org. Chem., 2007, 72, 4288–4291 CrossRef CAS; (b) C. X. Miao, L. N. He, J. L. Wang and F. Wu, J. Org. Chem., 2010, 75, 257–260 CrossRef CAS.
- (a) S. I. Murahashi, T. Naota and N. Hirai, J. Org. Chem., 1993, 58, 7318–7319 CrossRef CAS; (b) T. Iwahama, Y. Yoshino, T. Keitoku, S. Sakaguchi and Y. Ishii, J. Org. Chem., 2000, 65, 6502–6507 CrossRef CAS; (c) P. Liu, Y. Guan, R. A. Santen, C. Li and E. J. M. Hensen, Chem. Commun., 2011, 47, 11540–11542 RSC.
- (a) S. Yurdakal, G. Palmisano, V. Loddo, V. Augugliaro and L. Palmisano, J. Am. Chem. Soc., 2008, 130, 1568–1569 CrossRef CAS; (b) M. Zhang, Q. Wang, C. Chen, L. Zang, W. Ma and J. Zhao, Angew. Chem., Int. Ed., 2009, 48, 6081–6084 CrossRef CAS; (c) A. Tanaka, K. Hashimoto and H. Kominami, Chem. Commun., 2011, 47, 10446–10448 RSC; (d) C. Parmeggiani and F. Cardona, Green Chem., 2012, 14, 547–564 RSC.
- B. Karimi, A. Biglari, J. H. Clark and V. Budarin, Angew. Chem., Int. Ed., 2007, 46, 7210–7213 CrossRef CAS.
- (a) J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102–113 RSC; (b) D. Nicewicz and D. W. C. MacMillan, Science, 2008, 322, 77–80 CrossRef CAS; (c) C. Dai, J. M. R. Narayanam and C. R. J. Stephenson, Nat. Chem., 2011, 3, 140–145 CrossRef CAS; (d) A. G. Condie, J. C. G. Gomez and C. R. J. Stephenson, J. Am. Chem. Soc., 2010, 132, 1464–1465 CrossRef CAS; (e) M. A. Ischay, M. E. Anzovino, J. Du and T. P. Yoon, J. Am. Chem. Soc., 2008, 103, 12886–12887 CrossRef; (f) Z. Lu, M. Shen and T. P. Yoon, J. Am. Chem. Soc., 2011, 133, 1162–1164 CrossRef CAS; (g) D. A. Nagib and D. W. C. MacMillan, Nature, 2011, 480, 224–228 CrossRef CAS; (h) A. McNally, C. K. Prier and D. W. C. MacMillan, Science, 2011, 334, 1114–1117 CrossRef CAS; (i) M. Neumann, S. Fuldner, B. Konig and K. Zeitler, Angew. Chem., Int. Ed., 2011, 50, 951–954 CrossRef CAS; (j) K. Ohkubo, K. Mizushima, R. Iwata and S. Fukuzumi, Chem. Sci., 2011, 2, 715–722 RSC.
- (a) S. Gazi and R. Ananthakrishnan, Appl. Catal., B, 2011, 105, 317–325 CrossRef CAS; (b) R. Ananthakrishnan and S. Gazi, Catal. Sci. Technol., 2012, 2, 1463 RSC.
- J. M. Hoover and S. S. Stahl, J. Am. Chem. Soc., 2011, 133, 16901–16910 CrossRef CAS.
- (a) F. Minisci, O. Porta, F. Recupero, C. Punta, C. Gambarotti, M. Pierini and L. Galimberti, Synlett, 2004, 2203–2205 Search PubMed; (b) M. Uyanik, R. Fukatsu and K. Ishihara, Chem.–Asian J., 2010, 5, 456–460 CrossRef CAS; (c) A. Itoh, S. Hashimoto, K. Kuwabara, T. Kodama and Y. Masaki, Green Chem., 2005, 7, 830–432 RSC.
- S. Hirashima, S. Hashimoto, Y. Masaki and A. Itoh, Tetrahedron, 2006, 62, 7887–7891 CrossRef CAS.
- S. Hirashima and A. Itoh, Green Chem., 2007, 9, 318–320 RSC.
- L. H. Choudhury, T. Parvin and A. T. Khan, Tetrahedron, 2009, 65, 9513–9526 CrossRef CAS.
- A. T. Khan, Md. A. Ali, P. Goswami and L. H. Choudhury, J. Org. Chem., 2006, 71, 8961–8963 CrossRef CAS.
- A. T. Khan, T. Parvin, S. Gazi and L. H. Choudhury, Tetrahedron Lett., 2007, 48, 3805–3808 CrossRef CAS.
- A. T. Khan, T. Parvin and L. H. Choudhury, Eur. J. Org. Chem., 2008, 834–839 CrossRef CAS.
- N. Furukawa, T. Inoue, T. Aida and S. Oae, J. Chem. Soc., Chem. Commun., 1973, 212 RSC.
- Y. M. Zhong, H. C. Ma, J. X. Wang, X. J. Jia, W. F. Li and Z. Q. Lei, Catal. Sci. Technol., 2011, 1, 927–931 CAS.
- C. G. Hatchard and C. A. Parker, Proc. R. Soc. London, Ser. A, 1956, A235, 518–536 CrossRef.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental procedure for lamp intensity measurement by actinometry, 1H NMR and 13C NMR spectra of the compounds. |
| This journal is © The Royal Society of Chemistry 2012 |

