Dansyl-containing boronate hydrogel film as fluorescent chemosensor of copper ions in water†
Ryuhei
Nishiyabu
,
Hiroyasu
Kobayashi
and
Yuji
Kubo
*
Department of Applied Chemistry, Graduate School of Urban Environmental Sciences, Tokyo Metropolitan University, 1-1 Minami-ohsawa, Hachioji, Tokyo, 192-0397, Japan. E-mail: yujik@tmu.ac.jp; Fax: +81-42-677-3134; Tel: +81-42-677-3134
First published on 14th May 2012
Abstract
A new type of boronate hydrogel with covalently bound dansyldiethylenetriamine as an indicator has been developed; the gel networks are based on boronate esterification of poly(vinylalcohol) with benzene-1,4-diboronic acid. In this approach, phenylboronic acid-appended N-dansyldiethylenetriamine 1 was newly synthesized to be incorporated into the gel matrix. The resulting gel film showed an absorption band at 336 nm and fluorescence at 511 nm when excited at 340 nm in water. The fluorescence measurements indicated that at neutral conditions using a HEPES buffer, the gel film was selectively quenched after immersion in an aqueous solution of Cu2+ for 30 min. The response had minimal interference from other metal ions such as Na+, K+, Mg2+, Ca2+, Fe3+, Co2+, Ni2+, Zn2+, Cd2+, Hg2+, Al3+, and Pb2+, which was noteworthy because dansyldiethylenetriamine alone responds to Hg2+ and Ni2+ in addition to Cu2+ in water. The reversible sensing capability was also evaluated by rinsing the film with an aqueous solution of ethylenediaminetetraacetic acid (EDTA). The gel was found to be a reusable and free-standing film capable of visually detecting Cu2+, providing a simple and expedient tool for on-site monitoring of Cu2+ in environmental applications such as water analysis.
Introduction
Chemosensors capable of fluorometrically detecting transition metal ions in water1 have been recognized as powerful sensing tools for applications in environmental and biological fields. Accordingly, numerous efforts have been devoted to the design and development of selective fluorescent sensors in the disciplinary areas of supramolecular chemistry and analytical chemistry.2 Dansyl fluorophore (5-dimethylamino-1-naphthalenesulfonate) is one of the most useful candidates for designing the desired chemosensors, and it is characterized by a charge transfer excited state that exhibits solvatochromism and high emission quantum yields.3 The synthetic availability of the sulfonic acid group, together with its optical properties, led to the development of fluorescent sensors for the detection of not only cations4–8 but also anions9 in water. In particular, Cu2+ is a significant metal pollutant due to its widespread use, such as in plating technology.10 The use of the dansyl moiety has been a feasible approach for highly sensitive Cu2+ sensing by monitoring changes in fluorescence.5–8 However, a major drawback of dansyl-based systems is that they often suffer from cross-sensitivity toward other ions, particularly potential competitors containing Hg2+, due to their similar chemical behavior to Cu2+.7,8 Further, solution-based sensors are inconvenient for not only real-time and real-space measurements but also repetitive use. In this context, solid surface-supported fluorescent sensors are of growing interest for Cu2+ detection. For instance, Cu2+-binding ligands and dansyl reporters were immobilized on silica nanoparticles.11 Dansyl reporters have also been applicable to polymer-based12 as well as film-based13 fluorescent chemosensors for Cu2+.In an alternative approach, the use of boronate hydrogels14 has attracted our attention for film fabrication. Although it is well-known that the addition of borate as a cross-linking agent leads to the formation of a three-dimensional polyvinyl alcohol (PVA) network,15,16 to the best of our knowledge, gel indicators based on the PVA–borate network have been as yet unknown, possibly because PVA–borate gels are dissolved in water (vide infra). We realized that the employment of suitable boronic acid-appended cross-linkers and fluorophores would allow the fabrication of PVA-based gel films that provide not only a preferable microenvironment for the detection of the analyte in water but also immobilize the working units. Subsequent systems have potential as portable devices that would be user-friendly detection tools. As a proof-of-concept study, we report here a new type of reusable gel film that is composed of a covalently attached dansyl indicator in a PVA–boronate matrix. Importantly, the film was highly selective toward copper ion-induced fluorescence response in water, with minimal interference from competitive metal ions such as Hg2+.
Results and discussion
Synthesis of boronic acid-appended N-dansyldiethylenetriamine
To fabricate fluorescent boronate gels, it is required that the synthetic connection of phenylboronic acid into the dansyl indicator skeleton does not interfere with the metal ion binding and optical properties. With this in mind, we decided to use N-dansyldiethylenetriamine 26,17 because of the easy connection of phenylboronic acid at the terminal amine moiety of 2; the reductive amination of 2 with MIDI (N-methyliminodiacetic acid)-protected formylphenylboronic acid in the presence of NaBH4 afforded 1 after purification, in 18% yield. The assignment of the new compound 1 was carried out using several spectroscopic data (see ESI,† Fig. S1–4). The optical properties came from UV-vis and fluorescence measurements; 1 shows an absorption band at 329 nm (ε = 4.4 × 103 M−1 cm−1) in 2% DMSO aqueous solution (5.0 mM HEPES, pH = 7.0), which is consistent with that of 2. This suggests that the incorporation of phenylboronic acid into 2 causes no perturbation toward dansyl fluorophore. Indeed, when excited at 340 nm in 2% DMSO aqueous solution (5.0 mM HEPES, pH = 7.0), an orange emission was observed with a fluorescence maximum at 545 nm and a quantum yield of 0.02 against a quinine sulphate (Φ = 0.546 in 0.5 M H2SO4 aqueous solution).18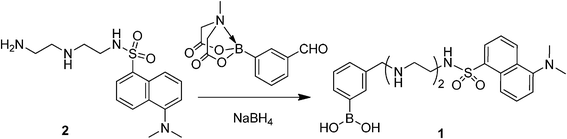 | ||
| Scheme 1 Synthesis of boronic acid-appended N-dansyldiethylenetriamine 1. | ||
Formation of boronate–PVA gel
We initially explored suitable cross-linkers for stable boronate gels in water. It is known that PVA–borate systems form homogeneous gels.15,16 Thus, when the phase diagram reported in the paper16 is considered, dansyl-containing PVA–borate gel was prepared from PVA (M.W. = 146![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–186
000–186![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 6.4 × 10−1 M19), borax (9.4 × 10−2 M), and 1 (1.3 × 10−4 M) (see ESI,† Fig. S5). The resulting fluorescent gel was found to dissolve in water within 90 min. To prepare stable boronate gels in water, we studied how benzene-1,4-diboronic acid (DBA) with a hydrophobic character would behave as a cross-linking agent in the PVA matrix. For this preparation, DBA was initially allowed to react with PVA in DMSO to provide an organogel because of its low solubility in water. Fig. 1 shows the phase diagram of the boronate–PVA gel in DMSO. 1.5 mL samples were prepared by mixing various amounts of PVA and DBA. For a solution containing a low concentration of PVA (<0.1 M), the sol phase was characterized even at a relatively high concentration of DBA. In contrast, when the PVA concentration became adequate to ensure intermolecular cross-linking through boronate esterification with DBA, gelation occurred. After several trials to obtain suitable gels to this end, we employed the following conditions: PVA = 4.0 × 10−1 M19 and DBA = 1.2 × 10−2 M. Under these conditions, excess amounts of free hydroxyl groups of PVA remained to participate in boronate esterification with 1.
000, 6.4 × 10−1 M19), borax (9.4 × 10−2 M), and 1 (1.3 × 10−4 M) (see ESI,† Fig. S5). The resulting fluorescent gel was found to dissolve in water within 90 min. To prepare stable boronate gels in water, we studied how benzene-1,4-diboronic acid (DBA) with a hydrophobic character would behave as a cross-linking agent in the PVA matrix. For this preparation, DBA was initially allowed to react with PVA in DMSO to provide an organogel because of its low solubility in water. Fig. 1 shows the phase diagram of the boronate–PVA gel in DMSO. 1.5 mL samples were prepared by mixing various amounts of PVA and DBA. For a solution containing a low concentration of PVA (<0.1 M), the sol phase was characterized even at a relatively high concentration of DBA. In contrast, when the PVA concentration became adequate to ensure intermolecular cross-linking through boronate esterification with DBA, gelation occurred. After several trials to obtain suitable gels to this end, we employed the following conditions: PVA = 4.0 × 10−1 M19 and DBA = 1.2 × 10−2 M. Under these conditions, excess amounts of free hydroxyl groups of PVA remained to participate in boronate esterification with 1.
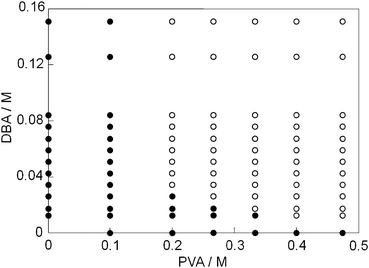 | ||
| Fig. 1 Phase diagram of the PVA–DBA system in DMSO (1.5 mL) at room temperature; (●) sol, (○) gel. | ||
The DMSO gel could be transferred to the corresponding hydrogel by immersing it in water (see experimental section). The obtained hydrogels were stable in water and their characterization was based on the FT-IR analysis. When the xerogel was measured by the KBr method, a characteristic intense peak assigned to the boronate ester formation between PVA and DBA was observed at 664 cm−1, together with a peak corresponding to the B–O stretch at 1304 cm−1 (Fig. 2).20 This result confirmed that the gel formation was driven by benzene diboronate-diol cross-linking in PVA. The gel is stable in water and pH stability tests indicated that the gel never decomposes between pH 1 and 11 (see ESI,† Fig. S6).
 | ||
| Fig. 2 FT-IR spectrum of xerogel produced by PVA and DBA. A characteristic intense peak at 664 cm−1 (*) was assignable to the boronate esters. | ||
Fabrication of boronate gel films with dansyl moieties
Taking the phase diagram of the PVA–DBA system into account (Fig. 1), the target boronate gels with indicators were fabricated as follows. A DMSO solution (0.15 mL) of indicator 1 (6.7 × 10−4 M) and DBA (6.0 × 10−2 M) at 25 °C was added to a DMSO solution (0.60 mL) of PVA (5.0 × 10−1 M19). The resulting solution containing PVA (4.0 × 10−1 M19), DBA (1.2× 10−2 M), and 1 (1.3 × 10−4 M) was cast into a slab at room temperature to immediately afford DMSO gel films with a molar cross-linking ratio of DBA to the PVA monomer unit of 0.12. After aging for 24 h at room temperature, the film was again immersed in DMSO, and then in solvents of increasing water ratio to obtain the desired fluorescent hydrogel. The film was a swollen gel with dimensions of 30 × 10 × 1 mm (Fig. 3a).21 The content of the indicator that was immobilized in the PVA matrix was estimated via UV-vis measurement, and the absorbance of the gel film suggested that (3.8 ± 0.3) × 10−8 mol of the indicator was incorporated into the boronate gel. A plausible structure is shown in Fig. 3b. The morphological analysis of the xerogel was performed by field-emission scanning electron microscopy (FE-SEM), which suggested the formation of a fibrous network structure (Fig. 3c).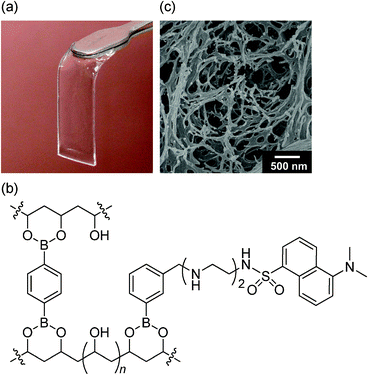 | ||
| Fig. 3 (a) Photograph of the boronate hydrogel film with dansyl moieties; (b) proposed structure of the indicator hydrogel wherein OH-inserted sp3-hybridized tetrahedral boronate esters would be partly produced; (c) FE-SEM image of the xerogel. | ||
Chemosensor capability of boronate hydrogel film
The boronate film exhibited fluorescence at 511 nm when excited at 340 nm in a HEPES aqueous solution; the emission maxima was blue shifted by 34 nm and the fluorescence quantum yield increased significantly (Φ = 0.37) in comparison with dye 1 in 2% DMSO aqueous solution (5.0 mM HEPES, pH = 7.0), possibly due to the hydrophobic microenvironment of the gel matrix.22 These optical properties enabled us to detect green emission from the film upon irradiation with UV light. We thus investigated how Cu2+ would induce a change in the fluorescence spectra of the gel film. The boronate film was immersed in a HEPES aqueous solution (5 mM, pH = 7.0) containing various amounts of Cu2+. The resulting solution was shaken at 125 rpm for 30 min. As shown in Fig. 4, the fluorescence of the gel was significantly quenched upon addition of incremental amounts of Cu2+. A non-linear curve fitting procedure (Fig. 4, inset) was carried out under the assumption of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric complex formation between the dansyldiethylenetriamine moiety and Cu2+,6 and we reproduced the fluorescence titration data wherein the association constant of the film with Cu2+ was estimated to be (3.74 ± 1.02) × 106 M−1. For practical applicability, the detection limit was estimated to be 8.83 × 10−7 M (883 nM) from the fluorescence profile in Fig. 4.
1 stoichiometric complex formation between the dansyldiethylenetriamine moiety and Cu2+,6 and we reproduced the fluorescence titration data wherein the association constant of the film with Cu2+ was estimated to be (3.74 ± 1.02) × 106 M−1. For practical applicability, the detection limit was estimated to be 8.83 × 10−7 M (883 nM) from the fluorescence profile in Fig. 4.
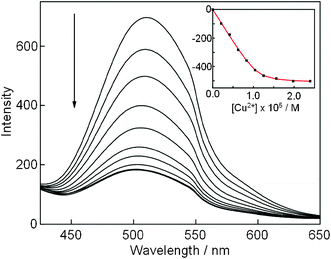 | ||
| Fig. 4 Change in fluorescence spectra of gel film (30 × 10 × 1 mm, 1 = 1.25 × 10−5 M) after immersion in Cu2+ in HEPES aqueous solution (5 mM, pH = 7.0, 3.0 mL) for 30 min: λex = 340 nm. Inset: fluorescent intensity changes at 511 nm plotted as a function of incremental amounts of Cu2+. | ||
The time dependency for Cu2+-induced fluorescence quenching was also examined. The gel film was immersed in a HEPES aqueous solution of Cu2+ (1.25 × 10−5 M), being almost consistent with the amount of dansyl moiety incorporated into the gel. After shaking (125 rpm) the resulting solution, the emission intensity was recorded at different times (Fig. 5a). A good linear relation between ln[(I0 − Ilim)/(Ilim − I)] versus time was obtained to estimate the first-order rate constant, 8.7 × 10−2 min−1. This means that the quenching of fluorescence is almost saturated (≈90%) for 30 min (Fig. 5b and 5c).
![(a) Time dependency for the Cu2+-induced fluorescent response of the gel film. (Inset: corresponding plot of ln[(I0 − Ilim)/(Ilim − I)] versus time for fluorescence quenching at 511 nm after immersion in a HEPES aqueous solution of Cu2+ (1.25 × 10−5 M).) Photograph of gel films under UV light (365 nm) after being immersed in HEPES aqueous solution (5 mM, pH = 7.0, 3.0 mL) for 30 min: (b) copper ion-free conditions; (c) in the presence of Cu2+ (1.25 × 10−5 M).](/image/article/2012/RA/c2ra20516e/c2ra20516e-f5.gif) | ||
| Fig. 5 (a) Time dependency for the Cu2+-induced fluorescent response of the gel film. (Inset: corresponding plot of ln[(I0 − Ilim)/(Ilim − I)] versus time for fluorescence quenching at 511 nm after immersion in a HEPES aqueous solution of Cu2+ (1.25 × 10−5 M).) Photograph of gel films under UV light (365 nm) after being immersed in HEPES aqueous solution (5 mM, pH = 7.0, 3.0 mL) for 30 min: (b) copper ion-free conditions; (c) in the presence of Cu2+ (1.25 × 10−5 M). | ||
Next, we investigated the selectivity of the gel toward other metal ions in water. Aqueous solutions (3 mL) of metal ions (1.25 × 10−5 M) such as Na+, K+, Mg2+, Ca2+, Fe3+, Co2+, Ni2+, Zn2+, Cd2+, Hg2+, Al3+ and Pb2+ were prepared in water. The gel film was immersed into each solution. After shaking the resulting solution (125 rpm) for 30 min, the fluorescence was measured. As shown in Fig. 6, the film exhibited a remarkable selectivity toward Cu2+. Other metal ions induced no fluorescence quenching of the film. Although it is known that dansyloligoamines respond to not only Cu2+ but also Hg2+ in water,7 dansyl-containing boronate hydrogel films respond to only Cu2+ in water.
![Fluorescence intensity changes in film gels after immersion in aqueous solutions of Na+, K+, Mg2+, Ca2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Hg2+, Al3+ and Pb2+, respectively. [Mn+] = 1.25 × 10−5 M, λex = 340 nm, λem = 511 nm.](/image/article/2012/RA/c2ra20516e/c2ra20516e-f6.gif) | ||
| Fig. 6 Fluorescence intensity changes in film gels after immersion in aqueous solutions of Na+, K+, Mg2+, Ca2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Hg2+, Al3+ and Pb2+, respectively. [Mn+] = 1.25 × 10−5 M, λex = 340 nm, λem = 511 nm. | ||
To obtain further evidence regarding selectivity, competitive experiments were carried out by adding Cu2+ (1.25 × 10−5 M) to the buffered solution in the presence of excess amounts of other metal ions (1.25 × 10−4 M). As shown in Fig. 7, there is almost no fluorescence quenching of the films in the presence of competitive metal ions, with the exception of 10 equiv. Ni2+, which causes a 17% decrease in the emission. This can be explained on the basis of energy transfer from dansyl to Ni2+, which should be attributed to quenching.6 In contrast, the addition of a relatively low amount of Cu2+ (1.25 × 10−5 M) to the solutions containing each metal ion other than Fe3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 quenched the emission to 30% of its initial value. As a result, although its selectivity toward Cu2+ slightly decreased in the case of Ni2+ and Fe3+, the film is still most responsive to Cu2+. Thus, co-existent ions of Hg2+ (Fig. 7) have some or a negligible interference effect on Cu2+-sensing in the boronate gel film.
23 quenched the emission to 30% of its initial value. As a result, although its selectivity toward Cu2+ slightly decreased in the case of Ni2+ and Fe3+, the film is still most responsive to Cu2+. Thus, co-existent ions of Hg2+ (Fig. 7) have some or a negligible interference effect on Cu2+-sensing in the boronate gel film.
 | ||
| Fig. 7 Competitive experiments for fluorescence quenching of film. The red bars represent the emission intensity of the film in the presence of other metal ions (1.25 × 10−4 M). The blue bars represent the emission intensity of the film that occurs upon addition of Cu2+ (1.25 × 10−5 M) in the presence of other metal ions (1.25 × 10−4 M). | ||
The fluorescence quenching could be recovered by simple rinsing of the film with an aqueous solution of ethylenediaminetetraacetic acid (EDTA). We investigated the reversible sensing capability by alternatively exposing the film to 3 mL of an aqueous solution (5 mM HEPES buffer, pH = 7.0) containing Cu2+ (1.25 × 10−5 M) and EDTA (1.25 × 10−5 M). As shown in Fig. 8, when the film was immersed in EDTA solution with shaking at 125 rpm for 30 min at room temperature, approximately 85% of emission could be restored as a result of a competitive binding phenomenon between the film and EDTA toward Cu2+. The reversible fluorescence switch was obtained after alternating treatment with Cu2+ and EDTA, although the fluorescence intensity of the film decreased gradually over several cycles. Compared to homogeneous solution-based chemosensors, the reversibility of the film has significant merits for practical use as an indicator.
 | ||
| Fig. 8 Fluorescence intensity change in the gel film upon alternating treatment by aqueous solutions of Cu2+ (1.25 × 10−5 M) and EDTA (1.25 × 10−5 M): λex = 340 nm, λem = 511 nm. | ||
Conclusion
We prepared for the first time a PVA–boronate hydrogel via boronate esterification between DBA and PVA. The incorporation of dansyldiethylenetriamine into the gel matrix using 1 allowed us to develop an emissive film through casting into a slab, exhibiting highly selective fluorescence quenching toward Cu2+ over other metal ions tested in aqueous HEPES solution (pH = 7.0). This result is worthwhile for developing chemosensors because dansyloligoamines significantly respond to Hg2+ as well as Cu2+ in a solution. In addition, the reusability of the sensing suggests that the film has potential as a simple and expedient tool for on-site monitoring of Cu2+ in environmental applications such as water analysis. We believe that the ready binding of functional phenylboronic acids into the PVA matrix would lead to the preparation of a variety of indicator gels; for instance, two different indicators could be incorporated into the gel matrix through boronate esterification wherein a synergistic effect between the indicators would provide advanced chemosensor systems. Further research in this regard is currently under way in our laboratory.Experimental section
Materials
Unless otherwise indicated, reagents and solvents used for this study were commercially available and used as supplied (Tokyo Kasei, Kanto Kagaku, Wako, Aldrich, and Merck). N-Dansyldiethylenetriamine was synthesized according to the procedure in a previous paper.17 As for the buffer solution, HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer solution (5 mM, pH = 7, I = 0.01) was used. The pH and ionic strength were adjusted by the addition of aqueous sodium hydroxide (1.0 M) and sodium chloride solution (1.0 M).Measurements
NMR spectra were recorded on a Bruker AVANCE-500 spectrometer using tetramethylsilane (TMS) as an internal standard (0 ppm) for 1H and 13C NMR analysis and BF3·OEt2 as an external standard (0 ppm) for 11B NMR analysis. All spectra were recorded at 298 K. Fast atom bombardment (FAB) mass spectra were obtained on a JEOL JMS-700 spectrometer where 3-nitrobenzyl alcohol was used as a matrix. Field-emission scanning electron microscopy (FE-SEM) was performed using a JEOL JSM-7500F (acceleration voltage of 5 kV). For FE-SEM measurement, xerogel of the boronate ester gel was prepared by a freeze-drying method and was coated with Au on an EIKO IB3 ION COATER. Infrared spectra were recorded on a JASCO FT-IR-5300 spectrometer using a KBr pellet technique. Elemental analysis was performed on a Yanaco CHNcoder MT-5 analyzer. Absorption and fluorescence spectra were measured on a Shimadzu UV-3100PC and a JASCO FP-6300 spectrophotometer, respectively. For UV-vis absorption measurement, the dansyl-functionalized boronate gel was put into a 1 cm × 1 cm cell filled with water. For fluorescence emission measurements, the films were put into a cell filled with the solution at an angle of 45° with respect to the incident light using a quartz glass plate (42 mm × 13 mm). The slit size for excitation and emission was 5 nm. Fluorescence quantum yields of dye 1 and dansyl-functionalized boronate gel were determined using quinine sulphate (Φ = 0.546 in 0.5 M H2SO4 aqueous solution) with 340 nm excitation at 25 °C as a reference.18Fabrication of dansyl-functionalized boronate hydrogel
A DMSO solution (0.6 mL) of PVA (M.W. = 146![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000∼186
000∼186![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 5.0 × 10−1 M)19 was added to a DMSO solution (0.15 mL) containing benzene-1,4-diboronic acid (6.0 × 10−2 M) and 1 (6.7 × 10−4 M) in a vial. The resulting solution was immediately cast into a slab to afford DMSO gel films (approximately 48 × 16 × 2 mm) and aged for 24 h at room temperature. The film was immersed in DMSO (5 mL) again, and then shaken at 100 rpm for 30 min. Similar procedures were carried out in solvents of increasing water ratio (5 mL, DMSO
000, 5.0 × 10−1 M)19 was added to a DMSO solution (0.15 mL) containing benzene-1,4-diboronic acid (6.0 × 10−2 M) and 1 (6.7 × 10−4 M) in a vial. The resulting solution was immediately cast into a slab to afford DMSO gel films (approximately 48 × 16 × 2 mm) and aged for 24 h at room temperature. The film was immersed in DMSO (5 mL) again, and then shaken at 100 rpm for 30 min. Similar procedures were carried out in solvents of increasing water ratio (5 mL, DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water = 50
water = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 , 25
50 , 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75, 12.5
75, 12.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 87.5 (v/v)) to obtain the desired fluorescent hydrogel. The film was a swollen gel with dimensions of 30 × 10 × 1 mm. The amount of immobilized 1 in the PVA–boronate gel matrix was calculated based on the absorption intensity of the immobilized 1 at 336 nm in the gel matrix and the extinction coefficient of 1 in DMSO (ε = 4.5 × 103 M−1 cm−1 at 341 nm).
87.5 (v/v)) to obtain the desired fluorescent hydrogel. The film was a swollen gel with dimensions of 30 × 10 × 1 mm. The amount of immobilized 1 in the PVA–boronate gel matrix was calculated based on the absorption intensity of the immobilized 1 at 336 nm in the gel matrix and the extinction coefficient of 1 in DMSO (ε = 4.5 × 103 M−1 cm−1 at 341 nm).
Fluorescence measurements of dansyl-functionalized boronate hydrogel
For the measurements, nitrate salts of metal ions (Na+, K+, Mg2+, Ca2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Hg2+, Al3+, and Pb2+) were used. The dansyl-functionalized boronate gel was immersed in HEPES buffer solutions (3 mL) containing the metal ions and shaken at 125 rpm with a shaking apparatus for 30 min at room temperature. The binding constant (K) of the immobilized dansyltriamine part with Cu2+ in a PVA–boronate matrix was determined from the titration curve in the fluorescence spectra upon the addition of incremental amounts of Cu2+ (Fig. 4) wherein the change in fluorescence intensity was expressed in the following equations.| A = K × M0 + K × S0 + 1 |
I, I0, and ΔIlim are the observed fluorescence intensity, the fluorescence intensity in the absence of a metal ion, and the saturation change in fluorescence intensity, respectively. In the equation, K, S0, and M0 are the binding constant, the initial concentration of 1, and the concentration of the metal ion added, respectively. The K and ΔIlim values were evaluated by a nonlinear least-squares method.
For the determination of the detection limit toward Cu2+, the intersection of the regression line obtained from the value of (I − I0) in the dynamic range of the titration curve allowed us to estimate the value of the detection limit by the following equation.
Y = Iavg − 3 × σ
I avg and σ are the average value and the standard deviation of fluorescence intensity at 511 nm, respectively, in the absence of a metal ion, measured three times.
Reusability of the film
For the estimation, after being immersed in a Cu2+ (1.25 × 10−5 M in 5 mM HEPES buffer (3 mL) at pH 7.0) aqueous solution for 30 min with shaking at 125 rpm at room temperature, the film was immersed in aqueous EDTA solution (1.25 × 10−5 M in 5 mM HEPES buffer (3 mL) at pH 7.0) for 30 min with shaking at 125 rpm at room temperature. The fluorescence measurement was then carried out.Acknowledgements
This research was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (Nos. 21550137, 23750167), the Yamada Science Foundation, and the JGC-S Scholarship Foundation.References
- (a) Current examples, see: B. K. Kanungo, M. Baral, R. K. Bera and S. K. Sahoo, Monatsh. Chem., 2010, 141, 157–168 CrossRef CAS; (b) C. Song, X. Zhang, C. Jia, P. Zhou, X. Quan and C. Duan, Talanta, 2010, 81, 643–649 CrossRef CAS; (c) C.-Y. Li, F. Xu and Y.-F. Li, Spectrochim. Acta, 2010, 76A, 197–201 CrossRef CAS; (d) H. Dai and H. Xu, Bioorg. Med. Chem. Lett., 2011, 21, 5141–5144 CrossRef CAS; (e) In addition, fluorescence sensors for the trace-level determination of toxic-metal ions have been reviewed; see: M. Dutta and D. Das, TrAC, Trends Anal. Chem., 2012, 32, 113–132 CrossRef CAS.
- (a) Current review articles, see: R. A. Bissell, A. P. de Silva, H. Q. N. Gunaratne, P. L. M. Lynch, G. E. M. Maguire and K. R. A. S. Sandanayake, Chem. Soc. Rev., 1992, 21, 187–195 RSC; (b) A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515–1566 CrossRef CAS; (c) B. Valeur and I. Leray, Coord. Chem. Rev., 2000, 205, 3–40 CrossRef CAS; (d) L. Prodi, F. Bolletta, M. Montalti and N. Zaccheroni, Coord. Chem. Rev., 2000, 205, 59–83 CrossRef CAS; (e) J. F. Callan, A. P. de Silva and D. C. Magri, Tetrahedron, 2005, 61, 8551–8588 CrossRef CAS; (f) E. M. Nolan and S. J. Lippard, Chem. Rev., 2008, 108, 3443–3480 CrossRef CAS; (g) A. P. de Silva, T. S. Moody and G. D. Wright, Analyst, 2009, 134, 2385–2393 RSC; (h) L.-J. Fan, Y. Zhang, C. B. Murphy, S. E. Angell, M. F. L. Parker, B. R. Flynn and W. E. Jones Jr., Coord. Chem. Rev., 2009, 253, 410–422 CrossRef CAS; (i) R. N. Dsouza, U. Pischel and W. M. Nau, Chem. Rev., 2011, 111, 7941–7980 CrossRef CAS; (j) N. Kaur and S. Kumer, Tetrahedron, 2011, 67, 9233–9264 CrossRef CAS.
- Y.-H. Li, L.-M. Chan, L. Tyer, R. T. Moody, C. M. Himel and D. M. Hercules, J. Am. Chem. Soc., 1975, 97, 3118–3126 CrossRef CAS.
- (a) G. K. Walkup and B. Imperiali, J. Am. Chem. Soc., 1997, 119, 3443–3450 CrossRef CAS; (b) L. Prodi, F. Bolletta, M. Montalti and N. Zaccheroni, Eur. J. Inorg. Chem., 1999, 455–460 CrossRef CAS; (c) S. Aoki, H. Kawatani, T. Goto, E. Kimura and M. Shiro, J. Am. Chem. Soc., 2001, 123, 1123–1132 CrossRef CAS; (d) R. Métivier, I. Leray and B. Valeur, Chem.–Eur. J., 2004, 10, 4480–4490 CrossRef; (e) Y. Zhao and Z. Zhong, Org. Lett., 2006, 8, 4715–4717 CrossRef CAS; (f) Y. Zhao and Z. Zhong, J. Am. Chem. Soc., 2006, 128, 9988–9989 CrossRef CAS; (g) H.-W. Li, Y. Li, Y.-Q. Dang, L.-J. Ma, Y. Wu, G. Hou and L. Wu, Chem. Commun., 2009, 4453–4455 RSC; (h) M. Kumar, R. Kumar and V. Bhalla, Chem. Commun., 2009, 7384–7386 RSC; (i) S. Pandey, A. Azam, S. Pandey and H. M. Chawla, Org. Biomol. Chem., 2009, 7, 269–279 RSC; (j) Ü. Ocak, M. Ocak, K. Surowiec, X. Liu and R. A. Bartsch, Tetrahedron, 2009, 65, 7038–7047 CrossRef; (k) M. Suresh, S. Mishra, S. K. Mishra, E. Suresh, A. K. Mandal, A. Shrivastav and A. Das, Org. Lett., 2009, 11, 2740–2743 CrossRef CAS; (l) P. Dinake, P. E. Prokhorova, V. S. Talanov, R. J. Butcher and G. G. Talanova, Tetrahedron Lett., 2010, 51, 5016–5019 CrossRef CAS; (m) B. P. Joshi, C. R. Lohani and K.-H. Lee, Org. Biomol. Chem., 2010, 8, 3220–3226 RSC; (n) D. Maity and T. Govindaraju, Chem. Commun., 2010, 46, 4499–4501 RSC; (o) L. Chen, L. Yang, H. Li, Y. Gao, D. Deng, Y. Wu and L.-J. Ma, Inorg. Chem., 2011, 50, 10028–10032 CrossRef CAS; (p) P. Anzenbacher Jr., F. Li and M. A. Palacois, Angew. Chem., Int. Ed., 2012, 51, 2345–2348 CrossRef.
- (a) A. Torrado, G. K. Walkup and B. Imperiali, J. Am. Chem. Soc., 1998, 120, 609–610 CrossRef CAS; (b) S. Bhattacharya and M. Thomas, Tetrahedron Lett., 2000, 41, 10313–10317 CrossRef CAS; (c) L. Kovbasyuk and R. Krämer, Inorg. Chem. Commun., 2006, 9, 586–590 CrossRef CAS; (d) B. R. White and J. A. Holcombe, Talanta, 2007, 71, 2015–2020 CrossRef CAS; (e) M. H. Lee, H. J. Kim, S. Yoon, N. Park and J. S. Kim, Org. Lett., 2008, 10, 213–216 CrossRef CAS; (f) V. S. Jisha, A. J. Thomas and D. Ramaiah, J. Org. Chem., 2009, 74, 6667–6673 CrossRef CAS; (g) K. Kaur and S. Kumar, Dalton Trans., 2011, 40, 2451–2458 RSC.
- R. Corradini, A. Dossena, G. Galaverna, R. Marchelli, A. Panagia and G. Sartor, J. Org. Chem., 1997, 62, 6283–6289 CrossRef CAS.
- L. Prodi, M. Montalti, N. Zaccheroni, F. Dallavalle, G. Folesani, M. Lanfranchi, R. Corradini, S. Pagliari and R. Marchelli, Helv. Chim. Acta, 2001, 84, 690–706 CrossRef CAS.
- (a) A. Dhir, V. Bhalla and M. Kumar, Org. Lett., 2008, 10, 4891–4894 CrossRef CAS; (b) L.-J. Ma, Y. Li, L. Li, J. Sun, C. Tian and Y. Wu, Chem. Commun., 2008, 6345–6347 RSC; (c) T. Gruber, C. Fischer, M. Felsmann, W. Seichter and E. Weber, Org. Biomol. Chem., 2009, 7, 4904–4917 RSC.
- (a) C.-F. Chen and Q.-Y. Chen, Tetrahedron Lett., 2004, 45, 3957–3960 CrossRef CAS; (b) R. Miao, Q.-Y. Zheng, C.-F. Chen and Z.-T. Huang, Tetrahedron Lett., 2004, 45, 4959–4962 CrossRef CAS; (c) S. Pagliari, R. Corradini, G. Galaverna, S. Sforza, A. Dossena, M. Montalti, L. Prodi, N. Zaccheroni and R. Marchelli, Chem.–Eur. J., 2004, 10, 2749–2758 CrossRef CAS; (d) S.-Y. Liu, Y.-B. He, G.-Y. Qing, K.-X. Xu and H.-J. Qin, Tetrahedron: Asymmetry, 2005, 16, 1527–1534 CrossRef CAS.
- F. Fu and Q. Wang, J. Environ. Manage., 2011, 92, 407–418 CrossRef CAS.
- (a) E. Brasola, F. Mancin, E. Rampazzo, P. Tecilla and U. Tonellato, Chem. Commun., 2003, 3026–3027 RSC; (b) E. Rampazzo, E. Brasola, S. Marcuz, F. Mancin, P. Tecilla and U. Tonellato, J. Mater. Chem., 2005, 15, 2687–2696 RSC; (c) M. Montalti, L. Prodi and N. Zaccheroni, J. Mater. Chem., 2005, 15, 2810–2814 RSC; (d) M. Arduini, S. Marcuz, M. Montolli, E. Rampazzo, F. Mancin, S. Gross, L. Armelao, P. Tecilla and U. Tonellato, Langmuir, 2005, 21, 9314–9321 CrossRef CAS; (e) R. Métivier, I. Leray, B. Lebeau and B. Valeur, J. Mater. Chem., 2005, 15, 2965–2973 RSC.
- (a) Y. Zheng, K. M. Gattás-Asfura, C. Li, F. M. Andreopoulos, S. M. Pham and R. M. Leblanc, J. Phys. Chem. B, 2003, 107, 483–488 CrossRef CAS; (b) J. Yin, X. Guan, D. Wang and S. Liu, Langmuir, 2009, 25, 11367–11374 CrossRef CAS.
- (a) Y. Zheng, J. Orbulescu, X. Ji, F. M. Andreopoulos, S. M. Pham and R. M. Leblanc, J. Am. Chem. Soc., 2003, 125, 2680–2686 CrossRef CAS; (b) L. Ding, X. Cui, Y. Han, F. Lü and Y. Fang, J. Photochem. Photobiol., A, 2007, 186, 143–150 CrossRef CAS.
- (a) D. Shiino, Y. Murata, A. Kubo, Y. J. Kim, K. Kataoka, Y. Koyama, A. Kikuchi, M. Yokoyama, Y. Sakurai and T. Okano, J. Controlled Release, 1995, 37, 269–276 CrossRef CAS; (b) A. Kikuchi, K. Suzuki, O. Okabayashi, H. Hoshino, K. Kataoka, Y. Sakurai and T. Okano, Anal. Chem., 1996, 68, 823–828 CrossRef CAS; (c) I. Hisamitsu, K. Kataoka, T. Okano and Y. Sakurai, Pharm. Res., 1997, 14, 289–293 CrossRef CAS; (d) M. D. Parris, B. A. MacKay, J. W. Rathke, R. J. Klingler and R. E. Gerald II, Macromolecules, 2008, 41, 8181–8186 CrossRef CAS; (e) W. M. J. Ma, M. P. P. Morais, F. D'Hooge, J. M. H. v. d. Elsen, J. P. L. Cox, T. D. James and J. S. Fossey, Chem. Commun., 2009, 532–534 RSC; (f) S. Manju, M. Antony and K. Sreenivasan, J. Mater. Sci., 2010, 45, 4006–4012 CrossRef CAS; (g) A. Matsumoto, K. Yamamoto, R. Yoshida, K. Kataoka, T. Aoyagi and Y. Miyahara, Chem. Commun., 2010, 46, 2203–2205 RSC; (h) J. Xu, D. Yang, W. Li, Y. Gao, H. Chen and H. Li, Polymer, 2011, 52, 4268–4276 CrossRef CAS.
- (a) M. Shibayama, H. Yoshizawa, H. Kurokawa, H. Fujiwara and S. Nomura, Polymer, 1988, 29, 2066–2071 CrossRef CAS; (b) H. Kurokawa, M. Shibayama, T. Ishimaru, S. Nomura and W.-L. Wu, Polymer, 1992, 33, 2182–2188 CrossRef CAS; (c) M. Shibayama, T. Takeuchi and S. Nomura, Macromolecules, 1994, 27, 5350–5358 CrossRef CAS; (d) E. T. Wise and S. G. Weber, Macromolecules, 1995, 28, 8321–8327 CrossRef CAS; (e) C. Y. Chen and T.-L. Yu, Polymer, 1997, 38, 2019–2025 CrossRef CAS; (f) E. Carretti, S. Grassi, M. Cossalter, I. Natali, G. Caminati, R. G. Weiss, P. Baglioni and L. Dei, Langmuir, 2009, 25, 8656–8662 CrossRef CAS.
- G. Keita, A. Ricard, R. Audebert, E. Pezron and L. Leibler, Polymer, 1995, 36, 49–54 CrossRef CAS.
- C. J. Morrison, S. K. Park, C. Simocko, S. A. McCallum, S. M. Cramer and J. A. Moore, J. Am. Chem. Soc., 2008, 130, 17029–17037 CrossRef CAS.
- J. N. Demas and G. A. Crosby, J. Phys. Chem., 1971, 75, 991–1024 CrossRef.
- The concentration was based on the monomer unit.
- (a) B. M. Rambo and J. J. Lavigne, Chem. Mater., 2007, 19, 3732–3739 CrossRef CAS; (b) R. Nishiyabu, S. Teraoka, Y. Matsushima and Y. Kubo, ChemPlusChem, 2012, 77, 201–209 CrossRef CAS.
- When phenylboronic acid-free dansyldiethylenetriamine 2 was used for the fabrication instead of 1, dansyl fluorophores were not embedded in the PVA matrix; see Fig. S7 in ESI†.
- (a) D. L. Bernik and R. M. Negri, J. Colloid Interface Sci., 1998, 203, 97–105 CrossRef CAS; (b) E. Abel, G. E. M. Maguire, O. Murillo, I. Suzuki, S. L. De Wall and G. W. Gokel, J. Am. Chem. Soc., 1999, 121, 9043–9052 CrossRef CAS; (c) J. González-Benito, A. Aznar and J. Baselga, J. Fluoresc., 2001, 11, 307–314 CrossRef; (d) P. M. Page, C. A. Munson and F. V. Bright, Langmuir, 2004, 20, 10507–10516 CrossRef CAS.
- The quenching effect by Cu2+ is somewhat diminished in the presence of 10 equiv of Fe3+, presumably due to competitive interaction of Fe3+ toward dansyl moieties.
Footnote |
| † Electronic supplementary information (ESI) available: NMR and FAB mass spectra of compound 1 and experimental details. See DOI: 10.1039/c2ra20516e |
| This journal is © The Royal Society of Chemistry 2012 |

