Chiral phosphine-squaramide-catalyzed Morita–Baylis–Hillman reaction: enantioselective synthesis of 3-hydroxy-2-oxindoles†
Jing-Ying
Qian
a,
Ci-Ci
Wang
a,
Feng
Sha
a and
Xin-Yan
Wu
*ab
aKey Laboratory for Advanced Materials and Institute of Fine Chemicals, East China University of Science and Technology, Shanghai 200237, P. R. China. E-mail: xinyanwu@ecust.edu.cn; Fax: +86 21 64252758; Tel: +86 21 64252011
bState Key Laboratory of Elemento-organic Chemistry, Nankai University, Tianjin 300071, P. R. China
First published on 31st May 2012
Abstract
Phosphine-squaramide derivatives were developed to catalyze the enantioselective Morita–Baylis–Hillman reaction of acrylates with isatins to construct 3-hydroxy-2-oxindoles with quaternary stereocenters. In the presence of 2 mol% H–bonding catalyst 3e, the desired products were achieved in high yields and good-to-excellent enantioselectivities (up to 95% ee).
Introduction
3-Substituted-3-hydroxy-2-oxindoles are important structural motifs found in many alkaloid natural products and pharma-ceutical molecules.1 Over the past decade, organometallic and metal-free catalytic asymmetric methods have been developed for the construction of compounds bearing a quaternary stereocenter at the 3-position.2–4 However, enantioselective organocatalysis mainly focused on the aldol additions to isatins.4a–h The Morita–Baylis–Hillman (MBH) reaction is one of the most useful carbon–carbon bond forming reactions providing densely functionalized alcohols.5 The MBH reaction of electron-deficient olefins to isatin derivatives could obtain 3-substituted-3-hydroxy-2-oxindoles.6 Very recently, enantioselective versions were reported, using cinchona alkaloids7 or phosphinothiourea8 as chiral catalysts. Herein, we describe the first example of phosphine-squaramide catalyzed intermolecular MBH reaction with isatins as electrophiles, providing 3-substituted-3-hydroxy-2-oxindole derivatives in excellent yields with high enantioselectivities.In our previous work, bifunctional phosphine was first used as a chiral organocatalyst to promote the enantioselective MBH reaction involving isatin as an electrophile.8 In the presence of phosphinothiourea 1 (Fig. 1), this MBH reaction was achieved in excellent chemical yields but moderate enantioselectivities. As a continuous work, we developed the novel chiral bifunctional phosphine organocatalysts bearing squaramide as H–bond donator. Compared with thiourea, the squaramide has a greater difference in duality, rigidity, H–bond length, H–bond angle, and pKa, which endowed it with a unique catalophore for dual H–bonding catalysts.9,10
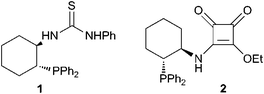 | ||
| Fig. 1 Structure of bifunctional phosphine 1 and 2. | ||
Results and discussion
Phosphine-squaramides 3a–h were easily prepared by the condensation of (1R,2R)-2-amino-1-(diphenylphosphino)cyclo-hexane11 with the corresponding squaramide derived from diethyl squarate in CH2Cl2 (Scheme 1).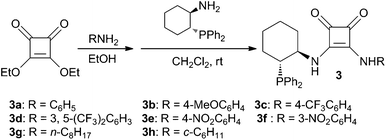 | ||
| Scheme 1 Synthetic route of the squaramide catalysts 3a–h. | ||
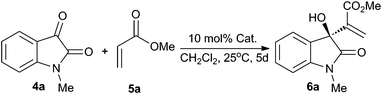
With the new chiral bifunctional phosphines at hand, we initially conducted a MBH reaction of N-methyl isatin with methyl acrylate in dichloromethane at room temperature in the presence of 10 mol% of catalyst for 5 days (Table 1). The phosphine-squaramide 2 (Fig. 1), which was highly efficient for the intramolecular MBH reaction,12 gave the MBH adduct with good yield but moderate enantioselectivity (entry 1). Pleasingly, phosphine-squaramide 3 with a dual H–bonding donator13 exhibited high catalytic activity, and more importantly, promising enantioselectivity (entries 2–9). Among the screened phosphine-squaramide organocatalysts, 3e was the best one, providing the desired product in 81% ee with 97% yield (entry 6). It is obvious that phosphine-squaramides 3 achieved better enantioselectivity than the phosphinothiourea 1 containing the same chiral backbone (entry 10).8
| Entry | Catalyst | Yield %b | ee %c |
|---|---|---|---|
| a The reactions were performed with 4a (0.2 mmol), 5a (1 mmol) and 10 mol% of catalyst 1–3 in 1 mL CH2Cl2 at 25 °C for 5 days. b Isolated yield. c Determined by chiral HPLC analysis using Chiralcel OD-H column. | |||
| 1 | 2 | 74 | 42 |
| 2 | 3a | 85 | 77 |
| 3 | 3b | 89 | 74 |
| 4 | 3c | 96 | 78 |
| 5 | 3d | 94 | 77 |
| 6 | 3e | 97 | 81 |
| 7 | 3f | 90 | 77 |
| 8 | 3g | 96 | 65 |
| 9 | 3h | 97 | 58 |
| 10 | 1 | 91 | 45 |
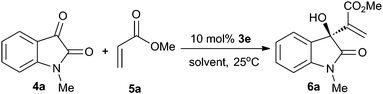
The effect of solvent was next explored with 10 mol% of catalyst 3e (Table 2). The MBH adducts were obtained in excellent yields in the screened aprotic polar solvents (entries 1–7). When a non-polar solvent such as toluene was involved, the MBH reaction became sluggish and resulted in lower yield (entry 8). In a protic polar solvent such as MeOH, a decrease of chemical yield resulted from the side-reaction, and the enantioselectivity was lower than other cases (entry 9 vs. 2–8). The solvent survey indicated that ethyl acetate was the optimal solvent, providing product 6a in 87% ee and 99% yield (entry 6).
| Entry | Solvent | Time/d | Yield %b | ee %c |
|---|---|---|---|---|
| a Unless stated otherwise, the reactions were performed with 4a (0.2 mmol), 5a (0.4 mmol) and 10 mol% of catalyst 3e in 1 mL solvent at 25 °C. b Isolated yield. c Determined by chiral HPLC analysis using Chiralcel OD-H column. d 1 mmol scale. | ||||
| 1 | CH2Cl2 | 5 | 97 | 81 |
| 2 | CH2Cl2d | 5 | 97 | 81 |
| 3 | CHCl3 | 5 | 95 | 83 |
| 4 | THF | 2 | 99 | 77 |
| 5 | Ether | 3 | 97 | 82 |
| 6 | EtOAc | 2 | 99 | 87 |
| 7 | CH3CN | 6 | 92 | 77 |
| 8 | Toluene | 6 | 79 | 85 |
| 9 | CH3OH | 3 | 85 | 74 |
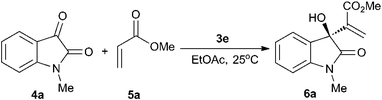
Further optimization of the reaction conditions was focused on the examination of substrate concentration, catalyst loading and reaction temperature (Table 3). The results indicated that the different substrate concentration has no effect on neither chemical yield nor stereoselectivity (entries 1–3). When the catalyst loading was reduced to 2 mol%, same results were obtained (entries 2, 4 and 5). In fact, the phosphine-squaramide catalyst has poor solubility in organic solvent. However, with 1 mol% catalyst 3e, the reaction rate decreased observably (entry 6). In the presence of 2 mol% 3e, the higher reaction temperature (40 °C) resulted in a decrease of reaction rate and enantioselectivity, probably due to the decomposition of catalyst (entry 8 vs. 5), while the lower reaction temperature (0 °C) resulted in slower reaction rate and better enantioselectivity (entry 9 vs. 5).
| Entry | Conc./M | 3e/mol (%) | Time/d | Yield %b | ee %c |
|---|---|---|---|---|---|
| a Unless stated otherwise, the reactions were performed with 4a (0.2 mmol), 5a (0.4 mmol) and catalyst 3e in EtOAc at 25 °C. b Isolated yield. c Determined by chiral HPLC analysis using Chiralcel OD-H column. d The reaction was conducted at 40 °C. e The reaction was conducted at 0 °C. | |||||
| 1 | 0.1 | 10 | 2 | 99 | 87 |
| 2 | 0.2 | 10 | 2 | 99 | 87 |
| 3 | 0.3 | 10 | 1.7 | 99 | 87 |
| 4 | 0.2 | 5 | 3 | 99 | 87 |
| 5 | 0.2 | 2 | 3 | 99 | 87 |
| 6 | 0.2 | 1 | 6.5 | 94 | 88 |
| 7 | 0.2 | 0.5 | 8 | 81 | 88 |
| 8d | 0.2 | 2 | 4.5 | 93 | 81 |
| 9e | 0.2 | 2 | 6.5 | 39 | 93 |
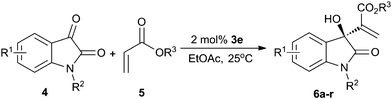
Under the optimized reaction conditions (2 mol% 3e, 2 equiv. of acrylate, ethyl acetate as solvent, 25 °C), we examined the substrate scope of various acrylates (Table 4, entries 1–6). Almost the same level of enantioselectivities and chemical yields were observed when alkyl acrylates were used as nucleophiles (Table 4, entries 1–3, 6), except t-butyl acrylate due to its steric effect. However, the MBH reaction of phenyl acrylate exhibited poor reactivity under the typical reaction conditions, only 12% yield was obtained after reacting for one week (entry 5).
| Entry | R1 | R2 | R3 | Product | Time/d | Yield %b | ee %c | |
|---|---|---|---|---|---|---|---|---|
| a The reactions were performed with 4 (0.2 mmol), 5 (0.4 mmol) and 2 mol% of catalyst 3e in 1 mL EtOAc at 25 °C. b Isolated yield. c Determined by chiral HPLC analysis, and the data in parentheses were obtained after being recrystallized once from ethanol and petroleum ether. | ||||||||
| 1 | H | Me | Me | 6a | 3 | 99 | 87 | |
| 2 | H | Me | Et | 6b | 2 | 98 | 86 | |
| 3 | H | Me | n-Bu | 6c | 2 | 99 | 83 | |
| 4 | H | Me | t-Bu | 6d | 3 | 77 | 71 | |
| 5 | H | Me | Ph | 6e | 7 | 12 | 80 | |
| 6 | H | Me | Bn | 6f | 2 | 99 | 89 | |
| 7 | H | n-Bu | Bn | 6g | 4.5 | 88 | 87 | |
| 8 | H | Bn | Bn | 6h | 3.5 | 92 | 88 | |
| 9 | 4-Cl | Me | Bn | 6i | 3.5 | 92 | 94 (99) | |
| 10 | 4-Br | Me | Bn | 6j | 4.5 | 91 | 95 (99) | |
| 11 | 5-F | Me | Bn | 6k | 3.5 | 99 | 76 (99) | |
| 12 | 5-Cl | Me | Bn | 6l | 1.5 | 99 | 71 | |
| 13 | 5-Br | Me | Bn | 6m | 1.5 | 98 | 72 (91) | |
| 14 | 6-Br | Me | Bn | 6n | 2.5 | 91 | 83 (99) | |
| 15 | 7-Br | Me | Bn | 6o | 3 | 81 | 82 (99) | |
| 16 | 5-Me | Me | Bn | 6p | 2.5 | 98 | 89 (98) | |
| 17 | 5-MeO | Me | Bn | 6q | 4.5 | 90 | 85 | |
| 18 | 7-Me | Me | Bn | 6r | 3.5 | 80 | 89 (99) | |
Next the substrate scope of different isatin derivatives was investigated by reacting them with benzyl acrylate (Table 4, entries 6–18). The N-alkyl groups of isatin affected the reaction rate rather than the enantioselectivity, and good results were attained (entries 6–8). For the MBH reaction of benzyl acrylate to N-methyl isatins with different aromatic moieties, good-to-excellent yields (80–99%) were achieved in all the examples examined, although different reaction times were required (entries 9–18 and 6). Regarding the enantioselectivity, the introduction of substituents at 4-position had a positive effect (entries 9 and 10 vs. 6), while the electron-attracting group at other position exhibited a negative effect, especially at 5-postion (entries 11–15 vs. 6). The presence of an electron-donating group at the phenyl unit affected the reactivity instead of the enantioselectivity (entries 16–18 vs. 6). For some of the solid products, their ee values could reach up to 99% after a simple recrystallization. The absolute configuration of product 6m was determined to be S by X-ray analysis (Fig. 2). And the configurations of other compounds were tentatively assigned by comparing to 6m.
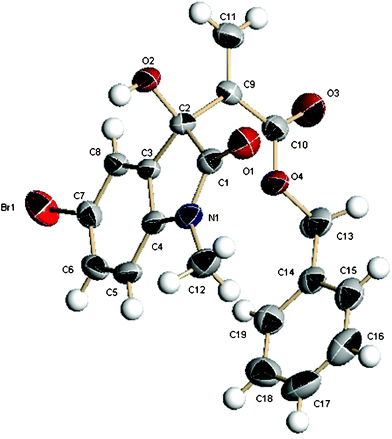 | ||
| Fig. 2 X-ray crystal structure of MBH product 6m. | ||
In addition, we tried the MBH reaction of N-Ac isatin with benzyl acrylate. However, the isatin with an electron-withdrawing group at the 1-position was inert under the typical reaction conditions. Other nucleophiles were also examined. The MBH reaction between acrolein and N-methyl isatin was accomplished in 3 h, but the enantioselectivity was unsatisfied (6% ee and 74% yield). The methyl vinyl ketone resulted in complicated side-reactions.
Conclusions
In summary, we have developed phosphine-squaramide compounds as a novel class of chiral organocatalysts for the asymmetric Morita–Baylis–Hillman reaction using isatins as electrophiles. A variety of chiral 3-hydroxy-2-oxindoles were efficiently obtained in good-to-excellent yields (up to 99%) with high enantioselectivities (up to 99% ee after a simple recrystallization).Experimental
General information
Melting points were taken without correction. Optical rotations were measured on a WZZ-2A digital polarimeter at the wavelength of the sodium D-line (589 nm). 1H, 13C and 31P NMR spectra were recorded on Bruker 400 spectrometer. The chemical shifts of 1H NMR and 13C NMR spectra were referenced to tetramethylsilane (δ 0.00 ppm) using CDCl3 or (CD3)2SO as solvent. The chemical shifts of 31P NMR spectra were referenced to an external H3PO4 signal (0.00 ppm). IR spectra were recorded on Nicolet Magna-I 550 spectrometer. High Resolution Mass spectra (HRMS) were recorded on Micromass GCT with Electron Spray Ionization (ESI) resource. HPLC analysis was performed on Waters equipment using Daicel Chiralcel OD-H, Chiralpak AS-H or AD-H column.Toluene, THF and ether were freshly distilled from sodium-benzophenone. Dichloromethane, chloroform, ethyl acetate and acetonitrile were freshly distilled from CaH2. Methanol was distilled from magnesium. Thin-layer chromatography (TLC) was performed on 10–40 μm silica gel plates. Column chromatography was performed using silica gel (300–400 mesh) eluting with ethyl acetate, petroleum ether and CH2Cl2.
General procedure for the synthesis of chiral phosphine-squaramide catalysts
To a solution of diethyl squarate (374 mg, 2.2 mmol) in EtOH (10 mL) was added amine (2 mmol) in EtOH (5 mL). The reaction mixture was stirring at room temperature or under reflux (monitoring by TLC), then the resulting solution was concentrated and purified by column chromatography to afford the corresponding squaramide. A solution of (1R,2R)-2-amino-1-(diphenylphosphino)-cyclohexane12 (85 mg, 0.3 mmol) in CH2Cl2 (5 mL) was added to a solution of squaramide (0.33 mmol) in CH2Cl2 (5 mL). After stirring at room temperature for 4 days, the reaction mixture was filtered, and the precipitate was washed with CH2Cl2 (3 × 2 mL) to afford phosphine-squaramide 3 as solid.3-((1R,2R)-2-(diphenylphosphino)cyclohexylamino)-4-(phenylamino)cyclobut-3-ene-1,2-dione (3a)
White solid, 47% yield. M.p.: > 300 °C. [α]25D +6.0 (c 0.54, DMSO). 1H NMR (DMSO-d6, 400 MHz): δ 9.13 (s, 1H), 7.62 (d, J = 8.4 Hz, 1H), 7.56–7.40 (m, 4H), 7.40–7.20 (m, 9H), 7.15 (t, J = 7.1 Hz, 1H), 7.01 (t, J = 6.5 Hz, 1H), 3.94 (br s, 1H), 2.67 (t, J = 9.8 Hz, 1H), 2.09–1.91 (m, 1H), 1.84–1.46, (m, 4H), 1.42–1.20 (m, 2H), 1.07–0.86 (m, 1H); 13C NMR (DMSO-d6, 100 MHz): δ 183.3, 179.8, 168.1, 163.2, 138.9, 136.7 (d, J = 13.3 Hz), 135.8 (d, J = 15.7 Hz), 134.0 (d, J = 21.0 Hz), 132.7 (d, J = 19.7 Hz), 129.2, 128.5, 128.4, 128.3 (×2), 128.2, 122.4, 117.8, 55.9 (d, J = 17.6 Hz), 40.4 (d, J = 14.2 Hz), 34.7 (d, J = 6.2 Hz), 27.7 (d, J = 6.5 Hz), 24.6 (d, J = 6.2 Hz), 24.3, 15.8; 31P NMR (DMSO-d6, 162 MHz): δ −7.90; IR (KBr, cm−1): ν 3423, 2972, 2935, 2852, 1796, 1658, 1604, 1568, 1479, 1433, 1089, 1050, 754, 735, 698, 513, 476; HRMS (ESI) Calcd for C28H28N2O2P ([M+H]+): 455.1888; Found: 455.1895.3-((1R,2R)-2-(diphenylphosphino)cyclohexylamino)-4-(4-methoxyphenylamino)cyclobut-3-ene-1,2-dione (3b)
White solid, 40% yield. M.p.: > 300 °C. [α]25D +10.0 (c 0.56, DMSO). 1H NMR (DMSO-d6, 400 MHz): δ 9.04 (s, 1H), 7.51–7.14 (m, 13H), 6.88 (d, J = 8.9 Hz 2H), 3.92–3.89 (m, 1H), 3.71 (s, 3H), 2.65 (t, J = 10.0 Hz, 1H), 1.99–1.96 (m, 1H), 1.72–1.50 (m, 4H), 1.33–1.27 (m, 2H), 0.98–0.89 (m, 1H); 13C NMR (DMSO-d6, 100 MHz): δ 182.7, 180.0, 167.7, 163.2, 155.1, 136.7 (d, J = 13.3 Hz), 135.8 (d, J = 15.9 Hz), 134.1 (d, J = 21.1 Hz), 132.7 (d, J = 19.6 Hz), 132.1, 129.0, 128.4, 128.3 (×2), 128.2, 119.4, 114.4, 55.8 (d, J = 17.7 Hz), 55.2, 40.3 (d, J = 14.3 Hz), 34.7 (d, J = 7.2 Hz), 27.7 (d, J = 5.4 Hz), 24.6 (d, J = 5.2 Hz), 24.3; 31P NMR (DMSO-d6, 162 MHz): δ −8.01; IR (KBr, cm−1): ν 3198, 3112, 3047, 2934, 2853, 1794, 1650, 1610, 1567, 1518, 1456, 1366, 1319, 1297, 1251, 1181, 1116, 1039, 828, 734, 697, 513; HRMS (ESI) Calcd for C29H30N2O3P ([M+H]+): 485.1994; Found: 485.1989.3-((1R,2R)-2-(diphenylphosphino)cyclohexylamino)-4-(4-(trifluoromethyl)phenylamino)cyclobut-3-ene-1,2-dione (3c)
White solid, 51% yield. M.p.: > 300 °C. [α]25D +23.7 (c 0.47, DMSO). 1H NMR (DMSO-d6, 400 MHz): δ 9.37 (s, 1H), 7.66 (d, J = 8.6 Hz, 3H), 7.54–7.44 (m, 6H), 7.36–7.34 (m, 3H), 7.25 (t, J = 7.3 Hz, 2H), 7.20 (t, J = 7.3 Hz, 1H), 4.02–3.92 (m, 1H), 2.69 (t, J = 10.6 Hz, 1H), 2.02–1.99 (m, 1H), 1.76–1.53 (m, 4H), 1.33–1.24 (m, 2H), 1.00–0.97 (m, 1H); 13C NMR (DMSO-d6, 100 MHz): δ 184.0, 179.7, 168.6, 162.5, 142.5, 136.7 (d, J = 13.5 Hz), 135.8 (d, J = 15.4 Hz), 134.0 (d, J = 21.0 Hz), 132.9 (d, J = 20.0 Hz), 129.1, 128.5, 128.4, 128.3 (×2), 128.2, 126.6 (d, J = 3.6 Hz), 117.8, 56.3 (d, J = 18.0 Hz), 40.3 (d, J = 14.3 Hz), 34.6 (d, J = 7.2 Hz), 27.8 (d, J = 6.6 Hz), 24.6 (d, J = 5.8 Hz), 24.4; 31P NMR (DMSO-d6, 162 MHz): δ −3.08; IR (KBr, cm−1): ν 3184, 2937, 1798, 1666, 1610, 1574, 1545, 1449, 1322, 1163, 1119, 1070, 838, 741, 697; HRMS (ESI) Calcd for C29H27N2O2F3P ([M+H]+): 523.1762; Found: 523.1764.3-(3,5-Bis(trifluoromethyl)phenylamino)-4-((1R,2R)-2-(diphenylphosphino)cyclohexylamino)cyclobut-3-ene-1,2-dione (3d)
White solid, 64% yield. M.p.: > 300 °C. [α]25D +19.5 (c 0.41, DMSO). 1H NMR (DMSO-d6, 400 MHz): δ 9.66 (s, 1H), 7.91 (s, 2H), 7.70–7.66 (m, 2H), 7.54–7.43 (m, 4H), 7.48–7.43 (m, 3H), 7.25 (t, J = 7.3 Hz, 2H), 7.09 (t, J = 7.3 Hz, 1H), 4.00–3.94 (m, 1H), 2.70 (t, J = 10.8 Hz, 1H), 2.03–2.00 (m, 1H), 1.76–1.53 (m, 4H), 1.34–1.23 (m, 2H), 0.99–0.96 (m, 1H); 13C NMR (DMSO-d6, 100 MHz): δ 184.1, 179.9, 168.8, 161.9, 141.0, 136.8 (d, J = 13.6 Hz), 135.7 (d, J = 15.6 Hz), 134.1 (d, J = 21.2 Hz), 132.9 (d, J = 19.8 Hz), 131.3 (d, J = 32.9 Hz), 129.1, 128.4, 128.3 (×2), 128.0, 124.5, 121.8, 117.7, 114.6–114.5 (m), 56.3 (d, J = 17.9 Hz), 40.3 (part in DMSO-d6 residual peak), 34.5 (d, J = 7.4 Hz), 27.7 (d, J = 5.4 Hz), 24.6 (d, J = 4.0 Hz), 24.4; 31P NMR (162 MHz, DMSO-d6): δ -8.03; IR (KBr, cm−1): ν 3137, 3069, 2947, 1797, 1666, 1568, 1485, 1463, 1434, 1378, 1280, 1189, 1126, 749, 699, 684; HRMS (ESI) Calcd for C30H26N2O2PF6 ([M+H]+): 591.1636; Found: 591.1637.3-((1R,2R)-2-(diphenylphosphino)cyclohexylamino)-4-(4-nitrophenylamino)cyclobut-3-ene-1,2-dione (3e)
Yellow solid, 53% yield. M.p.: > 300 °C. The product 3e in DMSO is brown, and specific rotation data can not be obtained for its dark color. 1H NMR (DMSO-d6, 400 MHz): δ 9.60 (s, 1H), 8.20 (d, J = 8.9 Hz, 2H), 7.73 (d, J = 9.4 Hz, 1H), 7.55–7.45 (m, 6H), 7.36–7.35 (m, 3H), 7.23 (t, J = 7.4 Hz, 2H), 7.09 (t, J = 7.3 Hz, 1H), 4.04–3.94 (m, 1H), 2.71 (t, J = 10.8 Hz, 1H), 2.02–1.99 (m, 1H), 1.76–1.75 (m, 4H), 1.38–1.23 (m, 2H), 1.04–0.96 (m, 1H); 13C NMR (DMSO-d6, 100 MHz): δ 184.6, 179.6, 169.0, 161.7, 145.2, 141.3, 136.8 (d, J = 13.3 Hz), 135.8 (d, J = 15.2 Hz), 134.1 (d, J = 21.1 Hz), 132.9 (d, J = 20.0 Hz), 129.1, 128.4, 128.3 (×2), 125.6, 117.4, 56.6 (d, J = 17.6 Hz), 40.4 (d, J = 14.4 Hz), 34.5 (d, J = 7.4 Hz), 27.8 (d, J = 6.7 Hz), 24.7 (d, J = 6.0 Hz), 24.4; 31P NMR (DMSO-d6, 162 MHz): δ −7.8; IR (KBr, cm−1): ν 3195, 3143, 3069, 2936, 2851, 1797, 1668, 1619, 1601, 1577, 1509, 1440, 1345, 1317, 1273, 1192, 1114, 847, 749, 702, 514; HRMS (ESI) Calcd for C28H27N3O4P ([M+H]+): 500.1739; Found: 500.1734.3-((1R,2R)-2-(diphenylphosphino)cyclohexylamino)-4-(3-nitrophenylamino)cyclobut-3-ene-1,2-dione (3f)
Yellow solid, 35% yield. M.p.: > 300 °C. [α]25D +31.3 (c 0.32, DMSO). 1H NMR (DMSO-d6, 400 MHz): δ 9.47 (s, 1H), 8.28 (s, 1H), 7.83 (d, J = 7.8 Hz, 1H), 7.66–7.44 (m, 7H), 7.36–7.35 (m, 3H), 7.25 (t, J = 7.4 Hz, 2H), 7.11 (t, J = 7.3 Hz, 1H), 4.02–3.92 (m, 1H), 2.70 (t, J = 10.6 Hz, 1H), 2.02–1.99 (m, 1H), 1.76–1.53 (m, 4H), 1.39–1.23 (m, 2H), 1.03–0.94 (m, 1H); 13C NMR (DMSO-d6, 100 MHz): δ 183.9, 179.8, 163.5, 162.3, 148.6, 140.3, 136.8 (d, J = 13.3 Hz), 135.8 (d, J = 15.3 Hz), 134.0 (d, J = 21.2 Hz), 132.9 (d, J = 20.0 Hz), 130.7, 129.2, 128.5, 128.4, 128.3 (×2), 128.1, 123.7, 116.5, 112.1, 56.3 (d, J = 17.0 Hz), 40.3 (d, J = 14.3 Hz), 34.6 (d, J = 8.4 Hz), 27.8 (d, J = 5.8 Hz), 24.6 (d, J = 4.1 Hz), 24.4; 31P NMR (DMSO-d6, 162 MHz): δ −7.90; IR (KBr, cm−1): ν 3167, 3070, 2940, 2850, 1798, 1659, 1602, 1569, 1482, 1455, 1350, 1260, 1115, 1101, 999, 811, 797, 736, 698, 507, 490; HRMS (ESI) Calcd for C28H27N3O4P ([M+H]+): 500.1739; Found: 500.1735.3-((1R,2R)-2-(diphenylphosphino)cyclohexylamino)-4-(octylamino)cyclobut-3-ene-1,2-dione (3g)
White solid, 36% yield. M.p.: 251–252 °C. [α]25D −11.2 (c 0.33, DMSO). 1H NMR (DMSO-d6, 400 MHz): δ 7.51–7.28 (m, 10H), 6.95 (br s 1H), 3.78 (br, 1H), 3.41 (m, 3H, part in water peak), 2.58 (t, J = 10.0 Hz, 1H, part in DMSO-d6 residual peak), 1.95–1.92 (m, 1H), 1.70–1.26 (m, 18H), 0.89–0.83 (m, 4H); 13C NMR data can not be obtained for the poor solubility of 3g in organic solvent. 31P NMR (DMSO-d6, 162 MHz): δ −8.09; IR (KBr, cm−1): ν 3169, 3069, 2927, 2853, 1642, 1567, 1472, 1434, 1362, 1120, 742, 697; HRMS (ESI) Calcd for C30H40N2O2P ([M+H]+): 491.2827; Found: 491.2828.3-(Cyclohexylamino)-4-((1R,2R)-2-(diphenylphosphino) cyclohexylamino)cyclobut-3-ene-1,2-dione (3h)
White solid, 38% yield. M.p.: > 300 °C. Specific rotation data can not be obtained for the poor solubility of 3h in organic solvent. 1H NMR (DMSO-d6, 400 MHz): δ 7.76 (br s, 1H), 7.49–7.31 (m, 10H), 6.98 (br s, 1H), 3.81 (s, 1H), 3.65 (s, 1H), 2.67–2.58 (m, 1H, part in DMSO-d6 residual peak), 1.95–1.52 (m, 9H), 1.28–1.18 (m, 8H), 0.92–0.84 (m, 1H); 13C NMR data can not be obtained for the poor solubility of 3h in organic solvent. 31P NMR (DMSO-d6, 162 MHz): δ −8.05; IR (KBr, cm−1): ν 3187, 3052, 2926, 2852, 1797, 1644, 1562, 1480, 1433, 1346, 1314, 1261, 1101, 1029, 801, 737, 699; HRMS (ESI) Calcd for C28H34N2O2P ([M+H]+): 461.2358; Found: 461.2350.General procedure for the enantioselective Morita–Baylis–Hillman reaction
To a solution of phosphine-squaramide 3e (0.004 mmol) in EtOAc (1.0 mL) was added acrylate (0.4 mmol) at 25 °C. After 10 min stirring at this temperature, isatin derivative (0.2 mmol) was added. The reaction mixture was stirred at 25 °C (monitoring by TLC). Then the resulting solution was concentrated under reduced pressure and the residue was purified by a flash column chromatography on silica gel to afford the desired adducts and the ee values were determined by HPLC analysis with chiral column.(S)-methyl 2-(3-hydroxy-1-methyl-2-oxoindolin-3-yl)acrylate (6a)
White solid, 99% yield, 87% ee, [α]25D +55.4 (c 0.35, CH2Cl2). 1H NMR (CDCl3, 400 MHz): δ 7.37–7.34 (m, 1H), 7.20 (d, J = 6.4 Hz, 1H), 7.05 (t, J = 7.2 Hz, 1H), 6.88 (d, J = 7.6 Hz, 1H), 6.57 (s, 1H), 6.42 (s, 1H), 3.65 (s, 3H), 3.59 (s, 1H), 3.26 (s, 3H); HPLC analysis (OD-H column, λ = 254 nm, eluent: hexane/2-propanol = 90/10, flow rate: 1.0 mL min−1): tR = 12.12 min (minor), 19.81 min (major).(S)-ethyl 2-(3-hydroxy-1-methyl-2-oxoindolin-3-yl)acrylate (6b)
Amber syrup, 98% yield, 86% ee, [α]25D +41.4 (c 0.42, CH2Cl2). 1H NMR (CDCl3, 400 MHz): δ 7.37–7.34 (m, 1H), 7.20 (d, J = 6.8 Hz, 1H), 7.05 (t, J = 7.2 Hz, 1H),6.87 (d, J = 8.0 Hz, 1H), 6.59 (s, 1H), 6.41 (s, 1H), 4.10–4.01 (m, 2H), 3.56 (s, 1H), 3.26 (s, 3H), 1.14 (t, J = 7.2 Hz, 3H); HPLC analysis (OD-H column, λ = 254 nm, eluent: hexane/2-propanol = 90/10, flow rate: 1.0 mL min−1): tR = 11.24 min (minor), 17.87 min (major).(S)-butyl 2-(3-hydroxy-1-methyl-2-oxoindolin-3-yl)acrylate (6c)
Amber syrup, 99% yield, 83% ee, [α]25D +29.5 (c 0.53, CH2Cl2). 1H NMR (CDCl3, 400 MHz): δ 7.34–7.30 (m, 1H), 7.17 (d, J = 6.4 Hz, 1H), 7.02 (t, J = 8.0 Hz, 1H), 6.85 (d, J = 7.6 Hz, 1H), 6.58 (s, 1H), 6.44 (s, 1H), 4.22 (s, 1H), 4.04–3.92 (m, 2H), 3.23 (s, 3H), 1.50–1.43 (m, 2H), 1.24–1.18 (m, 2H), 0.85 (t, J = 7.2 Hz, 3H); HPLC analysis (OD-H column, λ = 254 nm, eluent: hexane/2-propanol = 95/5, flow rate: 1.0 mL min−1): tR = 10.46 min (minor), 13.55 min (major).(S)-tert-butyl 2-(3-hydroxy-1-methyl-2-oxoindolin-3-yl) acrylate (6d)
Amber syrup, 77% yield, 71% ee, [α]25D +14.5 (c 0.45, CH2Cl2). 1H NMR (CDCl3, 400 MHz): δ 7.35–7.30 (m, 1H), 7.18 (d, J = 6.9 Hz, 1H), 7.03 (t, J = 7.2 Hz, 1H), 6.83 (d, J = 8.0 Hz, 1H), 6.50 (s, 1H), 6.20 (s, 1H), 4.16 (s, 1H), 3.20 (s, 3H), 1.22 (s, 9H); HPLC analysis (AS-H column, λ = 254 nm, eluent: hexane/2-propanol = 90/10, flow rate: 1.0 mL min−1): tR = 7.31 min (major), 11.29 min (minor).(S)-phenyl 2-(3-hydroxy-1-methyl-2-oxoindolin-3-yl)acrylate (6e)
Amber syrup, 12% yield, 80% ee, [α]20D +12.6 (c 0.26, CH2Cl2). 1H NMR (CDCl3, 400 MHz): δ 7.38–7.27 (m, 4H), 7.19 (t, J = 7.6 Hz, 1H), 7.11–7.07 (m, 1H), 6.93–6.90 (m, 2H), 6.86–6.84 (m, 2H), 6.66 (s, 1H), 3.47 (s, 1H), 3.22 (s, 3H); HPLC analysis (OD-H column, λ = 254 nm, eluent: hexane/2-propanol = 90/10, flow rate: 1.0 mL min−1): tR = 19.21 min (minor), 31.34 min (major).(S)-benzyl 2-(3-hydroxy-1-methyl-2-oxoindolin-3-yl)acrylate (6f)
Amber syrup, 99% yield, 89% ee, [α]25D +34.0 (c 0.53, CH2Cl2). 1H NMR (CDCl3, 400 MHz): δ 7.36–7.32 (m, 4H), 7.19 (d, J = 6.8 Hz, 1H), 7.13–7.10 (m, 2H), 7.05 (t, J = 7.5 Hz, 1H), 6.73 (d, J = 8.1 Hz, 1H), 6.67 (s, 1H), 6.47 (s, 1H), 5.00 (d, J = 12.4 Hz, 1H), 4.96 (d, J = 12.3 Hz, 1H), 3.43 (s, 1H), 2.98 (s, 3H); HPLC analysis (AD-H column, λ = 254 nm, eluent: hexane/2-propanol = 90/10, flow rate: 1.0 mL min−1): tR = 25.00 min (minor), 29.50 min (major).(S)-benzyl 2-(1-butyl-3-hydroxy-2-oxoindolin-3-yl)acrylate (6g)
Amber syrup, 88% yield, 87% ee, [α]18D +38.0 (c 0.65, CH2Cl2). 1H NMR (CDCl3, 400 MHz): δ 7.31–7.25 (m, 4H), 7.12–7.16 (m, 1H), 7.09–7.06 (m, 2H), 7.03–7.00 (m, 1H), 6.75 (d, J = 7.8 Hz, 1H), 6.61 (s, 1H), 6.45 (s, 1H), 5.05–5.02 (m, 1H), 4.92–4.89 (m, 1H), 4.12 (s, 1H), 3.60–3.54 (m, 1H), 3.45–3.37 (m, 1H), 1.62–1.54 (m, 2H), 1.41–1.31 (m, 2H), 0.92 (t, J = 7.3 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 176.2, 164.4, 144.0, 139.1, 135.2, 130.0, 129.7, 128.5, 128.2 (×2), 123.9, 122.7, 109.0, 76.1, 66.8, 39.9, 29.1, 20.1, 13.8; IR (KBr, cm−1): ν 3333, 2961, 2929, 1728, 1698, 1613, 1495, 1470, 1456, 1382, 1280, 1175, 951, 744; HRMS (ESI) calcd for C22H23NO4Na ([M+Na]+): 388.1525; Found: 388.1529. HPLC analysis (OD-H column, λ = 254 nm, eluent: hexane/2-propanol = 90/10, flow rate: 1.0 mL min−1): tR = 8.96 min (minor), 12.33 min (major).(S)-benzyl 2-(1-benzyl-3-hydroxy-2-oxoindolin-3-yl)acrylate (6h)
Amber syrup, 92% yield, 88% ee, [α]15D +37.0 (c 0.73, CH2Cl2). 1H NMR (CDCl3, 400 MHz): δ 7.31–7.16 (m, 10H), 7.12–7.09 (m, 2H), 6.70–6.96 (m, 1H), 6.63 (s, 1H), 6.60 (d, J = 7.8 Hz, 1H), 6.47 (s, 1H), 5.06–5.03 (m, 1H), 4.91–4.83 (m, 2H), 4.52–4.48 (m, 1H), 3.98 (s, 1H); 13C NMR (CDCl3, 100 MHz): δ 176.5, 164.4, 143.6, 139.0, 135.5, 135.1, 130.1, 129.5, 128.8, 128.6 (×2), 128.4, 128.3, 127.6, 127.3, 123.9, 123.0, 109.9, 76.2, 66.9, 43.8; IR (KBr, cm−1): ν 3339, 1705, 1615, 1492, 1460, 1373, 1357, 1317, 1176, 1163, 1057, 972, 939, 758, 694; HRMS (ESI) calcd for C25H21NO4Na ([M+Na]+): 422.1368; Found: 422.1370. HPLC analysis (OD-H column, λ = 254 nm, eluent: hexane/2-propanol = 90/10, flow rate: 1.0 mL min−1): tR = 14.33 min (minor), 19.08 min (major).(R)-benzyl 2-(4-chloro-3-hydroxy-1-methyl-2-oxoindolin-3-yl) acrylate (6i)
White solid, 92% yield, 94% ee, [α]24D +39.3 (c 0.66, CH2Cl2). After recrystallization: 99% ee. 1H NMR (CDCl3, 400 MHz): δ 7.32–7.30 (m, 3H), 7.23 (t, J = 8.0 Hz, 1H), 7.13–7.11 (m, 2H), 6.96–6.94 (m, 1H), 6.79 (s, 1H), 6.60–6.57 (m, 2H), 4.99–4.92 (m, 2H), 4.00 (s, 1H), 2.94 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 175.3, 164.3, 146.2, 136.9, 134.9, 131.4, 131.1, 130.9, 128.6, 128.4 (×2), 125.7, 124.1, 107.2, 76.5, 67.0, 26.4; IR (KBr, cm−1): ν 3338, 1709, 1608, 1591, 1460, 1325, 1174, 1119, 1065, 777, 732; HRMS (ESI) calcd for C19H16NO4NaCl ([M+Na]+): 380.0666; Found: 380.0662. HPLC analysis (OD-H column, λ = 254 nm, eluent: hexane/2-propanol = 90/10, flow rate: 1.0 mL min−1): tR = 19.17 min (minor), 28.07 min (major).(R)-benzyl 2-(4-bromo-3-hydroxy-1-methyl-2-oxoindolin-3-yl) acrylate (6j)
White solid, 91% yield, 95% ee, [α]23D +28.9 (c 0.73, CH2Cl2). After recrystallization: 99% ee. 1H NMR (CDCl3, 400 MHz): δ 7.32–7.30 (m, 3H), 7.18–7.11 (m, 4H), 6.82 (s, 1H), 6.62 (d, J = 7.0 Hz, 1H), 6.59 (s, 1H), 4.99–4.92 (m, 2H), 3.92 (s, 1H), 2.94 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 175.1, 164.3, 146.4, 136.8, 135.0, 131.3 (×2), 128.6, 128.4 (×2), 127.4, 127.2, 119.4, 107.7, 77.2, 67.0, 26.3; IR (KBr, cm−1): ν 3435, 1712, 1605, 1582, 1460, 1315, 1159, 1113, 1045, 988, 951, 787, 761, 697; HRMS (ESI) calcd for C19H17NO4Br ([M+H]+): 402.0341; Found: 402.0347. HPLC analysis (OD-H column, λ = 254 nm, eluent: hexane/2-propanol = 90/10, flow rate: 1.0 mL min−1): tR = 16.53 min (minor), 25.60 min (major).(S)-benzyl 2-(5-fluoro-3-hydroxy-1-methyl-2-oxoindolin-3-yl)acrylate (6k)
White solid, 99% yield, 76% ee, [α]17D +50.0 (c 0.68, CH2Cl2). After recrystallization: 99% ee. 1H NMR (CDCl3, 400 MHz): δ 7.32–7.30 (m, 3H), 7.12–7.10 (m, 2H), 7.00 (td, J = 2.5 Hz, J = 8.8 Hz, 1H), 6.92 (dd, J = 2.6 Hz, J = 7.4 Hz, 1H), 6.65 (s, 1H), 6.61 (dd, J = 4.0 Hz, J = 8.3 Hz, 1H), 6.48 (s, 1H), 5.00–4.92 (m, 2H), 4.26 (s, 1H), 2.94 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 176.1, 164.1, 159.4 (d, J = 242.1 Hz), 140.3, 138.7, 134.8, 131.0 (d, J = 8.1 Hz), 129.0, 128.6, 128.5 (×2), 116.1 (d, J = 23.5 Hz), 112.1 (d, J = 29.4 Hz), 109.3 (d, J = 8.1 Hz), 76.2, 67.1, 26.2; IR (KBr, cm−1): ν 3331, 1726, 1706, 1619, 1491, 1459, 1365, 1287, 1186, 1106, 1050, 966, 819, 699; HRMS (ESI) calcd for C19H17NO4F ([M+H]+): 342.1142; Found: 342.1140. HPLC analysis (OD-H column, λ = 254 nm, eluent: hexane/2-propanol = 90/10, flow rate: 1.0 mL min−1): tR = 14.67 min (minor), 18.18 min (major).(S)-benzyl 2-(5-chloro-3-hydroxy-1-methyl-2-oxoindolin-3-yl) acrylate (6l)
White solid, 99% yield, 71% ee, [α]17D +26.2 (c 0.71, CH2Cl2). 1H NMR (CDCl3, 400 MHz): δ 7.33–7.26 (m, 4H), 7.14–7.10 (m, 3H), 6.66 (s, 1H), 6.62 (d, J = 8.3 Hz, 1H), 6.48 (s, 1H), 5.01–4.92 (m, 2H), 4.02 (s, 1H), 2.94 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 175.9, 164.1, 143.0, 138.6, 134.8, 131.0, 129.9, 129.0, 128.6, 128.5, 128.3, 124.4, 109.7, 76.0, 67.1, 26.2; IR (KBr, cm−1): ν 3335, 1727, 1705, 1608, 1487, 1359, 1288, 1188, 1105, 1049, 967, 818, 751, 697; HRMS (ESI) calcd for C19H16NO4NaCl ([M+Na]+): 380.0666; Found: 380.0668. HPLC analysis (OD-H column, λ = 254 nm, eluent: hexane/2-propanol = 90/10, flow rate: 1.0 mL min−1): tR = 16.00 min (minor), 19.53 min (major).(S)-benzyl 2-(5-bromo-3-hydroxy-1-methyl-2-oxoindolin-3-yl) acrylate (6m)
White solid, 98% yield, 72% ee, [α]17D +17.2 (c 0.78, CH2Cl2), After recrystallization: 91% ee. 1H NMR (CDCl3, 400 MHz): δ 7.43 (dd, J = 2.0 Hz, J = 8.3 Hz, 1H), 7.33–7.31 (m, 3H), 7.28–7.27 (m, 1H), 7.13–7.11 (m, 2H), 6.64 (s, 1H), 6.58 (d, J = 8.3 Hz, 1H), 6.48 (s, 1H), 5.02–4.92 (m, 2H), 3.87 (s, 1H), 2.94 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 175.7, 164.1, 143.5, 138.6, 134.8, 132.9, 131.3, 129.0, 128.6, 128.5, 127.1, 155.5, 110.2, 75.9, 67.1, 26.2; IR (KBr, cm−1): ν 3329, 2937, 1704, 1630, 1484, 1456, 1421, 1357, 1287, 1185, 1103, 1051, 966, 814, 755, 696; HRMS (ESI) calcd for C19H16NO4NaBr ([M+Na]+): 424.0160; Found: 424.0161. HPLC analysis (OD-H column, λ = 254 nm, eluent: hexane/2-propanol = 95/5, flow rate: 1.0 mL min−1): tR = 33.38 min (minor), 39.82 min (major).(S)-benzyl 2-(6-bromo-3-hydroxy-1-methyl-2-oxoindolin-3-yl) acrylate (6n)
White solid, 91% yield, 83% ee, [α]23D +31.4 (c 0.72, CH2Cl2), After recrystallization: 99% ee. 1H NMR (CDCl3, 400 MHz): δ 7.35–7.32 (m, 3H), 7.17–7.15 (m, 1H), 7.08–7.06 (m, 2H), 7.01–6.99 (m, 1H), 6.78 (d, J = 1.8 Hz, 1H), 6.65 (s, 1H), 6.47 (s, 1H), 5.00–4.98 (m, 1H), 4.89–4.86 (m, 1H), 4.06 (s, 1H), 2.88 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 176.1, 164.1, 145.6, 138.6, 134.7, 129.0, 128.6 (×2), 128.3, 125.7, 125.0, 123.8, 112.4, 75.7, 67.1, 26.2; IR (KBr, cm−1): ν 3301, 1732, 1712, 1602, 1373, 1286, 1180, 1098, 1053, 984, 962, 763; HRMS (ESI) calcd for C19H16NO4NaBr ([M+Na]+): 424.0160; Found: 424.0157. HPLC analysis (OD-H column, λ = 254 nm, eluent: hexane/2-propanol = 90/10, flow rate: 0.8 mL min−1): tR = 17.61 min (minor), 22.45 min (major).(S)-benzyl 2-(7-bromo-3-hydroxy-1-methyl-2-oxoindolin-3-yl) acrylate (6o)
White solid, 81% yield, 82% ee, [α]24D +74.5 (c 0.65, CH2Cl2), After recrystallization: 99% ee. 1H NMR (CDCl3, 400 MHz): δ 7.40 (dd, J = 1.3 Hz, J = 8.3 Hz, 1H), 7.34–7.31 (m, 3H), 7.12–7.05 (m, 3H), 6.88–6.85 (m, 1H), 6.66 (s, 1H), 6.49 (s, 1H), 5.00–4.91 (m, 2H), 4.16 (s, 1H), 3.33 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 176.8, 164.1, 138.8, 135.7, 134.7, 132.4, 129.0, 128.7, 128.5, 128.4, 124.2, 122.9, 102.9, 75.3, 67.2, 29.7; IR (KBr, cm−1): ν 3374, 1711, 1608, 1579, 1461, 1313, 1165, 1113, 1045, 965, 782, 747, 703; HRMS (ESI) calcd for C19H16NO4NaBr ([M+Na]+): 424.0160; Found: 424.0163. HPLC analysis (OD-H column, λ = 254 nm, eluent: hexane/2-propanol = 90/10, flow rate: 1.0 mL min−1): tR = 10.62 min (minor), 13.15 min (major).(S)-benzyl 2-(3-hydroxy-1,5-dimethyl-2-oxoindolin-3-yl) acrylate (6p)
White solid, 99% yield, 89% ee, [α]16D +28.4 (c 0.67, CH2Cl2), After recrystallization: 98% ee. 1H NMR (CDCl3, 400 MHz): δ 7.31–7.29 (m, 3H), 7.11–7.08 (m, 3H), 6.99 (s, 1H), 6.63 (s, 1H), 6.60 (d, J = 8.0 Hz, 1H), 6.46 (s, 1H), 5.00–4.91 (m, 2H), 3.82 (s, 1H), 2.93 (s, 3H), 2.29 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 176.1, 164.4, 142.0, 139.2, 135.0, 132.6, 130.3, 129.3, 128.5 (×2), 128.4 (×2), 124.6, 108.5, 76.2, 67.0, 26.1, 21.0; IR (KBr, cm−1): ν 3322, 1727, 1700, 1622, 1497, 1367, 1288, 1187, 1106, 1049, 962, 807, 698; HRMS (ESI) calcd for C20H19NO4Na ([M+Na]+): 360.1212; Found: 360.1212. HPLC analysis (AD-H column, λ = 254 nm, eluent: hexane/2-propanol = 90/10, flow rate: 1.0 mL min−1): tR = 27.54 min (minor), 31.92 min (major).(S)-benzyl 2-(3-hydroxy-5-methoxy-1-methyl-2-oxoindolin-3-yl)acrylate (6q)
White solid, 90% yield, 85% ee, [α]27D +21.2 (c 0.64, CH2Cl2), 1H NMR (CDCl3, 400 MHz): δ 7.23–7.22 (m, 3H), 7.03–7.01 (m, 2H), 6.76–6.70 (m, 2H), 6.56–6.52 (m, 2H), 6.40 (s, 1H), 4.92–4.83 (m, 2H), 4.19 (s, 1H), 3.67 (s, 3H), 2.83 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 176.0, 164.3, 156.2, 139.0, 137.7, 134.9, 130.6, 128.7, 128.5 (×2), 128.4, 114.6, 110.9, 109.2, 76.4, 67.0, 55.8, 26.1; IR (KBr, cm−1): ν 3342, 3266, 1722, 1691, 1500, 1465, 1373, 1282, 1186, 1029, 961, 810, 754, 698; HRMS (ESI) calcd for C20H19NO5Na ([M+Na]+): 376.1161; Found: 376.1159. HPLC analysis (AD-H column, λ = 254 nm, eluent: hexane/2-propanol = 90/10, flow rate: 1.0 mL min−1): tR = 38.07 min (minor), 48.60 min (major).(S)-benzyl 2-(3-hydroxy-1,7-dimethyl-2-oxoindolin-3-yl) acrylate (6r)
White solid, 80% yield, 89% ee, [α]27D +54.8 (c 0.55, CH2Cl2), After recrystallization: 99% ee. 1H NMR (CDCl3, 400 MHz): δ 7.31–7.29 (m, 3H), 7.09–7.01 (m, 2H), 7.03–6.98 (m, 2H), 6.92–6.89 (m, 1H), 6.64 (s, 1H), 6.48 (s, 1H), 4.99–4.88 (m, 2H), 4.01 (s, 1H), 3.18 (s, 3H), 2.39 (s, 3H); 13C NMR (CDCl3, 100 MHz): δ 177.0, 164.4, 142.0, 139.3, 135.0, 133.9, 130.0, 128.6. 128.5, 128.4 (×2), 122.9, 121.8, 120.3, 75.4, 67.0, 29.5, 18.9; IR (KBr, cm−1): ν 3374, 1706, 1602, 1456, 1369, 1288, 1184, 1111, 1071, 1025, 962, 744, 700; HRMS (ESI) calcd for C20H19NO4Na ([M+Na]+): 360.1212; Found: 360.1205. HPLC analysis (AD-H column, λ = 254 nm, eluent: hexane/2-propanol = 90/10, flow rate: 1.0 mL min−1): tR = 29.48 min (minor), 37.65 min (major).Acknowledgements
We are grateful for the financial support from National Natural Science Foundation of China (20772029), and the Fundamental Research Funds for the Central Universities.References
- (a) For reviews, see: S. Peddibhotla, Curr. Bioact. Compd., 2009, 5, 20 CrossRef CAS; (b) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748 CrossRef CAS; (c) C. Marti and E. M. Carreira, Eur. J. Org. Chem., 2003, 2209 CrossRef CAS.
- (a) For reviews, see: A. B. Dounay and L. E. Overman, Chem. Rev., 2003, 103, 2945 CrossRef CAS; (b) F. Zhou, Y.-L. Liu and J. Zhou, Adv. Synth. Catal., 2010, 352, 1381 CrossRef CAS; (c) J. S. Russel, Top. Heterocycl. Chem., 2010, 26, 397 CrossRef CAS.
- (a) For organometallic catalysis, see: R. Shinatani, K. Takatsu and T. Hayashi, Chem. Commun., 2010, 46, 6822 RSC; (b) X.-P. Fu, L. Liu, D. Wang, Y.-J. Chen and C.-J. Li, Green Chem., 2011, 13, 549 RSC; (c) H. Lai, Z. Huang, Q. Wu and Y. Qin, J. Org. Chem., 2009, 74, 283 CrossRef CAS; (d) D. Tomita, K. Yamatsugu, M. Kanai and M. Shibasaki, J. Am. Chem. Soc., 2009, 131, 6946 CrossRef CAS; (e) T. Ishimaru, N. Shibata, J. Nagai, S. Nakamura, T. Toru and S. Kanemasa, J. Am. Chem. Soc., 2006, 128, 16488 CrossRef CAS; (f) D. Sano, K. Nagata and T. Itoh, Org. Lett., 2008, 10, 1593 CrossRef CAS; (g) J. Itoh, S. B. Han and M. J. Krische, Angew. Chem., Int. Ed., 2009, 48, 6313 CrossRef CAS; (h) V. Hanhan, A. H. Sahin, T. W. Chang, J. C. Fettinger and A. K. Franz, Angew. Chem., Int. Ed., 2010, 49, 744 CrossRef; (i) H. M. Meshram, P. Ramesh, B. C. Reddy, B. Sridhar and J. S. Yadav, Tetrahedron, 2011, 67, 3150 CrossRef CAS; (j) X.-C. Qiao, S.-F. Zhu and Q.-L. Zhou, Tetrahedron: Asymmetry, 2009, 20, 1254 CrossRef CAS; (k) K. Aikawa, S. Mimura, Y. Numata and K. Mikami, Eur. J. Org. Chem., 2011, 62 CrossRef CAS; (l) K. Zheng, C. Yin, X. Liu, L. Lin and X. Feng, Angew. Chem., Int. Ed., 2011, 50, 2573 CrossRef CAS; (m) Z.-Y. Cao, Y. Zhang, C.-B. Ji and J. Zhou, Org. Lett., 2011, 13, 6398 CrossRef CAS.
- (a) For organocatalysis, see: G. Luppi, P. G. Cozzi, M. Monari, B. Kaptein, Q. B. Broxterman and C. Tomasini, J. Org. Chem., 2005, 70, 7418 CrossRef CAS; (b) S. Nakamura, N. Hara, H. Nakashima, K. Kubo, N. Shibata and T. Toru, Chem.–Eur. J., 2008, 14, 8079 CrossRef CAS; (c) G. Luppi, M. Monari, F. A. Violante, A. C. Pinto, B. Kaptein, Q. B. Broxterman, S. J. Garden and C. Tomasini, Tetrahedron, 2006, 62, 12017 CrossRef CAS; (d) A. V. Malkov, M. A. Kabeshov, M. Bella, O. Kysilka, D. A. Malyshev, K. Pluháčková and P. Kočovský, Org. Lett., 2007, 9, 5473 CrossRef CAS; (e) F. Xue, S. Zhang, L. Liu, W. Duan and W. Wang, Chem.–Asian J., 2009, 4, 1664 CrossRef CAS; (f) N. Hara, S. Nakamura, N. Shibata and T. Toru, Chem.–Eur. J., 2009, 15, 6790 CrossRef CAS; (g) Q. Guo, M. Bhanushali and C.-G. Zhao, Angew. Chem., Int. Ed., 2010, 49, 9460 CrossRef CAS; (h) C. Shen, F. Shen, H. Xia, P. Zhang and X. Chen, Tetrahedron: Asymmetry, 2011, 22, 708 CrossRef CAS; (i) P. Chauhan and S. S. Chimni, Chem.–Eur. J., 2010, 16, 7709 CrossRef CAS; (j) J. Deng, S. Zhang, P. Ding, H. Jiang, W. Wang and J. Li, Adv. Synth. Catal., 2010, 352, 833 CrossRef CAS; (k) Y.-L. Liu and J. Zhou, Chem. Commun., 2012, 48, 1919 RSC; (l) G. Bergonzini and P. Melchiorre, Angew. Chem., Int. Ed., 2012, 51, 971 CrossRef CAS.
- (a) For recent reviews on asymmetric MBH reactions, see: G. Masson, C. Housseman and J. Zhu, Angew. Chem., Int. Ed., 2007, 46, 4614 CrossRef CAS; (b) D. Basavaiah, K. V. Rao and R. J. Reddy, Chem. Soc. Rev., 2007, 36, 1581 RSC; (c) P. R. Krishna, R. Sachwani and P. S. Reddy, Synlett, 2008, 2897 CrossRef CAS; (d) V. Carrasco-Sanchez, M. J. Simirgiotis and L. S. Santos, Molecules, 2009, 14, 3989 CrossRef CAS; (e) Y. Wei and M. Shi, Acc. Chem. Res., 2010, 43, 1005 CrossRef CAS.
- S. J. Garden and J. M. S. Skakle, Tetrahedron Lett., 2002, 43, 1969 CrossRef CAS.
- (a) Y.-L. Liu, B.-L. Wang, J.-J. Cao, L. Chen, Y.-X. Zhang, C. Wang and J. Zhou, J. Am. Chem. Soc., 2010, 132, 15176 CrossRef CAS; (b) X.-Y. Guan, Y. Wei and M. Shi, Chem.–Eur. J., 2010, 16, 13617 CrossRef CAS; (c) F. Zhong, G.-Y. Chen and Y. Lu, Org. Lett., 2011, 13, 82 CrossRef CAS.
- C.-C. Wang and X.-Y. Wu, Tetrahedron, 2011, 16, 2974 CrossRef.
- J. P. Malerich, K. Hagihara and V. H. Rawal, J. Am. Chem. Soc., 2008, 130, 14416 CrossRef CAS.
- (a) For reviews on squaramide catalysis, see: R. I. Storer, C. Aciro and L. H. Jones, Chem. Soc. Rev., 2011, 40, 2330 RSC; (b) J. Alemán, A. Parra, H. Jiang and K. A. Jørgensen, Chem.–Eur. J., 2011, 17, 6890 CrossRef.
- (a) A. Caiazzo, S. Dalili and A. K. Yudin, Org. Lett., 2002, 4, 2597 CrossRef CAS; (b) Y.-Q. Fang and E. N. Jacobsen, J. Am. Chem. Soc., 2008, 130, 5660 CrossRef CAS; (c) K. Yuan, L. Zhang, H.-L. Song, Y. Hu and X.-Y. Wu, Tetrahedron Lett., 2008, 49, 6262 CrossRef CAS.
- H.-L. Song, K. Yuan and X.-Y. Wu, Chem. Commun., 2011, 47, 1012 RSC.
- (a) For recent reviews on hydrogen bond activation, see: P. I. Dalko, Hydrogen Bonding in Organic Synthesis, Wiley-VCH, Weinheim, 2009 Search PubMed; (b) X. H. Yu and W. Wang, Chem.–Asian J., 2008, 3, 516 CrossRef CAS; (c) Z. G. Zhang and P. R. Schreiner, Chem. Soc. Rev., 2009, 38, 1187 RSC; (d) D. Leow and C.-H. Tan, Chem.–Asian J., 2009, 4, 488 CrossRef CAS; (e) S. J. Connon, Synlett, 2009, 354 CAS; (f) Y. Sohtome and K. Nagasawa, Synlett, 2010, 1 CAS; (g) M. Zeng and S.-L. You, Synlett, 2010, 1289 CAS; (h) M. Terada, Synthesis, 2010, 1929 CrossRef CAS; (i) Y. Takemoto, Chem. Pharm. Bull., 2010, 58, 593 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: NMR spectra for the phosphine-squaramides 3a–h, HPLC spectra for the Morita–Baylis–Hillman products and the crystallographic data. See DOI: 10.1039/c2ra20521a |
| This journal is © The Royal Society of Chemistry 2012 |