Hard carbon: a promising lithium-ion battery anode for high temperature applications with ionic electrolyte
Honghe
Zheng
*ab,
Qunting
Qu
a,
Li
Zhang
a,
Gao
Liu
b and
Vincent S.
Battaglia
b
aSchool of Energy, Soochow University, Suzhou, Jiangsu 215006, P. R. China. E-mail: hhzheng66@yahoo.com.cn
bLawrence Berkeley National Laboratory, 1 Cyclotron Rd, Berkeley, CA 94720, USA
First published on 23rd March 2012
Abstract
Electrochemical behavior of a hard carbon plate in an electrolyte based on a room temperature ionic liquid consisting of trimethyl-n-hexylammonium (TMHA) cation and bis(trifluoromethanesulfone) imide (TFSI) anion was investigated. Hard carbon is found to be less prone to passivation due to the high electrochemical stability of the ionic liquid. Lithiation and de-lithiation of the carbon anode is strongly affected by temperature. At room temperature, the hard carbon is difficult to lithiate and a high potential hysteresis is observed between charge and discharge curves. Increasing temperature contributes to higher reversible capacity, higher coulombic efficiency, and lower potential hysteresis. At 80 °C, a reversible capacity of 16.2 mAh cm−2 (equivalent to 675.0 mAh g−1) was obtained with 73.6% of the first cycle coulombic efficiency. Considering that graphitic anodes do not work at this temperature due to the decomposition of the solid electrolyte interphase (SEI), the combination of hard carbon with ionic electrolyte can meet some special requirements for high temperature applications with improved safety. The activation energy for Li-ion transfer across the hard carbon/ionic electrolyte interface was measured by ac impedance spectroscopy; a value of 80 ± 5 kJ mol−1 was obtained. The large activation energy implies a high barrier of activation at the electrode/electrolyte interface, which accounts for the poor rate performance of the hard carbon anode in the ionic electrolyte.
1. Introduction
Carbonaceous materials have been extensively studied for use as negative electrodes in lithium-ion batteries.1 Among them, graphitic anodes are widely used in commercial lithium ion cells owing to their low working potential, high reversible capacity, good rate performance, and superior cycling behavior.2–5 Other carbonaceous materials have also been studied to enhance the performance of lithium-ion batteries. Non-graphitizable (“hard”) carbons, as possible alternatives to graphitic carbon materials, have shown advantages of even higher reversible capacity and better cycling performance.6–8 However, this kind of material suffers from some major deficiencies: large irreversible capacity in the first cycle and high potential hysteresis between charge and discharge. Intercalation and de-intercalation of lithium into and from hard carbon in conventional organic electrolyte has been extensively investigated. Researchers have correlated the electrochemical characteristics with their micro-structures to find the insertion mechanism of Li into hard carbons. However, to our knowledge, few studies relating to the electrochemical behavior of hard carbon in electrolytes based on pure room temperature ionic liquids (RTILs) have been reported.RTILs are known as green solvents having the advantages of non-flammability, high thermal and electrochemical stability, wide liquid phase range, negligible vapor pressure, high conductivity, and easy recycling.9–13 The new functional, non-aqueous liquid systems are believed to allow for the electrochemical intercalation of lithium into carbon materials to meet special requirements for chemical and industrial purposes.14,15 Ionic liquids with very stable cations such as tetraalkyl ammonium, pyrrolidinium, and piperidinium cations have wide electrochemical windows (often more than 5 V). Electrochemical intercalation of lithium into graphitic carbon anodes in these ionic liquids has shown promising prospects.16–19 Graphite anodes were found less prone to being passivated in the ionic electrolyte. Intercalation of the cations of the ionic liquids into the graphene interlayer occurs prior to Li intercalation, which can lead to blocking of the layered material. Graphite anodes are very hard to be lithiated in these pure ionic solutions. To solve this problem, organic film-forming additives have to be used to form a stable solid electrolyte interphase (SEI) to prevent the intercalation of ionic liquid cations.16–19 There is no doubt that addition of inflammable organic solvents spoils the non-flammability property of the ionic electrolyte. From this point of view, investigation of carbon anodes in pure ionic liquids is of great significance to improve the safety of lithium-ion batteries. However, up to today, no satisfactory results have been achieved for graphitic anodes in pure ionic electrolytes.
Hard carbon materials have no graphitic structure; structural exfoliation is not aroused by solvent intercalation. Therefore, the electrochemical properties of this kind of carbon material are less sensitive to electrolyte compositions.20,21 Film-forming additives are not always necessary for hard carbon, even in conventional organic electrolytes. In this study, the electrochemical behavior of lithiation and de-lithiation for a hard carbon in an ammonium-based ionic liquid containing 1.0 M Li salt was examined under different temperatures. Good electrochemical performance was obtained under 80 °C, at which graphitic anodes do not work due to the decomposition of SEI film. It shows that hard carbon in a pure ionic liquid is promising for some special uses requiring high temperatures and high safety. Li-ion transfer at the interface between the hard carbon electrode and the ionic electrolyte was determined. A high activation barrier for interfacial Li-ion transfer accounts for the poor rate performance of the electrode.
2. Experimental
Trimethylhexylammonium bistrifluoro-methane sulfonylimide (TMHA-TFSI) ionic liquid was prepared according to previous reports.22,23 The obtained sample was purified and dehydrated before use. Water content of the TMHA-TFSI sample was determined to be less than 20 ppm. 1.0 M LiTFSI/TMHA-TFSI ionic electrolyte was prepared by dissolving 1.0 M LiTFSI as supporting electrolyte. A hard carbon plate prepared from phenolic resin at 2273 K (GC-20) with a thickness of around 160 μm was obtained from Tokai Carbon Co., Ltd., Japan. The material features are amorphous structure with an apparent density of 1.51 g cm−3 and an electrical conductivity of 238.1 S cm−1. Electrochemical windows of the ionic liquid were measured by cyclic voltammetry. The GC-20 hard carbon was employed as the working electrode. Lithium foil was used as both counter and reference electrode. The electrode was polarized in the potential range of 0 to 5 V vs. Li/Li+ with a scan rate of 0.1 mV s−1. The temperature was controlled between 20 and 80 °C with a precision of ± 0.5 °C.Conductivity of the ionic solutions was measured by the ac impedance method in the frequency range of 10 MHz to 100 Hz, using a conductivity cell (cell constant = 1.02). The cell was calibrated with a 0.1 M KCl solution. The temperature was controlled with a thermostatic oil bath and the estimated error of experimental conductivity is ± 1%. Thermal analysis of the 1.0 M LiTFSI/TMHA-TFSI ionic electrolyte was conducted by a thermal gravimetric analysis (TGA). The analysis of the sample was performed in an alumina (Al2O3) pan with a heating rate of 5 °C min−1 under a N2 atmosphere.
Three electrode cells were assembled in an argon-filled glove box with a dew point below −80 °C. The effective electrode surface area was 1.0 cm2 as limited by an O-ring. Li metal plate was used as the counter and reference electrodes. Electrochemical behavior of the hard carbon plate was investigated by cyclic voltammetry (CV) and galvanostatic charge-discharge tests at different temperatures. Cyclic voltammetry of the graphite electrode was conducted at the scan rate of 0.1 mV s−1 over the potential range of 0 to 3 V vs. Li/Li+. Galvanostatic charge and discharge tests were conducted at a current density of 0.4 mA cm−2 between 0 and 2 V vs. Li/Li+. Rate performance of the anode at 80 °C consisted of full discharges at 0.4, 1.0, 2.0, and 5.0 mA cm−2, respectively. A charge of 0.4 mA cm−2 to 0 V preceded each discharge.
Fourier transform infrared spectroscopy (FTIR) measurements of the hard carbon anode were conducted by using both the transmittance (hard carbon powders pelletized with KBr) and diffuse reflectance modes using a Bio-RAD-FTS-60V spectrometer. After the first charge-discharge test in the ionic liquid at each temperature, the hard carbon plates were thoroughly washed with DEC solvent under an argon atmosphere and then dried under vacuum for 2 h. The carbon plates were then ground into fine particles and mixed with high-quality KBr (Sigma) with a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. The pellets were pressed under argon atmosphere and then quickly transferred to the spectrometer's measurement chamber.
100. The pellets were pressed under argon atmosphere and then quickly transferred to the spectrometer's measurement chamber.
Li-ion transfer at the interface between the hard carbon electrode and the ionic electrolyte was investigated by using a Sorlatron 1255 with a three-electrode cell. After formation cycles between 0 and 2.0 V, the electrode was swept to a potential of 800 mV and kept at that potential for at least 3 h to reach a steady-state. The impedance spectra were then determined with an amplitude of 5 mV over a frequency range from 100 kHz to 10 mHz under different temperatures.
3. Results and discussion
Electrochemical stability of the TMHA-TFSI ionic liquid against the hard carbon plate was investigated by the cyclic voltammetry method. As shown in Fig. 1, TMHA-TFSI ionic liquid used in this study is fairly stable against hard carbon from 0 to 5 V at room temperature. No significant reduction or oxidation reaction is observed in the investigated potential range. With increasing temperature, a cathodic peak at around 0 V and an anodic peak at around 5 V begin to appear. When the temperature was increased to 80 °C, two cathodic peaks were observed at around 0.8 V and 0 V vs. Li/Li+. There are a couple of reasons associated with the cathodic peaks under high temperature. One is attributed to the intercalation TMHA cations into the carbon anode. There is no doubt, TMHA intercalation into the hard carbon in the cathodic process is intensified with temperature rise. The small anodic current peak at around 4 V could be related to the deintercalation of the TMHA cations from the hard carbon. However, considering the peak intensity is fairly low (less than 0.1 mA cm−2), the intercalation and de-intercalation of the TMHA cations into and from the hard carbon, if true, is almost negligible. The other one reason may be related to the reduction reaction of the ionic liquid at the carbon surface. Considering the good stability of the ionic liquid against graphite, Al, and Pt, which was demonstrated in our previous studies,22,23 the reduction of the ionic liquid at the hard carbon surface is less probable. Overall, the CV result shows that the ionic liquid is quite stable against hard carbon. No significant reduction or oxidation reactions are observed in the 0–5 V range, even up to 80 °C. This result is consistent with the literature in which glassy carbon is often employed as an electrode to evaluate the stability of ionic liquids.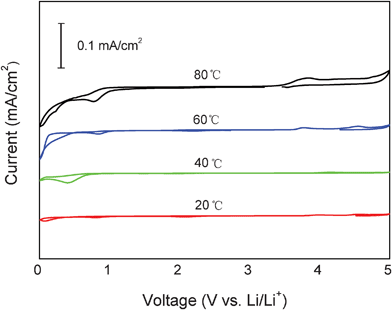 | ||
| Fig. 1 Electrochemical windows of the TMHA-TFSI ionic liquid against the hard carbon plate under different temperatures at a scan rate of 0.1 mV s−1. | ||
It is worth noting that there is a strong reduction current for the ionic liquid on acetylene black surface at potentials below 0.75 V vs. Li/Li+,23 indicating that the ionic liquid may be reduced on acetylene black surface. In this sense, acetylene black, which is widely employed in the Li-ion battery industry, is not particularly suitable for use as a conductive additive in the anode when it is combined with the ionic electrolyte, especially under high temperatures. For this reason, a hard carbon plate was selected as the electrode in order to avoid interference from the conductive additive and polymeric binder. In addition, hard carbon plates are known to have good ionic and electronic conductivity, which is particularly suited for use in electrochemical systems.
Fig. 2 shows the TGA curves of TMHA-TFSI containing 1.0 M LiTFSI from 25 to 500 °C. It is seen that the ionic electrolyte is stable up to 270 °C and decomposed rapidly at 350 °C. Compared with conventional organic electrolyte, the ionic electrolyte is very stable and safe for use as electrolytes for electrochemical devices over a wide range of temperatures. With advantages of nonflammability, high thermal and electrochemical stability, and wide liquid phase range, the ionic electrolyte will have a low heat of reaction during electrochemical cycles compared to conventional organic electrolytes. In this sense, safety should not be the major issue for an electrochemical system based on a combination of hard carbon and pure ionic electrolyte.24–27
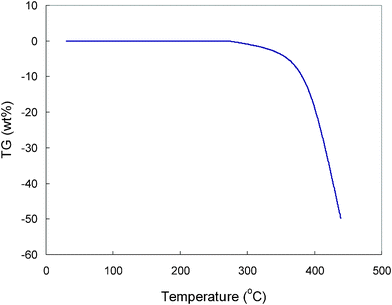 | ||
| Fig. 2 TGA curve of the TMHA-TFSI ionic liquid containing 1.0 M LiTFSI salt. | ||
Electrolyte conductivity is an important factor relating to Li diffusion within the electrolyte. The variation of ionic conductivity for TMHA-TFSI without and with 1.0 M LiTFSI salt is displayed in Fig. 3. At room temperature (30 °C), 14.1 × 10−4 S cm−1 was measured for the pure TMHA-TFSI ionic liquid. LiTFSI salt addition significantly decreases the conductivity of the solution. Addition of 1.0 M LiTFSI salt brought the conductivity down to 2.52 × 10−4 S cm−1 at the same temperature. This indicates a strong interaction between ions within the 1.0 M TFSI/TMHA-TFSI ionic electrolyte.
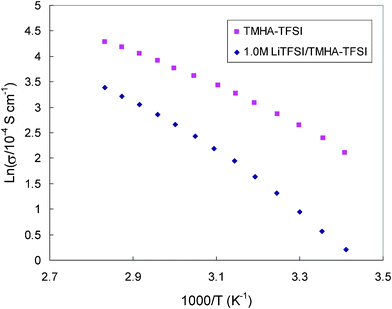 | ||
| Fig. 3 Arrhenius plots between the ionic conductivity of TMHA-TFSI ionic liquid without and with 1.0 M LiTFSI salt and 1/T. | ||
As expected, conductivity of the ionic electrolyte is strongly dependant on temperature. Temperature dependence of the ionic conductivity for the ionic solution doesn't obey the Arrhenius equation very well. Slightly downward curvature reflects its Vogel–Tamman–Fulcher (VTF) behavior, σ = AT1/2 exp[−B/(T−T0)]. Roughly, the conductivity activation energies determined from the Arrhenius fits can be calculated from the angle of the plots. Activation energies of 34 ± 5 and 49 ± 5 kJ mol−1 were obtained for the TMHA-TFSI ionic liquid without and with 1.0 M LiTFSI salt, respectively. The activation energy of conductivity for LiTFSI/TMHA-TFSI ionic electrolyte is significantly higher than the ca. 20 kJ mol−1 typically obtained from conventional organic electrolyte, reflecting a high resistance for Li ion migration within the ionic electrolyte.
Fig. 4 shows cyclic voltammograms of the hard carbon in the 1.0 M LiTFSI/THMA-TFSI ionic electrolyte. The voltammograms were measured with a sweep rate of 0.1 mV s−1 in the potential range between 0 and 3.0 V vs. Li/Li+. As seen in the figure, a cathodic current was observed below 0.9 V, which is ascribed to Li-ion intercalation into the hard carbon electrode. A corresponding anodic current identified as Li-ion extraction appeared in the following reverse sweep. Intensity of both the cathodic and the anodic peaks is strongly temperature dependent. At 30 °C, the intensity of the cathodic peak and the subsequent anodic peak is very small. It reveals that the non-graphitizable carbon is difficult to lithiate. With increasing temperature, both the cathodic and the anodic peaks are significantly intensified. At 80 °C, Li intercalation in the cathodic sweep and the subsequent lithium extraction in the anodic sweep are the predominant reactions. The intensity of the cathodic peak near 0 V is 20 times as strong as that of the cathodic peak obtained from the polarization of the hard carbon in the TMHA-TFSI ionic liquid without LiTFSI at the same scan-rate under 80 °C. It indicates that the predominant cathodic reaction originates from the interaction between hard carbon and the Li ions in the electrolyte. The subsequent strong anodic peak at around 0.7 V (Fig. 3), characteristic of the lithium ion de-intercalation, supports the theory that the main reaction in the cathodic process is Li intercalation, not the irreversible side reaction or TMHA intercalation. Also, it should be noted that, with increasing temperature, the anodic peak potential corresponding to the de-intercalation of Li ion from the hard carbon shifts toward 0 V, indicating a decrease in over-potential. An increase of the area ratio between the anodic and the cathodic peaks towards 1 implies the increase of the coulombic efficiency of the electrochemical cycle. All of the information obtained from the CV study shows that the hard carbon can be lithiated and de-lithiated at high temperature. The reversible capacity and coulombic efficiency can be enhanced and the over-potential can be decreased by elevating temperature.
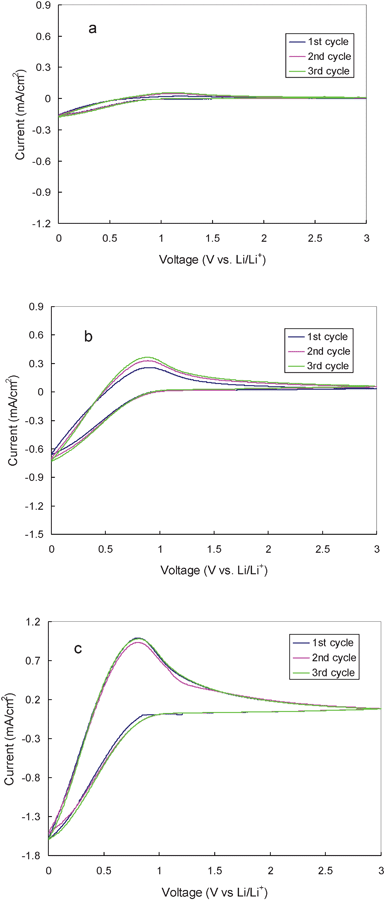 | ||
| Fig. 4 Cyclic voltammogram of the hard carbon in 1.0 M LiTFSI/TMHA-TFSI ionic electrolyte under different temperatures (a: 30 °C, b: 50 °C, c: 80 °C) at a scan rate 0.1 mV s−1 | ||
Charge-discharge profiles of the hard carbon anode in the 1.0 M LiTFSI/TMHA-TFSI ionic electrolyte at different temperatures are displayed in Fig. 5. Clearly, the charge-discharge capacity and efficiency are strongly affected by temperature. At 30 °C, the first charge capacity is 1.85 mAh cm−2 (equivalent to 77.1 mAh g−1) and the first discharge capacity is only 0.49 mAh cm−2 (equivalent to 20.4 mAh g−1). As a result, the first cycle coulombic efficiency is only 26.5%. Although lithiation and de-lithiation of the hard carbon in the ionic electrolyte is not denied, the electrochemical reaction is very weak and most of the charged capacity was not released. The charge-discharge process exhibits very low reversible capacity, low coulombic efficiency, and severe voltage hysteresis. When the temperature was increased to 50 °C, both the capacity and the first cycle coulombic efficiency are considerably improved. The first charge capacity is increased to 6.58 mAh cm−2 (equivalent to 274.2 mAh g−1) and the first discharge capacity is 2.55 mAh cm−2 (equivalent to 106.2 mAh g−1). Correspondingly, the first cycle coulombic efficiency is improved to 38.8%. Further increasing temperature contributes to even high electrochemical performance of the hard carbon anode. At 80 °C, the first charge capacity is obtained to be 22.0 mAh cm−2 (equivalent to 916.7 mAh g−1) and the first discharge capacity is increased to 16.2 mAh cm−2 (equivalent to 675.0 mAh g−1). As a result, the first coulombic efficiency is 73.6%. The voltage hysteresis between charge and discharge is greatly reduced and more flattened charge-discharge profiles are observed.
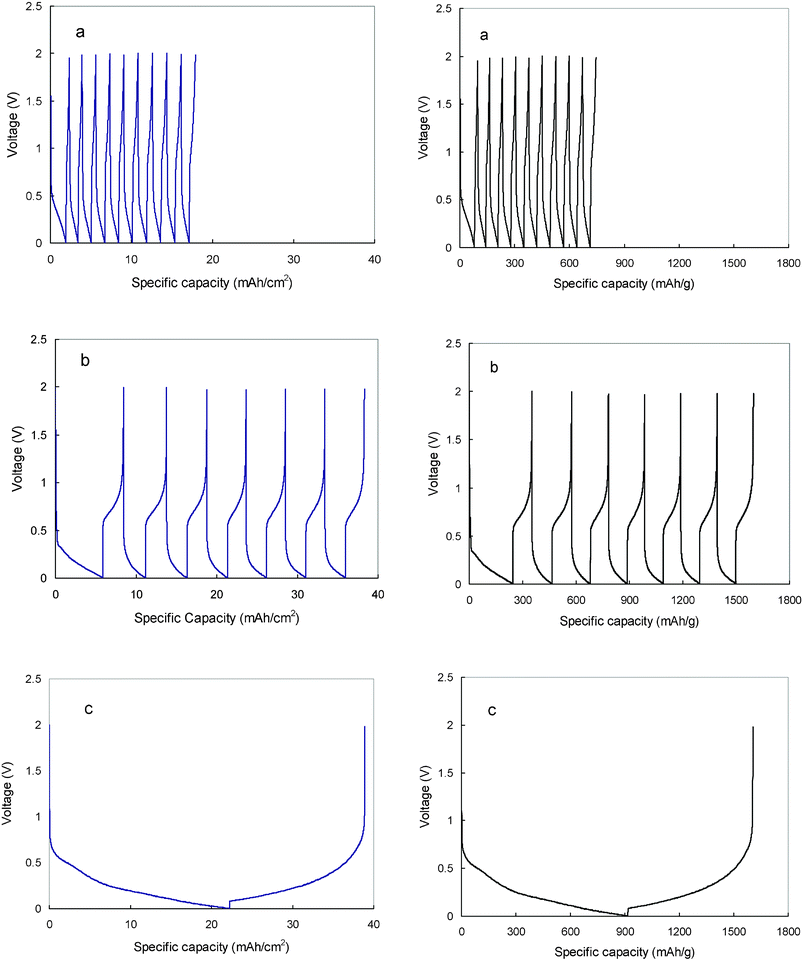 | ||
| Fig. 5 Chronopotential profiles of the hard carbon plate in 1.0 M LiTFSI/TMHA-TFSI ionic electrolyte under different temperatures (a: 30 °C, b: 50 °C, c: 80 °C) at a current density of 0.4 mA cm−2. | ||
Irreversible capacity is one of the most important factors preventing the widespread commercialization of hard carbon for Li-ion batteries. For hard carbon in conventional organic electrolyte, a plateau at around 0.8 V is always observed, which is ascribed to the formation of solid electrolyte interface (SEI) film through the decomposition of electrolyte. By observation, no noticeable plateau at 0.8 V is detected in this study, implying that the electrolyte is not reduced in the first charge. In this sense, the irreversible capacity is not the result of decomposition of the electrolyte as is generally believed. The irreversible capacity loss is more likely to be associated with the irreversible Li capture or absorption within the hard carbon in the first cycle. As we understand, there are five different kinds of lithium storage mechanisms for hard carbons.28–30 These include: (I) absorption of Li at the surface site of cluster and disordered hexagonal planes; (II) Li intercalation into hexagonal clusters; (III) Li accumulation in cluster gap between edges of carbon hexagon clusters; (IV) Li capture in the microvoid surrounded by the hexagonal plane; and (V) Li capture in the atomic defect in heteroatom void. Generally, Li storage mechanism of hard carbon has more of adsorption and capture character than the intercalation. Except for the intercalated Li into graphene inter-layers, the captured Li by other different mechanisms can not be fully released in the first cycle. From this, most of the irreversible capacity should be ascribed to the Li capture or absorption within the hard carbon. In addition, we noticed that the some of the irreversible capacity (trapped lithium) can be reversibly discharged by elevating temperature. It supports the claim that the irreversible Li is not in the form of Li salts, as it is in conventional organic electrolytes.
The galvanostatic charge-discharge test shows that, although the hard carbon plate is difficult to lithiate in the ionic electrolyte at room temperature, lithiation/de-lithiation is the dominant reaction of the hard carbon in the quaternary ammonium-based ionic electrolyte during electrochemical cycles. The reversible capacity and the first coulombic efficiency can be improved by elevating the temperature. At 80 °C, the hard carbon anode delivers a capacity of 16.2 mAh cm−2 at 0.4 mA cm−2 with the first coulombic efficiency of 73.6%. This result is attractive as the commercial graphite anodes for lithium ion cells deliver no more than 4–6 mAh cm−2 capacity at 0.4 mA cm−2 current depending on the electrode thickness. Considering that graphitic anodes in conventional organic electrolytes will be completely destroyed at 60 to 80 °C due to the decomposition of SEI components,31,32 hard carbon with the pure ionic electrolyte is a promising use at high temperatures. In addition, the safety of the cell is greatly improved as the electrolyte is stable and non-flammable.
Fig. 6 shows the FTIR spectra between wave numbers 400 and 2000 cm−1 obtained from the hard carbon after being cycled in 1.0 M LiTFSI/TMHA-TFSI ionic liquid at different temperatures. Few common discernable symmetrical and asymmetrical infrared vibrations are observed for the hard carbon sample cycled at 30 °C. The only typical peak, appearing at 867 cm−1, may be assigned to C–H vibrations. After cycling at high temperature, a new pronounced peak appeared at 1200 cm−1. This peak is known to be associated with the TMHA cations.33 Other typical peaks relating to SEI components such as Li2CO3 are not observed, even after the electrode was cycled at 80 °C. This indicates that the weak side reaction of the hard carbon anode in the ionic electrolyte at high temperature (as shown in Fig. 1) is more likely to be ascribed to TMHA intercalation, rather than the ionic liquid decomposition.
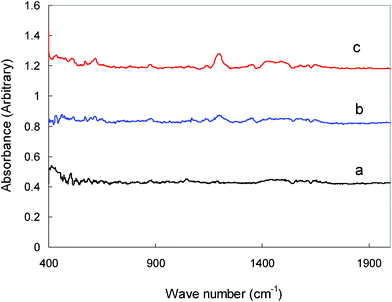 | ||
| Fig. 6 FTIR spectra of the hard carbon plate after electrochemical cycles in 1.0 M LiTFSI/TMHA-TFSI ionic electrolyte under different temperatures (a: 30 °C, b: 50 °C, c: 80 °C). | ||
The FTIR study confirms that the hard carbon is less prone to passivation in the ionic liquid. Nevertheless, high reversible capacity can still be obtained, which demonstrates that the formation of an SEI is not a necessary condition for the cycling of hard carbon in the ionic electrolyte. This is because hard carbon has no graphitic interlayer structure. It still works well without the protection of a compact SEI film produced from the reduction reaction of electrolyte components. For this reason, the hard carbon anode doesn't fail due to SEI decomposition as graphite anodes do at higher temperatures. This advantage of hard carbon in pure ionic electrolyte makes it possible for use to meet some special requirements under high temperatures with improved safety.
Cycling properties of the hard carbon anode in 1.0 M LiTFSI/TMHA-TFSI ionic electrolyte under different temperature are displayed in Fig. 7. As can be distinguished from the initial 10 cycles, cycling behavior of the hard carbon is quite stable. Although there is a capacity fade at 80 °C, the cycling capability is still satisfactory. The slight capacity decline with increasing electrochemical cycles at high temperature may be attributed to the TMHA intercalation as discussed above. It is known that the intercalated TMHA cations have a blocking effect on the migration of Li ion into the electrode.34 In this sense, preventing TMHA cation intercalation is also of great significance for hard carbon anode to stabilize its capacity and prolong its cycle life, which needs thorough and in-depth study in the future.
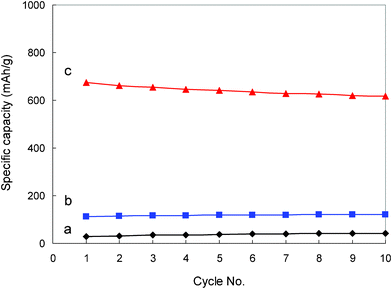 | ||
| Fig. 7 Cycling performance of the hard carbon anode in 1.0 M LiTFSI/TMHA-TFSI ionic electrolyte under different temperatures (a: 30 °C, b: 50 °C, c: 80 °C) at a current of 0.4 mA cm−2. | ||
Rate capability of the hard carbon in the electrolyte at 80 °C is shown in Fig. 8. As expected, the discharge capacity depends strongly on discharge rate. The rapid increase of the discharge curve to a high voltage level at higher current is attributed to the severe polarization of the cell. By comparison, rate performance of the hard carbon in the ionic electrolyte seems to be worse than most of the reported results for hard carbon in conventional organic electrolytes.7,8 At 1 mA cm−2 current, almost half of the reversible capacity is lost. The most important reason for the poor rate performance is associated with the carbon plate nature of the electrode. The electrolyte cannot penetrate into the electrode and the specific area of the electrode directly contacted with the electrolyte is very low. This leads to a very high real current density at the electrode surface even at relatively low rate. In addition, the extremely long Li-ion diffusion length is also a factor affecting the rate capability of the electrode. However, the two factors can be overcome by using a thinner hard carbon plate.
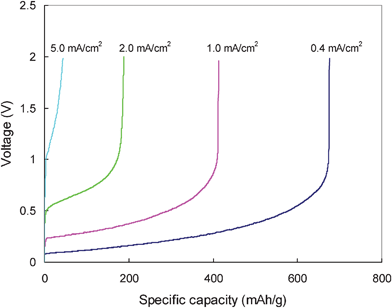 | ||
| Fig. 8 Rate capability of the hard carbon plate in 1.0 M LiTFSI/TMHA-TFSI ionic electrolyte at 80 °C. | ||
To explain the strong temperature effect on some of the electrochemical behavior, the charge transfer of the hard carbon redox reaction was investigated with EIS. Fig. 9 shows the influence of temperature on the Nyquist plots of the hard carbon after formation at 800 mV vs. Li/Li+. According to the literature, the high frequency intercept with the abscissa refers to the total amount of ohmic resistance of the cell, particularly electrolyte resistance. The large semicircle is attributed to the charge transfer process at the hard carbon/ionic electrolyte interface.35,36 The magnitude of the charge transfer resistance (Rct) is observed to be strongly dependent on the electrode potential and cell temperature. At a fixed potential of 800 mV, the high frequency limit shifts rapidly in the negative direction on the real axis illustrating the decrease in ohmic resistance of the cell with increasing temperature. The shrinking of the semicircle with increasing temperature is indicative of the decrease in charge transfer resistance. The total internal resistance obtained is several times larger than that found in the traditional organic electrolyte used in Li-ion cells. It is known that the electrolyte resistance through the separator and contact resistance contribute to a large fraction of the ohmic potential drop while the charge transfer resistance may control the rate of lithium insertion. The high internal resistance is thus an important factor responsible for the poor electrochemical performance of the hard carbon in the ionic electrolyte, especially at low temperature.
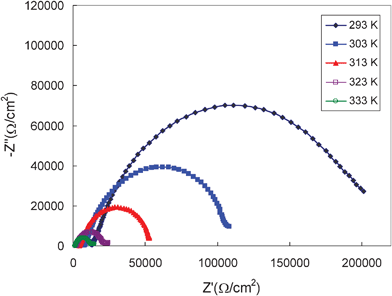 | ||
| Fig. 9 Nyquist plots of the hard carbon plate measured in 1.0 M LiTFSI/TMHA-TFSI ionic electrolyte at different temperatures at 800 mV vs. Li/Li+. | ||
The activation energy for the interfacial Li ion transfer at 800 mV vs. Li/Li+ was measured from the temperature dependence of the charge transfer resistances as shown in Fig. 10. Good linearity of the Arrhennius plot between log(1/Rct) and 1/T is observed. The apparent activation energy of the charge transfer can be calculated from the slope of the plot. As a result, a value of 80 ± 5 kJ mol−1 was obtained. The activation energy is much higher than those obtained for a graphite/organic electrolyte system. At a graphite electrode/EC-based electrolyte interface, the activation energy was measured to be no more than 60 kJ mol−1.37 Compared with the reported activation energy of lithium ion transfer at hard carbon or graphite interface in conventional organic liquid electrolyte (40–60 kJ mol−1),38–40 this value of activation energy is significantly high. The large activation energy implies that high energy barrier exists for lithium ion transfer at the interface between the hard carbon and the ionic electrolyte. For the Li ion transfer from the electrolyte to the bulk hard carbon electrode, the energy barrier should be related to the properties of the electrolyte, electrode/electrolyte interface and the Li-ion transport within the electrode. The high activation energy of the Li+ transport in the electrolyte (as shown in Fig. 3) plays a role in the high barrier of Li transfer at the electrolyte/electrode interface. In addition to bulk transport properties, the electrode surface condition and the wettability of the electrode by the electrolyte also considerably influences the Li migration through the electrode/electrolyte interface. As has been pointed out by Ogumi et al.,35,36 the high activation energy for Li ion charge transfer may be reduced by surface modification of the electrode, as the surface structure may greatly affect the Li-ion transfer kinetics at the electrode/electrolyte interface. From this point of view, the electrochemical performance of hard carbon can be improved by reducing the Li ion charge transfer resistance and the activation energy.
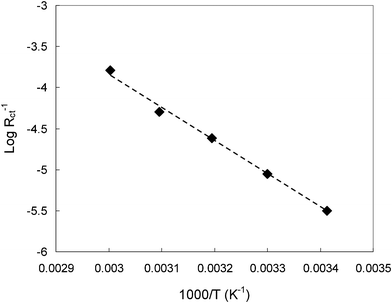 | ||
| Fig. 10 Temperature dependence of the hard carbon electrode impedance measured in 1.0 M LiTFSI/TMHA-TFSI ionic electrolyte at 800 mV. | ||
4. Conclusion
Lithiation and delithiantion of a hard carbon plate in TMHA-TFSI ionic electrolyte containing 1.0 M LiTFSI salt was investigated at different temperatures. At room temperature, the system shows a lot of polarization and the charge-discharge profile shows a large potential hysteresis. The polarization as well as the high first cycle irreversible capacity loss can be reduced by moving to higher operating temperatures. The electrode delivered a capacity of 675.0 mAh g−1 with a first cycle coulombic efficiency of 73.6% at 80 °C. Considering that graphitic carbon anodes do not work at this temperature due to the instability of SEI components, the hard carbon/ionic electrolyte combination can meet some special needs that require operation at higher temperatures with enhanced safety. In comparison to those carbon anodes in conventional electrolyte, the hard carbon works well without the protection of SEI film formed by the decomposition of electrolyte. The irreversible capacity loss seems more likely to be related to Li adsorption and capture at the first charge. This result may break new ground toward the development of Li-ion systems for and industrial purposes that required enhanced safety. A large activation energy of ca. 80 kJ mol−1 was obtained at the electrode/electrolyte interface, which partially accounts for the poor rate performance of the hard carbon in the ionic electrolyte.Acknowledgements
The authors are greatly indebted to the funding of Natural Science Foundation of China (NSFC, contract no. 21073129) and the Department of Science and technology of China for the 863 project (2009AA03Z225863).References
- M. Winter, J. O. Besenhard, M. E. Spahr and P. Nova'k, Insertion electrode materials for rechargeable lithium batteries, Adv. Mater., 1998, 10(10), 725–763 CrossRef CAS.
- N. Ohta, K. Nagaoka, K. Hoshi, S. Bitoh and M. Inagaki, Carbon-coated graphite for anode of lithium ion rechargeable batteries: Graphite substrates for carbon coating, J. Power Sources, 2009, 194(2), 985–990 CrossRef CAS.
- N. Ohta, T. Sogabe and K. Kuroda, A novel binder for the graphite anode of rechargeable lithium ion batteries for the improvement of reversible capacity, Carbon, 2001, 39(9), 1434–1436 CrossRef CAS.
- C.-K. Back and J. Prakash, Consideration of carbon structure effect on thermal stability of carbon anode for Li ion rechargeable batteries, Thermochim. Acta, 2011, 520(1–2), 93–98 CrossRef CAS.
- S. Komaba, M. Watanabe, H. Groult and N. Kumagai, Alkali carbonate-coated graphite electrode for lithium-ion batteries, Carbon, 2008, 46(9), 1184–1193 CrossRef CAS.
- H. Fujimoto, K. Tokumitsu, A. Mabuchi, N. Chinnasamy and T. Kasuh, The anode performance of the hard carbon for the lithium ion battery derived from the oxygen-containing aromatic precursors, J. Power Sources, 2010, 195(21), 7452–7456 CrossRef CAS.
- Y. Ohzawa, T. Suzuki, T. Achiha and T. Nakajima, Surface-modification of anode carbon for lithium-ion battery using chemical vapor infiltration technique, J. Phys. Chem. Solids, 2010, 71(4), 654–657 CrossRef CAS.
- F. Béguin, F. Chevallier, C. Vix-Guterl, S. Saadallah, V. Bertagna, J. N. Rouzaud and E. Frackowiak, Correlation of the irreversible lithium capacity with the active surface area of modified carbons, Carbon, 2005, 43(10), 2160–2167 CrossRef.
- J. Jin, H. H. Li, J. P. Wei, X. K. Bian, Z. Zhou and J. Yan, Li/LiFePO4 batteries with room temperature ionic liquid as electrolyte, Electrochem. Commun., 2009, 11(7), 1500–1503 CrossRef CAS.
- A. Fernicola, F. Croce, B. Scrosati, T. Watanabe and H. Ohno, LiTFSI-BEPyTFSI as an improved ionic liquid electrolyte for rechargeable lithium batteries, J. Power Sources, 2007, 174(1), 342–348 CrossRef CAS.
- S. Fang, Y. Tang, X. Tai, L. Yang, K. Tachibana and K. Kamijima, One ether-functionalized guanidinium ionic liquid as new electrolyte for lithium battery, J. Power Sources, 2011, 196(3), 1433–1441 CrossRef CAS.
- T. Sugimoto, Y. Atsumi, M. Kikuta, E. Ishiko, M. Kono and M. Ishikawa, Ionic liquid electrolyte systems based on bis(fluorosulfonyl)imide for lithium-ion batteries, J. Power Sources, 2009, 189(1), 802–805 CrossRef CAS.
- J. Wang, S. Y. Chew, Z. W. Zhao, S. Ashraf, D. Wexler, J. Chen, S. H. Ng, S. L. Chou and H. K. Liu, Sulfur–mesoporous carbon composites in conjunction with a novel ionic liquid electrolyte for lithium rechargeable batteries, Carbon, 2008, 46(2), 229–235 CrossRef CAS.
- K. Tsunashima, A. Kawabata, M. Matsumiya, S. Kodama, R. Enomoto, M. Sugiya and Y. Kunugi, Low viscous and highly conductive phosphonium ionic liquids based on bis(fluorosulfonyl)amide anion as potential electrolytes, Electrochem. Commun., 2011, 13(2), 178–181 CrossRef CAS.
- S. Arimoto, M. Sugimura, H. Kageyama, T. Torimoto and S. Kuwabata, Development of new techniques for scanning electron microscope observation using ionic liquid, Electrochim. Acta, 2008, 53(21), 6228–6234 CrossRef CAS.
- Y. Katayama, M. Yukumoto and T. Miura, Electrochemical intercalation of lithium into graphite in room-temperature molten salt containing ethylene carbonate, Electrochem. Solid-State Lett., 2003, 5, A96–97 CrossRef.
- H. Zheng, B. Li, Y. Fu, T. Abe and Z. Ogumi, Compatibility of quaternary ammonium-based ionic liquid electrolytes with electrodes in lithium ion batteries, Electrochim. Acta, 2006, 52(4), 1556–1562 CrossRef CAS.
- H. Zheng, K. Jiang, Y. Fu, T. Abe and Z. Ogumi, Electrochemical intercalation of lithium into a natural graphite anode in quaternary ammonium-based ionic liquid electrolytes, Carbon, 2006, 44(2), 203–210 CrossRef CAS.
- M. Holzapfel, C. Jost, A. Prodi-Schwab, F. Krumeich, A. Wursig, H. Buqa and P. Novak, Stabilisation of lithiated graphite in an electrolyte based on ionic liquids: an electrochemical and scanning electron microscopy study, Carbon, 2005, 43, 1488–1498 CrossRef CAS.
- S. Flandrois and B. Simon, Carbon materials for lithium-ion rechargeable batteries, Carbon, 1999, 37(2), 165–180 CrossRef CAS.
- H. F. Xiang, C. H. Chen, J. Zhang and K. Amine, Temperature effect on the graphite exfoliation in propylene carbonate based electrolytes, J. Power Sources, 2010, 195(2), 604–609 CrossRef CAS.
- H. Zheng, J. Qin, Y. Zhao, T. Abe and Z. Ogumi, Temperature dependence of the electrochemical behavior of LiCoO2 in quaternary ammonium-based ionic liquid electrolyte, Solid State Ionics, 2005, 176(29–30), 2219–2226 CAS.
- H. Zheng, H. Zhang, Y. Fu, T. Abe and Z. Ogumi, Temperature effects on the electrochemical behavior of spinel LiMn2O4 in quaternary ammonium-based ionic liquid electrolyte., J. Phys. Chem. B, 2005, 109(28), 13676–13684 CrossRef CAS.
- J. Sun, M. Forsyth and D. R. MacFarlane, Room temperature molten salts based on the quaternary ammonium ion, J. Phys. Chem. B, 1998, 102, 8858–8864 CrossRef CAS.
- T. Sato, T. Maruo, S. Marukane and K. Takagi, Ionic liquids containing carbonate solvent as electrolytes for lithium ion cells, J. Power Sources, 2004, 138, 253–261 CrossRef CAS.
- J.-H. Shin, W. A. Henderson and S. Passerini, Ionic liquids to the rescue? Overcoming the ionic conductivity limitations of polymer electrolytes, Electrochem. Commun., 2003, 5, 1016–1020 CrossRef CAS.
- Y. S. Fung and R. Q. Zhou, Room temperature molten salt as medium for lithium battery, J. Power Sources, 1999, 81, 891–895 CrossRef.
- I. Mochida, C.-H. Ku and Y. Korai, Anodic performance and insertion mechanism of hard carbons prepared from synthetic isotropic pitches, Carbon, 2001, 39, 399–410 CrossRef CAS.
- E. Buiel and J. R. Dahn, Li-insertion in hard carbon anode materials for Li-ion batteries, Electrochim. Acta, 1999, 45, 121–130 CrossRef CAS.
- J. Shu, M. Shui, D. Xu, S. Gao, X. Li, Y. Ren, L. Hou, J. Cui, J. Xu and Z. Zhu, Preparation of nano-sized hard carbon spherule and its electrochemical property, J. Electroanal. Chem., 2011, 657, 187–191 CrossRef CAS.
- H. Yang and X. D. Shen, Dynamic TGA–FTIR studies on the thermal stability of lithium/graphite with electrolyte in lithium-ion cell, J. Power Sources, 2007, 167(2), 515–519 CrossRef CAS.
- H.-Y. Lee, J.-K. Baek, S.-M. Lee, H.-K. Park, K.-Y. Lee and M.-H. Kim, Effect of carbon coating on elevated temperature performance of graphite as lithium-ion battery anode material, J. Power Sources, 2004, 128(1), 61–66 CrossRef CAS.
- H. Zheng, G. Liu and V. Battaglia, Film-forming properties of propylene carbonate in the presence of a quaternary ammonium ionic liquid on natural graphite anode, J. Phys. Chem. C, 2010, 114, 6182–6189 CAS.
- M. Nádherná, J. Reiter, J. Moškon and R. Dominko, Lithium bis(fluorosulfonyl) imide–PYR 14 TFSI ionic liquid electrolyte compatible with graphite, J. Power Sources, 2011, 196(18), 7700–7706 CrossRef.
- T. Doi, Y. Iriyama, T. Abe and Z. Ogumi, Lithium-ion transfer at a solid polymer electrolyte/non-graphitizable carbon electrode interface, J. Power Sources, 2005, 142(1–2), 329–332 CrossRef CAS.
- T. Doi, K. Miyatake, Y. Iriyama, T. Abe, Z. Ogumi and T. Nishizawa, Lithium-ion transfer at an electrolyte/non-graphitizable carbon electrode interface, Carbon, 2004, 42(15), 3183–3187 CrossRef CAS.
- T. Abe, H. Fukuda, Y. Iriyama and Z. Ogumi, Solvated Li-ion transfer at interface between graphite and electrolyte, J. Electrochem. Soc., 2004, 151(8), A1120–A1123 CrossRef CAS.
- Y. Yamada, Y. Iriyama, T. Abe and Z. Ogumi, Kinetics of lithium ion transfer at the interface between graphite and liquid electrolytes: effects of solvent and surface film, Langmuir, 2009, 25(21), 12766–12770 CrossRef CAS.
- P. P. Prosini and B. Branimir, Composite polyether electrolytes with a poly(styrenesulfonate) lithium salt and Lewis acid type additive, Electrochim. Acta, 2003, 48(13), 1899–1903 CrossRef CAS.
- T. Abe, S. Yamate, Y. Iriyama, M. Inaba, Z. Ogumi and T. Fukutsuka, Lithium ion transfer at carbon thin film electrode/electrolyte interface, Mol. Cryst. Liq. Cryst., 2002, 388, 555–560 Search PubMed.
| This journal is © The Royal Society of Chemistry 2012 |
