A novel method to reduce the influence of by-product water on the catalytic performance of PdCl2/Cu-HMS catalysts for the synthesis of diethyl carbonate†
Pingbo
Zhang
,
Mingming
Fan
* and
Pingping
Jiang
The Key Laboratory of Food Colloids and Biotechnology, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, 214122, Wuxi, PR China. E-mail: fanmm2000@126.com; Fax: +86-510-85917763; Tel: +86-510-85917090
First published on 23rd March 2012
Abstract
A novel method to synthesise diethyl carbonate is introduced by the coupling reaction, the hydrolysis of diethyl ether (DEE) and oxidative carbonylation of ethanol, which reduces the influence of by-product water and satisfies the energy coupling for the reaction system.
Diethyl carbonate (DEC) is recognized as an environmentally benign chemical because of its negligible ecotoxicity and low bioaccumulation and persistence. Because of its high oxygen content (40.6 wt%), DEC has been proposed as a replacement for tert-butyl ether (MTBE)1 (18.2 wt%) as an attractive oxygen-containing fuel additive. A further significant advantage of DEC over other fuel oxygenates such as MTBE is that DEC could slowly decompose to CO2 and ethanol, which have no environmental impact when released into the environment.2 In addition, the gasoline/water distribution coefficients for DEC are more favorable than those for dimethyl carbonate (DMC)3 and ethanol.4 Besides these applications as a fuel additive, DEC is also drawing attention as a safe solvent and an additive in lithium cell electrolyte.5
There are several synthetic methods for producing DEC, such as a phosgene process,6 carbonylation of ethyl nitrite,7 oxidative carbonylation of ethanol,8 and an ether exchange process.9 Among them, the vapor phase oxidative carbonylation of ethanol represents one of the proposed favorable processes based on “green chemistry” principles.8 Previous results have shown that the water produced stoichiometrically along with DEC during the oxidative carbonylation of ethanol inhibits the activity of the catalysts.10 Because of the presence of water altering the amount of surface ethanol and ethoxide on the catalyst, tailoring the catalyst to reduce water inhibition is a difficult task. Instead, changing the composition of the reactant species is a possible strategy that can be used to reduce the amount of water in the system.
Hydrolysis of diethyl ether is an important process for producing ethanol with the consumption of by-product water. Utilization of hydrolysis of diethyl ether decreases the content of water to enhance the catalytic performance of PdCl2/Cu-HMS, which has not been reported up to now. The hydrolysis of diethyl ether and oxidative carbonylation of ethanol was shown in the following expressions. The coupling reaction is shown in reaction (3).
| 2CH3CH2OH + 1/2O2 +CO → CH3CH2O(CO)OCH2CH3 + H2O | (1) |
| CH3CH2OCH2CH3 + H2O → 2CH3CH2OH | (2) |
| CH3CH2OCH2CH3 + 1/2O2 + CO → CH2CH2O(CO)OCH2CH3 | (3) |
From the expressions, the byproduct water from the oxidative carbonylation of ethanol was the reactant for the hydrolysis of diethyl ether. The main product of hydrolysis of diethyl ether was ethanol, which could be as the reactant of oxidative carbonylation of ethanol. Therefore, the method of synthesising DEC with diethyl ether and ethanol feeding is a reasonable technique, which overcomes the influence of water in the traditional oxidative carbonylation of ethanol for the synthesis of DEC and shows the dominance of the oxidative carbonylation of ethanol. The rate of water production from ethanol carbonylation is fairly slow.11 Therefore the rate of hydrolysis of diethyl ether reaction in the system will not need to be as fast as in a typical hydrolysis of diethyl ether reaction, because the use of the reaction is to remove water and the yield of ethanol is not a major concern. Therefore, the design of the system is very important.
For this reason, it may be interesting to introduce diethyl ether into reactants for the oxidative carbonylation of ethanol when preparing DEC. Up to now, there have been no references about the use of diethyl ether hydrolysis for the synthesis of DEC via the oxidative carbonylation of ethanol.
The present study was undertaken with the aim of assessing the effects of the hydrolysis of diethyl ether on the activity of PdCl2/Cu-HMS12 for the synthesis of DEC. In our work, the thermodynamic data13 was calculated to establish the theory groundwork for designing the coupling reaction. The performance of the PdCl2/Cu-HMS catalyst for the coupling reaction was investigated. In addition, the possible mechanism of coupling reaction was proposed.
As proposed above, the oxidative carbonylation of ethanol and hydrolysis of diethyl ether were shown in reaction (1) and reaction (2), respectively. Reaction (3) denoted the coupling reaction. The ΔfHmθ, ΔrSmθ, ΔrGmθ and ΔrCp,m of reaction (1)–(3) were shown in Table 1. From Table 1, reaction (1) and (2) are exothermic and endothermic, respectively. Therefore both reactions could enable coupling of the energy. In reaction (2), ΔrGmθ 0, which indicates that hydrolysis of diethyl ether is not a spontaneous reaction in the standard state. Therefore, the calculation of relation between ΔrGmθ and temperature is necessary in the next succession. The ΔrHmθ of reaction (3) is −285.64 KJ mol−1, which makes clear that the coupling reaction is an exothermic reaction. Enhancing the reaction temperature would go against the production of DEC. Therefore controlling the reaction temperature of the coupling reaction is important for improving the reaction performance. From the above analysis, in the range of certain temperature, reaction (1) and (2) were exothermic and endothermic, respectively, which could satisfy the energy coupling for reaction (1) and (2). In addition, the free energy of coupling reaction (3) was less than zero, which meant that the design of the coupling experiments was reasonable and the coupling reaction could be realized.
| ΔrHmθ KJ mol−1 | ΔrSmθ J mol−1 K−1 | ΔrGmθ KJ mol−1 | ΔrCp,m = ΔA + ΔBT + ΔCT2 | |||
|---|---|---|---|---|---|---|
| ΔA | ΔB × 103 | ΔC × 106 | ||||
| Reaction (1) | −309.47 | −208.01 | −247.45 | −20.51 | 128.015 | −119.325 |
| Reaction (2) | 23.83 | 33.79 | 13.75 | −37.32 | 94.06 | −66.95 |
| Reaction (3) | −285.64 | −174.22 | −233.70 | −57.83 | 222.105 | −186.275 |
In order to give insight into the effect of hydrolysis of diethyl ether on the catalytic synthesis of DEC by the oxidative carbonylation of ethanol, the performance of catalyst with ethanol and diethyl ether feeding was investigated and compared with when ethanol is the reactant. A comparison of EtOH and EtOH adding with DEE as the reactant on the catalytic performance is shown in Table 2.
From Table 2, when DEE was added into the reactants, the space time yield (STY) of DEC were improved to some extent, which showed that the essential role of DEE promoted the improvement of catalytic activity for the synthesis of DEC. The conversion of EtOH and the water content decreased somewhat, which was because ethanol produced from the hydrolysis of DEE existed in the products. Therefore, the utilization of the coupling reaction could enhance the catalytic performance for the synthesis of DEC.
Because there were both exothermic and endothermic reactions, the coupling reaction was sensitive to temperature. Therefore the investigation of the effect of temperature on the reaction performance was important for designing the experiments. The dependence temperature for the coupling reaction is shown in Fig. 1. As expected, increased temperature affected the synthesis reaction for DEC very favorably. Only a trace of DEC was produced at room temperature, but its formation increased with the increasing reaction temperature up to 413 K. Whereupon the catalytic activity for DEC synthesis gradually decreased with a further increase of reaction temperature. When reaction temperature was above 423 K, the STY of DEC decreased greatly, the exothermic reaction was responsible for that, because the coupling reaction released a lot of heat. This result indicates that the reaction temperature played an important role in the catalytic behavior of the coupling reaction for the synthesis of DEC. In addition, the ethanol conversion was favorable in 403 K, which was lower than the favorable temperature for DEE conversion (413–423 K).
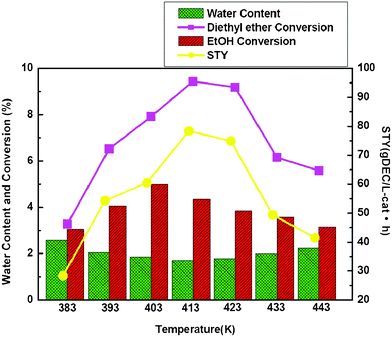 | ||
| Fig. 1 Effect of temperature on the catalytic performance and water content with EtOH and diethyl ether as reactants. Reaction condition: T = 413 K, P = 0.64 MPa, O2 = 10 sccm, CO = 80 sccm, N2 = 50 sccm. | ||
Fig. 1 shows the effect of temperature on water content in the reactor effluent. The water content decreased with increasing reaction temperature up to 413 K. Then the water content gradually increased by a further increase of reaction temperature. It has been reported that the optimal reaction temperature for ethanol gas phase oxidative carbonylation is 413 K, but the hydrolysis of DEE is generally performed at higher temperatures.14 It could be considered that the oxidative carbonylation of ethanol was faster among the reaction system under lower temperature, which was consistent with the favorable temperature for ethanol conversion. Because the hydrolysis of DEE was an endothermic reaction, it could need some heat to drive the reaction occurring. So when hydrolysis of diethyl ether proceeded under higher temperatures, the coupling reaction was realized with favorable DEC production.
Utilizing the hydrolysis of diethyl ether for DEC synthesis through the oxidative carbonylation of ethanol facilitated improvement of the catalyst’s performance. Scheme 1 shows a possible mechanism of the coupling reaction with ethanol and diethyl ether feeding. The coupling reaction system involves the hydrolysis of diethyl ether and oxidative carbonylation of ethanol. The oxidative carbonylation of ethanol takes place on the active centers of the catalyst. During this process, ethanol produced from the hydrolysis of diethyl ether was introduced into oxidative carbonylation of ethanol as reactant. Furthermore, the presence of water produced from the oxidative carbonylation of ethanol as reactant promotes the hydrolysis of diethyl ether. The B acid center is the Brønsted acid site of the catalysts, which is the active site for the hydrolysis of diethyl ether. Finally, the coupling reaction was realized to produce DEC. In our case, the PdCl2/Cu-HMS catalyst contains not only Cu and Pd as active species but also B acid centers, which would make it easy to sustain the catalytic cycle between the hydrolysis of diethyl ether and the oxidative carbonylation of ethanol. The proposed mechanism of the coupling reaction is an ideal mechanism which is propitious to explain the realization of coupling reaction. In fact, there is some ethanol introduced to the reaction system, and the oxidative carbonylation of ethanol was a start-up reaction among the system, which could provide not only heat energy but also water as the reactant for hydrolysis of diethyl ether. As a result, it is easy to understand that the hydrolysis of DEE has improved the catalytic performance of the synthesis of DEC by oxidative carbonylation of ethanol, as shown in Scheme 1.
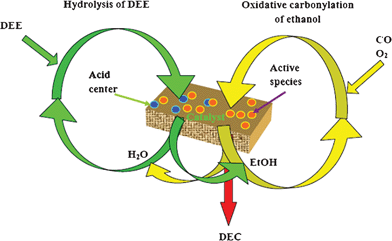 | ||
| Scheme 1 The possible mechanism of coupling reaction with ethanol and DEE feeding. | ||
Conclusions
The thermodynamics analysis indicated that oxidative carbonylation of ethanol and hydrolysis of DEE were exothermic and endothermic reactions, respectively, which satisfies the energy coupling for both reactions. In addition, the free energy of the coupling reaction was less than zero, which meant that the design of coupling experiments was reasonable and the coupling reaction could be realized.It was also shown that the coupling reactions of hydrolysis of diethyl ether (DEE) and oxidative carbonylation of ethanol, enhanced STY of DEC, and decreased the water content in the system. The coupling reaction showed sensitive dependence on the temperature and the favorable temperature was 413 K with less water in the system. The possible mechanism of coupling reaction was illuminated by the interaction between hydrolysis of diethyl ether and oxidative carbonylation of ethanol.
Acknowledgements
The financial supports from the National Natural Science Foundation of China (NSFC) (Grant No. 21106054), the Specialized Research Fund for the Doctoral Program of Higher Education (New Teachers) (Grant No. 20100093120003) and the Fundamental Research Funds for the Central Universities (No. JUSRP211A07) are gratefully acknowledged.References
- (a) M. A. Pacheco and C. L. Marshall, Energy Fuels, 1997, 11, 2 CrossRef CAS; (b) E. Leino, P. MäiArvela, V. Eta, D. Y. Murzin, T. Salmi and J. P. Mikkola, Appl. Catal. A, 2010, 383, 1 CrossRef CAS.
- (a) J. W. Crandall, J. E. Deitzler, L. A. Kapicak and F. Poppelsdorf, 1987, US Patent 4,663,477 Search PubMed; (b) A. Arteconi, A. Mazzarini and G. D. Nicola, Water Air Soil. Pollut., 2011, 221, 405 CrossRef CAS.
- H. Itoh, Y. Watanabe, K. Mori and H. Umino, Green Chem., 2003, 5, 558 RSC.
- (a) J. S. Gnanaraj, E. Zinigrad, L. Asraf, M. Sprecher, H. E. Gottlieb, W. Geissler, M. Schmidt and D. Aurbach, Electrochem. Commun., 2003, 5, 946 CrossRef CAS; (b) A. Arteconi, A. Mazzarini and G. D. Nicola, Water Air Soil. Pollut., 2011, 221, 405 CrossRef CAS.
- (a) B. M. Wiers, M. L. Foo, N. P. Balsara and J. R. Long, J. Am. Chem. Soc., 2011, 133, 14522 CrossRef CAS; (b) M. Herstedt, M. Stjerndahl, T. Gustafsson and K. Edstrom, Electrochem. Commun., 2003, 5, 467 CrossRef CAS; (c) G. Moumouzias, G. Ritzoulis, D. Siapkas and D. Terzidis, J. Power Sources, 2003, 122, 57 CrossRef CAS.
- I. E. Muskat and F. Strain, 1941, US Patent 2,379,250 Search PubMed.
- X. B. Ma, M. M. Fan and P. B. Zhang, Catal. Commun., 2004, 5, 765 CrossRef CAS.
- (a) U. Romano, R. Tessi, G. Ciprianni and L. Micucci, 1980, US Patent 4,218, 391 Search PubMed; (b) B. C. Dunn, C. Guenneau, S. A. Hilton, J. Pahnke, E. M. Eyring, J. Dworzanski, H. L. C. Meuzelaar, J. Z. Hu, M. S. Solum and R. J. Pugmire, Energy Fuels, 2001, 16, 177 CrossRef; (c) Z. Zhang, X. B. Ma, J. Zhang, F. He and S. P. Wang, J. Mol. Catal. A: Chem., 2005, 227, 141 CrossRef CAS; (d) N. S. Roh, B. C. Dunn, E. M. Eyring, R. J. Pugmire and H. L. C. Meuzelaar, Fuel Process. Technol., 2003, 83, 27 CrossRef CAS; (e) T. C. Liu and C. S. Chang, Appl. Catal. A, 2006, 304, 72 CrossRef CAS.
- (a) P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, London: Oxford University press, 1998, pp. 11 Search PubMed; (b) I. Z. Nadolska, K. Warmuzinski and J. Richter, Catal. Today, 2006, 114, 226 CrossRef.
- (a) S. A. Anderson and T. W. Root, J. Catal., 2003, 217, 396 CAS; (b) S. A. Anderson and T. W. Root, J. Mol. Catal. A: Chem., 2004, 220, 247 CrossRef CAS.
- (a) S. T. King, J. Catal., 1996, 161, 530 CrossRef CAS; (b) S. A. Anderson and T. W. Root, J. Catal., 2003, 217, 396 CAS.
- (a) P. B. Zhang, Z. Zhang, S. P. Wang and X. B. Ma, Catal. Commun., 2007, 8, 21 CrossRef CAS; (b) P. B. Zhang and X. B. Ma, Chem. Eng. J, 2010, 163, 93 CrossRef CAS.
- (a) I. Prigogine and R. Defay, Chemical Thermodynamics, London: Longmans Green, 1954, pp. 38–42 Search PubMed; (b) P. S. Ma, Chemical data, Bejing: China petrochemical press, 2003 Search PubMed; (c) S. Wang, The handbook of Petrochemical design (The first volume), Beijing: Chemical industry press, 2002, pp. 452–459 Search PubMed.
- (a) R. X. Jiang, S. F. Wang, X. Q. Zhao, Y. J. Wang and C. F. Zhang, Appl. Catal. A: Gen., 2003, 238, 131 CrossRef CAS; (b) G. Y. Cai and L. Y. Xu, Catalysis in C1 Chemistry, Beijing: Chemial Industry Press, 1995, pp. 367 Search PubMed.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra20529g/ |
| This journal is © The Royal Society of Chemistry 2012 |
