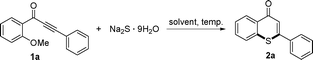
Domino reactions of 1-(2-alkoxyaryl)-3-akylprop-2-yn-1-ones with sodium sulfide leading to thiochromen-4-one derivatives†
Xiaobo
Yang
a,
Shangfu
Li
b,
Hongxia
Liu
b,
Yuyang
Jiang
b and
Hua
Fu
*ab
aKey Laboratory of Bioorganic Phosphorus Chemistry and Chemical Biology (Ministry of Education), Department of Chemistry, Tsinghua University, Beijing 100084, P. R. China. E-mail: fuhua@mail.tsinghua.edu.cn
bKey Laboratory of Chemical Biology (Guangdong Province), Graduate School of Shenzhen, Tsinghua University, Shenzhen 518057, P. R. China
First published on 11th May 2012
Abstract
A simple and practical approach to thiochromen-4-one derivatives has been developed through domino reactions of 1-(2-alkoxyaryl)-3-akylprop-2-yn-1-ones and sodium sulfide nonahydrate. The domino reactions underwent sequential addition and cyclization via cleavage of aromatic C–O bonds.
Introduction
Thiochromen-4-ones are biological active compounds and key intermediates, and some thiochromen-4-ones are antimicrobial and antifungal,1 antibacterial,2 antibiotic,3 anticarcinogenic,4 and antimalaria agents.5 The oxidized products of thiochromen-4-ones are used as the human cytomegalovirus protease inhibitors.6 Unfortunately, the previous methods for the synthesis of thiochromen-4-ones are very limited, they are usually prepared either by coupling of thiophenols with β-keto esters in the presence of polyphosphoric acid7 or by cyclization of β-substituted cinnamates derived from thiophenols and appropriate propiolates.5,8 However, the starting materials are often not readily available. Recently, Müller and co-workers reported an efficient palladium-catalyzed synthesis of 4H-thiochromen-4-ones and 4H-thiopyrano[2,3-b]pyridin-4-ones by a consecutive one-pot, three-component coupling-addition-SNAr sequence starting from aroyl chlorides, alkynes, and sodium sulfide nonahydrate.9 Herein, we report synthesis of thiochromen-4-one derivatives through sequential addition of substituted 1-(2-alkoxyaryl)-3-akylprop-2-yn-1-ones with sodium sulfide nonahydrate and intramolecular S-arylation via cleavage of aromatic C–O bonds in the absence of transition-metal catalysts.Results and discussion
First, a cascade reaction of 1-(2-methoxyphenyl)-3-phenylprop-2-yn-1-one (1a) with sodium sulfide leading to 2-phenyl-4H-thiochromen-4-one (2a) was used as a model example to optimize reaction conditions including catalysts, solvents, and temperature under nitrogen atmosphere in 24 h. As shown in Table 1, six solvents were tested at 80 °C (entries 1–6), and ethanol provided the highest yield (entry 6). Various reaction temperatures were investigated (compare entries 6–9), and 80 °C was found to be a suitable temperature (entry 6). When the reaction was performed under air, a slightly low yield was afforded (entry 10). In order to make out whether transition metals are involved in the reaction, the resulting solution from entry 6 was concentrated, the residue was determined by ICP-MS providing Ni (10 ppm), Rh (<10 ppm), Ru (<10 ppm), Pd (86 ppm), Co (12 ppm), Cu (2.2 ppm), Fe (26 ppm). We also attempted the reaction in the presence of various 10 mol% transition metals (entries 11–15), and the results showed that involvement of these metals did not obviously promote this reaction. Therefore, the cascade reaction of 1-(2-methoxyphenyl)-3-phenylprop-2-yn-1-one (1a) with sodium sulfide leading to 2-phenyl-4H-thiochromen-4-one (2a) underwent a transition metal-free process.| Entry | Cat. | Solvent | Temp. (°C) | Yield (%)b |
|---|---|---|---|---|
| a 1-(2-Methoxyphenyl)-3-phenylprop-2-yn-1-one (0.25 mmol), sodium sulfide nonahydrate (0.5 mmol), solvent (2 mL), catalyst (0.05 mmol), under nitrogen atmosphere, reaction time (24 h). b Isolated yield. c Under air. acac = acetylacetone. | ||||
| 1 | — | DMF | 80 | 47 |
| 2 | — | 1,4-Dioxane | 80 | 13 |
| 3 | — | Toluene | 80 | trace |
| 4 | — | H2O | 80 | 0 |
| 5 | — | MeOH | 80 | 75 |
| 6 | — | EtOH | 80 | 81 |
| 7 | — | EtOH | 60 | 39 |
| 8 | — | EtOH | 40 | 36 |
| 9 | — | EtOH | rt | 0 |
| 10 | — | EtOH | 80 | 72c |
| 11 | Ni(acac)2 | EtOH | 80 | 72 |
| 12 | RhCl3 | EtOH | 80 | 82 |
| 13 | Pd(OAc)2 | EtOH | 80 | 87 |
| 14 | Fe(NO3)3 | EtOH | 80 | 83 |
| 15 | CuI | EtOH | 80 | 89 |
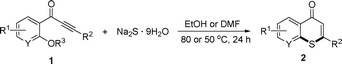
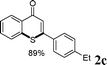
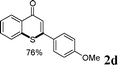
As shown in Table 2, the substrate scope for cascade reactions of substituted 1-(2-alkoxyaryl)-3-akylprop-2-yn-1-one (1) with sodium sulfide nonahydrate was investigated under our optimized conditions (using ethanol or DMF as the solvent at 80 or 50 °C under nitrogen atmosphere), the examined substrates provided good to excellent yields. For substituents R1 in 1, the substrates containing chloro groups (entries 7–12) showed slightly lower reactivity than those with OMe groups (entries 13–18). For substituents R2 in 1, aromatic groups provided higher yields than aliphatic ones (entries 20 and 21). We investigated reactions of 1 with different alkoxyl groups at the 2-site with sodium sulfide nonahydrate (entry 23), and good results were also observed. Actually, cleavage of the alkyl(C)–O bond in aryl-O-alkyl ethers was first in the previous researches under transition metal-free conditions.10 Interestingly, the cleavage of C–O bonds selectively occurred on the aromatic C–O bonds in the present reactions. The reaction conditions above are very simple, only a common solvent (ethanol or DMF) and a 80 or 50 °C temperature were required, and the reactions were performed under nitrogen atmosphere. The reactions could tolerate some functional groups including carbon–halogen bonds (entries 6–12, 18 and 20), ethers (entries 4, 5, 10, 11, 16–18), ketones (entry 19) and N-heterocycles (entry 22) in the substrates.
| Entry | 1 | 2 b |
|---|---|---|
| a Reaction conditions: under nitrogen atmosphere, 1 (0.25 mmol), sodium sulfide nonahydrate (0.5 mmol), solvent (2 mL) (DMF for entries 20 and 21; ethanol for others), reaction temperature (50 °C for entry 22; 80 °C for others), reaction time (24 h). b Isolated yield. | ||
| 1 |

|

|
| 2 |

|

|
| 3 |

|
|
| 4 |

|
|
| 5 |

|

|
| 6 |

|

|
| 7 |

|

|
| 8 |

|

|
| 9 |

|

|
| 10 |

|

|
| 11 |

|

|
| 12 |

|

|
| 13 |

|

|
| 14 |

|

|
| 15 |

|

|
| 16 |

|

|
| 17 |

|

|
| 18 |
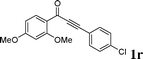
|

|
| 19 |

|

|
| 20 |

|

|
| 21 |
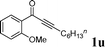
|

|
| 22 |

|

|
| 23 |

|
2a74% using 1w71% using 1x64% using 1y |
We attempted reactions of 1-(2-halophenyl)-3-phenylprop-2-yn-1-ones with sodium sulphide nonahydrate under the standard condition (Scheme 1), and the target product 2-phenyl-4H-thiochromen-4-one (2a) was also obtained in higher yields.
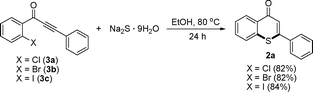 | ||
| Scheme 1 Reactions of 1-(2-halophenyl)-3-phenylprop-2-yn-1-ones with sodium sulfide nonahydrate leading to 2-phenyl-4h-thiochromen-4-one (2a) under the standard conditions. | ||
In order to explore the reaction mechanism for the synthesis of thiochromen-4-one derivatives, the following control experiments were performed under our standard conditions as shown in Scheme 2. When reaction of 1-(2-methoxyphenyl)-3-phenylprop-2-yn-1-one (1a) with sodium sulfide was carried out at room temperature (Scheme 2a), only addition product, 3-mercapto-1-(2-methoxyphenyl)-3-phenylprop-2-en-1-one (Ia), was provided in 97% yield, and no 2a was observed. The result showed that the first step reaction was addition of alkynes in 1 with Na2S. Treatment of 1-(2-hydroxyphenyl)-3-phenylprop-2-yn-1-one with Na2S under our standard conditions did not afford the target product and some unknown by-products were observed (Scheme 2b).
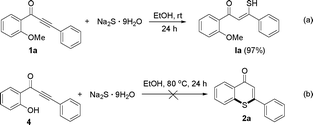 | ||
| Scheme 2 (a) Reaction of 1-(2-halophenyl)-3-phenylprop-2-yn-1-one with sodium sulfide at room temperature. (b) Reaction of 1-(2-hydroxyphenyl)-3-phenylprop-2-yn-1-one with sodium sulphide at 80 °C. | ||
A possible mechanism for the synthesis of thiochromen-4-one derivatives is proposed in Scheme 3 according to the results above. Addition of 1 with Na2S in ethanol or DMF provides intermediate I (here, ethanol as the solvent is used as the example in Scheme 3). Isomerization of I affords II, and intramolecular cycloaddition of II gives III. Re-aromatization of III by elimination of NaOR3 provides the target product (2).
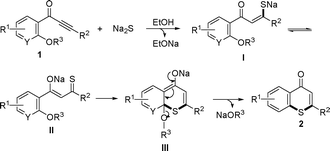 | ||
| Scheme 3 Possible mechanism for synthesis of thiochromen-4-one derivatives. | ||
Conclusions
We have developed a simple, practical and highly efficient transition metal-free method for the synthesis of thiochromen-4-one derivatives. The protocol uses readily available substituted 1-(2-alkoxyaryl)-3-akylprop-2-yn-1-ones and sodium sulfide nonahydrate as the starting materials, ethanol or DMF as the solvent, and the reactions were performed at 80 °C under a nitrogen atmosphere. The intramolecular cleavage of aromatic C–O bonds did not need aid of any transition-metal which avoided toxic metal contamination of the products, so this inexpensive and environmentally benign approach to thiochromen-4-one derivatives will find wide application in academic and industrial research.Experimental
General methods
All reactions were carried out under nitrogen atmosphere. Proton and carbon magnetic resonance spectra (1H NMR and 13C NMR) were recorded using tetramethylsilane (TMS) in the solvent of CDCl3 as the internal standard (1H NMR: TMS at 0.00 ppm, CDCl3 at 7.26 ppm; 13C NMR: CDCl3 at 77.0 ppm).Synthesis of compounds 1a–u
Compounds 1a–s and 1v–y were prepared according to the previous procedure;11 Compounds 1t and 1u were prepared according to the previous procedures.12,13General procedure for synthesis of compounds 2a–v
Substituted 1-(2-alkoxyaryl)-3-alkylprop-2-yn-1-one (1) (0.25 mmol), Na2S·9H2O (0.5 mmol, 120 mg) and DMF (2 mL) for entries 20 and 21, ethanol (2 mL) for others in Table 2, were added to a Schlenk tube with a magnetic stirrer. The mixture was allowed to stir under a nitrogen atmosphere (1 amt.) at 80 or 50 °C for 24 h (see Table 2). After cooling to room temperature, the resulting solution was concentrated via rotary evaporation, and the residue was purified by column chromatography on silica gel to provide the desired product.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 48 mg (81%). Light yellow solid, mp 123–125 °C (lit.14 122–123 °C). 1H NMR (CDCl3, 300 MHz) δ 8.55 (d, 1H, J = 7.2 Hz), 7.66 (m, 4H), 7.50 (m, 4H), 7.24 (s, 1H). 13C NMR (CDCl3, 75 MHz) δ 180.9, 153.1, 137.7, 136.6, 131.7, 131.1, 130.9, 129.4, 128.7, 127.9, 127.1, 126.6, 123.5. ESIMS [M + H]+m/z 239.1.
1). Yield 48 mg (81%). Light yellow solid, mp 123–125 °C (lit.14 122–123 °C). 1H NMR (CDCl3, 300 MHz) δ 8.55 (d, 1H, J = 7.2 Hz), 7.66 (m, 4H), 7.50 (m, 4H), 7.24 (s, 1H). 13C NMR (CDCl3, 75 MHz) δ 180.9, 153.1, 137.7, 136.6, 131.7, 131.1, 130.9, 129.4, 128.7, 127.9, 127.1, 126.6, 123.5. ESIMS [M + H]+m/z 239.1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 49 mg (79%). Light yellow solid, mp 124–126 °C. 1H NMR (CDCl3, 300 MHz) δ 8.51 (d, 1H, J = 7.9 Hz), 7.58 (m, 5H), 7.52 (m, 2H), 7.21 (s, 1H), 2.40 (s, 3H). 13C NMR (CDCl3, 75 MHz) δ 180.9, 153.1, 141.4, 137.8, 133.8, 131.6, 131.0, 130.1, 128.6, 127.8, 126.9, 126.5, 122.9, 21.5. ESIMS [M + H]+m/z 253.1, [M + Na]+m/z 275.1.
1). Yield 49 mg (79%). Light yellow solid, mp 124–126 °C. 1H NMR (CDCl3, 300 MHz) δ 8.51 (d, 1H, J = 7.9 Hz), 7.58 (m, 5H), 7.52 (m, 2H), 7.21 (s, 1H), 2.40 (s, 3H). 13C NMR (CDCl3, 75 MHz) δ 180.9, 153.1, 141.4, 137.8, 133.8, 131.6, 131.0, 130.1, 128.6, 127.8, 126.9, 126.5, 122.9, 21.5. ESIMS [M + H]+m/z 253.1, [M + Na]+m/z 275.1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 59 mg (89%). Light yellow solid, mp 70–72 °C. 1H NMR (CDCl3, 600 MHz) δ 8.52 (d, 1H, J = 8.2 Hz), 7.61 (m, 4H), 7.52 (m, 1H), 7.31 (m, 2H), 7.22 (s, 1H), 2.69 (dd, 2H, J = 7.6 Hz), 1.26 (t, 3H, J = 7.6 Hz). 13C NMR (CDCl3, 150 MHz) δ 181.0, 153.2, 147.7, 137.8, 134.0, 131.6, 131.0, 128.9, 128.6, 127.8, 126.9, 126.6, 123.0, 28.8, 15.4. HRMS for C17H15OS [M + H]+ 267.0844, found 267.0842.
1). Yield 59 mg (89%). Light yellow solid, mp 70–72 °C. 1H NMR (CDCl3, 600 MHz) δ 8.52 (d, 1H, J = 8.2 Hz), 7.61 (m, 4H), 7.52 (m, 1H), 7.31 (m, 2H), 7.22 (s, 1H), 2.69 (dd, 2H, J = 7.6 Hz), 1.26 (t, 3H, J = 7.6 Hz). 13C NMR (CDCl3, 150 MHz) δ 181.0, 153.2, 147.7, 137.8, 134.0, 131.6, 131.0, 128.9, 128.6, 127.8, 126.9, 126.6, 123.0, 28.8, 15.4. HRMS for C17H15OS [M + H]+ 267.0844, found 267.0842.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 51 mg (76%). Light yellow solid, mp 126–128 °C (lit.16 127–128 °C). 1H NMR (CDCl3, 300 MHz) δ 8.48 (d, 1H, J = 7.9 Hz), 7.56 (m, 5H), 7.14 (s, 1H), 6.94 (m, 2H), 3.81 (s, 3H). 13C NMR (CDCl3, 75 MHz) δ 180.9, 161.9, 152.7, 137.7, 131.5, 130.9, 128.8, 128.5, 128.3, 127.7, 126.5, 122.1, 114.7, 55.6. ESIMS [M + H]+m/z 269.0.
1). Yield 51 mg (76%). Light yellow solid, mp 126–128 °C (lit.16 127–128 °C). 1H NMR (CDCl3, 300 MHz) δ 8.48 (d, 1H, J = 7.9 Hz), 7.56 (m, 5H), 7.14 (s, 1H), 6.94 (m, 2H), 3.81 (s, 3H). 13C NMR (CDCl3, 75 MHz) δ 180.9, 161.9, 152.7, 137.7, 131.5, 130.9, 128.8, 128.5, 128.3, 127.7, 126.5, 122.1, 114.7, 55.6. ESIMS [M + H]+m/z 269.0.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 60 mg (85%). Light yellow solid, mp 110–112 °C. 1H NMR (CDCl3, 300 MHz) δ 8.51 (d, 1H, J = 8.6 Hz), 7.58 (m, 5H), 7.18 (s, 1H), 6.96 (m, 2H), 4.07 (dd, 2H, J = 6.9 Hz), 1.43 (t, 3H, J = 6.9 Hz). 13C NMR (CDCl3, 75 MHz) δ 180.9, 161.4, 152.9, 137.7, 131.5, 130.9, 128.5, 128.3, 127.7, 126.5, 122.1, 115.2, 63.9, 14.8. HRMS Calcd for C17H15O2S [M + H]+ 283.0793, found 283.0796.
1). Yield 60 mg (85%). Light yellow solid, mp 110–112 °C. 1H NMR (CDCl3, 300 MHz) δ 8.51 (d, 1H, J = 8.6 Hz), 7.58 (m, 5H), 7.18 (s, 1H), 6.96 (m, 2H), 4.07 (dd, 2H, J = 6.9 Hz), 1.43 (t, 3H, J = 6.9 Hz). 13C NMR (CDCl3, 75 MHz) δ 180.9, 161.4, 152.9, 137.7, 131.5, 130.9, 128.5, 128.3, 127.7, 126.5, 122.1, 115.2, 63.9, 14.8. HRMS Calcd for C17H15O2S [M + H]+ 283.0793, found 283.0796.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 55 mg (80%). Light yellow solid, mp 162–164 °C (lit.7 163–165 °C). 1H NMR (CDCl3, 300 MHz) δ 8.48 (d, 1H, J = 8.3 Hz), 7.55 (m, 5H), 7.42 (m, 2H), 7.15 (s, 1H). 13C NMR (CDCl3, 75 MHz) δ 180.7, 151.5, 137.5, 137.3, 135.0, 131.8, 130.9, 129.6, 128.7, 128.2, 127.9, 126.5, 123.6. ESIMS [M + H]+m/z 273.1, [M + Na]+m/z 295.1.
1). Yield 55 mg (80%). Light yellow solid, mp 162–164 °C (lit.7 163–165 °C). 1H NMR (CDCl3, 300 MHz) δ 8.48 (d, 1H, J = 8.3 Hz), 7.55 (m, 5H), 7.42 (m, 2H), 7.15 (s, 1H). 13C NMR (CDCl3, 75 MHz) δ 180.7, 151.5, 137.5, 137.3, 135.0, 131.8, 130.9, 129.6, 128.7, 128.2, 127.9, 126.5, 123.6. ESIMS [M + H]+m/z 273.1, [M + Na]+m/z 295.1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 37 mg (54%). Light yellow solid, mp 190–192 °C (lit.17 188–189 °C). 1H NMR (CDCl3, 600 MHz) δ 8.50 (d, 1H, J = 2.0 Hz), 7.67 (m, 2H), 7.59 (m, 2H), 7.51 (m, 3H), 7.23(s, 1H). 13C NMR (CDCl3, 150 MHz) δ 179.7, 153.3, 136.3, 135.9, 134.4, 132.1, 131.1, 129.5, 128.3, 128.0, 127.1, 123.3. ESIMS [M + H]+m/z 273.1, [M + Na]+m/z 295.1.
1). Yield 37 mg (54%). Light yellow solid, mp 190–192 °C (lit.17 188–189 °C). 1H NMR (CDCl3, 600 MHz) δ 8.50 (d, 1H, J = 2.0 Hz), 7.67 (m, 2H), 7.59 (m, 2H), 7.51 (m, 3H), 7.23(s, 1H). 13C NMR (CDCl3, 150 MHz) δ 179.7, 153.3, 136.3, 135.9, 134.4, 132.1, 131.1, 129.5, 128.3, 128.0, 127.1, 123.3. ESIMS [M + H]+m/z 273.1, [M + Na]+m/z 295.1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 49 mg (69%). Light yellow solid, mp 154–156 °C. 1H NMR (CDCl3, 600 MHz) δ 8.50 (s, 1H), 7.57 (m, 4H), 7.30 (m, 2H), 7.21 (s, 1H), 2.42 (s, 3H). 13C NMR (CDCl3, 150 MHz) δ 179.8, 153.4, 141.7, 135.9, 134.3, 133.5, 132.2, 132.0, 130.1, 128.2, 128.0, 126.9, 122.9, 21.5. HRMS Calcd for C16H12ClOS [M + H]+ 287.0297, found 287.0298.
1). Yield 49 mg (69%). Light yellow solid, mp 154–156 °C. 1H NMR (CDCl3, 600 MHz) δ 8.50 (s, 1H), 7.57 (m, 4H), 7.30 (m, 2H), 7.21 (s, 1H), 2.42 (s, 3H). 13C NMR (CDCl3, 150 MHz) δ 179.8, 153.4, 141.7, 135.9, 134.3, 133.5, 132.2, 132.0, 130.1, 128.2, 128.0, 126.9, 122.9, 21.5. HRMS Calcd for C16H12ClOS [M + H]+ 287.0297, found 287.0298.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 56 mg (74%). Light yellow solid, mp 110–112 °C. 1H NMR (CDCl3, 600 MHz) δ 8.51 (d, 1H, J = 2.1 Hz), 7.60 (m, 4H), 7.34(m, 2H), 7.23 (s, 1H), 2.72 (dd, 2H, J = 7.6 Hz), 1.28 (t, 3H, J = 7.6 Hz). 13C NMR (CDCl3, 150 MHz) δ 179.9, 153.5, 148.1, 136.0, 134.7, 133.8, 132.3, 132.1, 129.1, 128.4, 128.1, 127.1, 122.9, 29.0, 15.5. HRMS Calcd for C17H14ClOS [M + H]+ 301.0454, found 301.0460.
1). Yield 56 mg (74%). Light yellow solid, mp 110–112 °C. 1H NMR (CDCl3, 600 MHz) δ 8.51 (d, 1H, J = 2.1 Hz), 7.60 (m, 4H), 7.34(m, 2H), 7.23 (s, 1H), 2.72 (dd, 2H, J = 7.6 Hz), 1.28 (t, 3H, J = 7.6 Hz). 13C NMR (CDCl3, 150 MHz) δ 179.9, 153.5, 148.1, 136.0, 134.7, 133.8, 132.3, 132.1, 129.1, 128.4, 128.1, 127.1, 122.9, 29.0, 15.5. HRMS Calcd for C17H14ClOS [M + H]+ 301.0454, found 301.0460.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 46 mg (61%). Light yellow solid, mp 190–192 °C (lit.18 179–181 °C). 1H NMR (CDCl3, 600 MHz) δ 8.48 (d, 1H, J = 1.4 Hz), 7.61 (m, 2H), 7.53 (m, 2H), 7.17 (s, 1H), 6.99 (m, 2H), 3.86 (s, 3H). 13C NMR (CDCl3, 150 MHz) δ 179.7, 162.1, 153.0, 135.8, 134.3, 132.1, 131.9, 128.5, 128.4, 128.2, 127.9, 122.0, 114.8, 55.6. ESIMS [M + H]+m/z 303.2.
1). Yield 46 mg (61%). Light yellow solid, mp 190–192 °C (lit.18 179–181 °C). 1H NMR (CDCl3, 600 MHz) δ 8.48 (d, 1H, J = 1.4 Hz), 7.61 (m, 2H), 7.53 (m, 2H), 7.17 (s, 1H), 6.99 (m, 2H), 3.86 (s, 3H). 13C NMR (CDCl3, 150 MHz) δ 179.7, 162.1, 153.0, 135.8, 134.3, 132.1, 131.9, 128.5, 128.4, 128.2, 127.9, 122.0, 114.8, 55.6. ESIMS [M + H]+m/z 303.2.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 54 mg (68%). Light yellow solid, mp 149–151 °C. 1H NMR (CDCl3, 600 MHz) δ 8.48 (d, 1H, J = 2.0 Hz), 7.60 (m, 2H), 7.54 (m, 2H), 7.17 (s, 1H), 6.96 (m, 2H), 4.09 (dd, 2H, J = 6.9 Hz), 1.44 (t, 3H, J = 6.9 Hz). 13C NMR (CDCl3, 150 MHz) δ 179.7, 161.5, 153.1, 135.0, 134.2, 132.1, 131.9, 128.4, 128.3, 128.2, 127.9, 121.9, 115.3, 63.9, 14.8. HRMS Calcd for C17H14ClO2S [M + H]+ 317.0403, found 317.0401.
1). Yield 54 mg (68%). Light yellow solid, mp 149–151 °C. 1H NMR (CDCl3, 600 MHz) δ 8.48 (d, 1H, J = 2.0 Hz), 7.60 (m, 2H), 7.54 (m, 2H), 7.17 (s, 1H), 6.96 (m, 2H), 4.09 (dd, 2H, J = 6.9 Hz), 1.44 (t, 3H, J = 6.9 Hz). 13C NMR (CDCl3, 150 MHz) δ 179.7, 161.5, 153.1, 135.0, 134.2, 132.1, 131.9, 128.4, 128.3, 128.2, 127.9, 121.9, 115.3, 63.9, 14.8. HRMS Calcd for C17H14ClO2S [M + H]+ 317.0403, found 317.0401.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 41 mg (53%). Light yellow solid, mp 201–204 °C (lit.17 196–197 °C) 1H NMR (CDCl3, 600 MHz) δ 8.50 (s, 1H), 7.59 (m, 4H), 7.48 (m, 2H), 7.18 (s, 1H). 13C NMR (CDCl3, 150 MHz) δ 179.6, 151.9, 137.5, 135.5, 134.7, 134.6, 132.2, 132.0, 129.7, 128.2, 128.0, 123.5, 123.4. ESIMS [M + Na]+m/z 330.6.
1). Yield 41 mg (53%). Light yellow solid, mp 201–204 °C (lit.17 196–197 °C) 1H NMR (CDCl3, 600 MHz) δ 8.50 (s, 1H), 7.59 (m, 4H), 7.48 (m, 2H), 7.18 (s, 1H). 13C NMR (CDCl3, 150 MHz) δ 179.6, 151.9, 137.5, 135.5, 134.7, 134.6, 132.2, 132.0, 129.7, 128.2, 128.0, 123.5, 123.4. ESIMS [M + Na]+m/z 330.6.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 53 mg (79%). Light yellow solid, mp 135–137 °C (lit.18 150 °C). 1H NMR (CDCl3, 300 MHz) δ 8.44 (d, 1H, J = 8.9 Hz), 7.64 (m, 2H), 7.48 (m, 3H), 7.16 (s, 1H), 7.07 (m, 2H), 3.90 (s, 3H). 13C NMR (CDCl3, 75 MHz) δ 180.4, 162.0, 152.1, 140.0, 136.7, 130.7, 130.6, 129.3, 127.0, 124.8, 123.5, 116.6, 108.6, 55.8. ESIMS [M + H]+m/z 269.0.
1). Yield 53 mg (79%). Light yellow solid, mp 135–137 °C (lit.18 150 °C). 1H NMR (CDCl3, 300 MHz) δ 8.44 (d, 1H, J = 8.9 Hz), 7.64 (m, 2H), 7.48 (m, 3H), 7.16 (s, 1H), 7.07 (m, 2H), 3.90 (s, 3H). 13C NMR (CDCl3, 75 MHz) δ 180.4, 162.0, 152.1, 140.0, 136.7, 130.7, 130.6, 129.3, 127.0, 124.8, 123.5, 116.6, 108.6, 55.8. ESIMS [M + H]+m/z 269.0.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 59 mg (85%). Light yellow solid, mp 135–137 °C 1H NMR (CDCl3, 600 MHz) δ 8.41 (d, 1H, J = 8.9 Hz), 7.52 (m, 2H), 7.24 (m, 2H), 7.11 (s, 1H), 7.03 (m, 1H), 6.98 (m, 1H), 3.87 (s, 3H), 2.38 (s, 3H). 13C NMR (CDCl3, 150 MHz) δ 180.4, 162.0, 152.1, 141.2, 139.9, 133.7, 130.5, 130.0, 126.7, 124.8, 122.8, 116.5, 108.5, 55.8, 21.4. HRMS Calcd for C17H15O2S [M + H]+ 283.0793, found 283.0787.
1). Yield 59 mg (85%). Light yellow solid, mp 135–137 °C 1H NMR (CDCl3, 600 MHz) δ 8.41 (d, 1H, J = 8.9 Hz), 7.52 (m, 2H), 7.24 (m, 2H), 7.11 (s, 1H), 7.03 (m, 1H), 6.98 (m, 1H), 3.87 (s, 3H), 2.38 (s, 3H). 13C NMR (CDCl3, 150 MHz) δ 180.4, 162.0, 152.1, 141.2, 139.9, 133.7, 130.5, 130.0, 126.7, 124.8, 122.8, 116.5, 108.5, 55.8, 21.4. HRMS Calcd for C17H15O2S [M + H]+ 283.0793, found 283.0787.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 62 mg (84%). Light yellow solid, mp 86–88 °C 1H NMR (CDCl3, 300 MHz) δ 8.42 (d, 1H, J = 8.9 Hz), 7.55 (m, 2H), 7.27 (m, 2H), 7.13 (s, 1H), 7.01 (m, 2H), 3.87 (s, 3H), 2.69 (dd, 2H, J = 7.6 Hz), 1.25 (t, 3H, J = 7.6 Hz). 13C NMR (CDCl3, 75 MHz) δ 180.4, 162.0, 152.1, 147.5, 139.9, 133.9, 130.5, 128.8, 126.9, 124.8, 122.9, 116.5, 108.5, 55.8, 28.8, 15.4. HRMS Calcd for C18H17O2S [M + H]+ 297.0949, found 297.0952.
1). Yield 62 mg (84%). Light yellow solid, mp 86–88 °C 1H NMR (CDCl3, 300 MHz) δ 8.42 (d, 1H, J = 8.9 Hz), 7.55 (m, 2H), 7.27 (m, 2H), 7.13 (s, 1H), 7.01 (m, 2H), 3.87 (s, 3H), 2.69 (dd, 2H, J = 7.6 Hz), 1.25 (t, 3H, J = 7.6 Hz). 13C NMR (CDCl3, 75 MHz) δ 180.4, 162.0, 152.1, 147.5, 139.9, 133.9, 130.5, 128.8, 126.9, 124.8, 122.9, 116.5, 108.5, 55.8, 28.8, 15.4. HRMS Calcd for C18H17O2S [M + H]+ 297.0949, found 297.0952.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 64 mg (86%). Light yellow solid, mp 136–138 °C. 1H NMR (CDCl3, 600 MHz) δ 8.39 (d, 1H, J = 8.9 Hz), 7.55 (m, 2H), 7.06 (s, 1H), 7.01 (m, 1H), 6.94 (m, 3H), 3.85 (s, 3H), 3.81 (s, 3H). 13C NMR (CDCl3, 150 MHz) δ 180.4, 161.9, 161.8, 151.7, 139.9, 130.4, 128.8, 128.2, 124.7, 122.1, 116.4, 114.7, 108.5, 55.6, 55.5. HRMS Calcd for C17H15O3S [M + H]+ 299.0742, found 299.0747.
1). Yield 64 mg (86%). Light yellow solid, mp 136–138 °C. 1H NMR (CDCl3, 600 MHz) δ 8.39 (d, 1H, J = 8.9 Hz), 7.55 (m, 2H), 7.06 (s, 1H), 7.01 (m, 1H), 6.94 (m, 3H), 3.85 (s, 3H), 3.81 (s, 3H). 13C NMR (CDCl3, 150 MHz) δ 180.4, 161.9, 161.8, 151.7, 139.9, 130.4, 128.8, 128.2, 124.7, 122.1, 116.4, 114.7, 108.5, 55.6, 55.5. HRMS Calcd for C17H15O3S [M + H]+ 299.0742, found 299.0747.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 62 mg (80%). Light yellow solid, mp 135–136 °C. 1H NMR (CDCl3, 300 MHz) δ 8.42 (d, 1H, J = 8.9 Hz), 7.58 (m, 2H), 7.10 (s, 1H), 6.99 (m, 4H), 4.06 (dd, 2H, J = 6.9 Hz), 3.89 (s, 3H), 1.43 (t, 3H, J = 7.2 Hz). 13C NMR (CDCl3, 75 MHz) δ 180.4, 161.9, 161.2, 151.8, 139.9, 130.5, 128.7, 128.3, 124.8, 122.1, 116.4, 115.2, 108.6, 63.8, 55.8, 14.8. HRMS Calcd for C18H17O3S [M + H]+ 313.0898, found 313.0901.
1). Yield 62 mg (80%). Light yellow solid, mp 135–136 °C. 1H NMR (CDCl3, 300 MHz) δ 8.42 (d, 1H, J = 8.9 Hz), 7.58 (m, 2H), 7.10 (s, 1H), 6.99 (m, 4H), 4.06 (dd, 2H, J = 6.9 Hz), 3.89 (s, 3H), 1.43 (t, 3H, J = 7.2 Hz). 13C NMR (CDCl3, 75 MHz) δ 180.4, 161.9, 161.2, 151.8, 139.9, 130.5, 128.7, 128.3, 124.8, 122.1, 116.4, 115.2, 108.6, 63.8, 55.8, 14.8. HRMS Calcd for C18H17O3S [M + H]+ 313.0898, found 313.0901.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 58 mg (77%). Light yellow solid, mp 152–154 °C. 1H NMR (CDCl3, 300 MHz) δ 8.42 (d, 1H, J = 8.9 Hz), 7.56 (m, 2H), 7.43 (m, 2H), 7.07 (m, 2H), 6.99(m, 1H), 3.90 (s, 3H). 13C NMR (CDCl3, 75 MHz) δ 180.2, 162.1, 150.5, 139.6, 137.0, 135.0, 130.6, 129.6, 128.2, 124.7, 123.6, 116.7, 108.6, 55.8. HRMS Calcd for C16H12ClO2S [M + H]+ 303.0247, found 303.0254.
1). Yield 58 mg (77%). Light yellow solid, mp 152–154 °C. 1H NMR (CDCl3, 300 MHz) δ 8.42 (d, 1H, J = 8.9 Hz), 7.56 (m, 2H), 7.43 (m, 2H), 7.07 (m, 2H), 6.99(m, 1H), 3.90 (s, 3H). 13C NMR (CDCl3, 75 MHz) δ 180.2, 162.1, 150.5, 139.6, 137.0, 135.0, 130.6, 129.6, 128.2, 124.7, 123.6, 116.7, 108.6, 55.8. HRMS Calcd for C16H12ClO2S [M + H]+ 303.0247, found 303.0254.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 45 mg (64%). Light yellow solid, mp 173–175 °C. 1H NMR (CDCl3, 600 MHz) δ 8.55 (d, 1H, J = 8.3 Hz), 8.08 (m, 2H), 7.79 (m, 2H), 7.67 (m, 2H), 7.58 (m, 1H), 7.27 (s, 1H), 2.67 (s, 3H). 13C NMR (CDCl3, 150 MHz) δ 197.2, 180.7, 151.5, 140.7, 138.6, 137.4, 132.0, 130.9, 129.3, 128.8, 128.1, 127.4, 126.7, 124.3, 26.9. HRMS Calcd for C17H13O2S [M + H]+ 281.0636, found 281.0631.
1). Yield 45 mg (64%). Light yellow solid, mp 173–175 °C. 1H NMR (CDCl3, 600 MHz) δ 8.55 (d, 1H, J = 8.3 Hz), 8.08 (m, 2H), 7.79 (m, 2H), 7.67 (m, 2H), 7.58 (m, 1H), 7.27 (s, 1H), 2.67 (s, 3H). 13C NMR (CDCl3, 150 MHz) δ 197.2, 180.7, 151.5, 140.7, 138.6, 137.4, 132.0, 130.9, 129.3, 128.8, 128.1, 127.4, 126.7, 124.3, 26.9. HRMS Calcd for C17H13O2S [M + H]+ 281.0636, found 281.0631.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 29 mg (57%). Yellow oil. 1H NMR (CDCl3, 300 MHz) δ 8.49 (d, 1H, J = 7.9 Hz), 7.50 (m, 3H), 6.86 (s, 1H), 2.65 (t, 2H, J = 7.6 Hz), 1.76 (dt, 2H, J = 7.2, 7.6 Hz), 1.01 (t, 3H, J = 7.2 Hz). 13C NMR (CDCl3, 75 MHz) δ 180.7, 156.3, 137.8, 131.4, 131.0, 128.6, 127.5, 126.3, 124.3, 39.5, 23.2, 13.5. HRMS Calcd for C12H13OS [M + H]+ 205.0687, found 205.0684.
1). Yield 29 mg (57%). Yellow oil. 1H NMR (CDCl3, 300 MHz) δ 8.49 (d, 1H, J = 7.9 Hz), 7.50 (m, 3H), 6.86 (s, 1H), 2.65 (t, 2H, J = 7.6 Hz), 1.76 (dt, 2H, J = 7.2, 7.6 Hz), 1.01 (t, 3H, J = 7.2 Hz). 13C NMR (CDCl3, 75 MHz) δ 180.7, 156.3, 137.8, 131.4, 131.0, 128.6, 127.5, 126.3, 124.3, 39.5, 23.2, 13.5. HRMS Calcd for C12H13OS [M + H]+ 205.0687, found 205.0684.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 33 mg (53%). Yellow oil. 1H NMR (CDCl3, 300 MHz) δ 8.48 (d, 1H, J = 7.6 Hz), 7.51 (m, 3H), 6.86 (s, 1H), 2.66 (t, 2H, J = 7.6 Hz), 1.73 (m, 2H), 1.33 (m, 6H), 0.88 (t, 3H, J = 6.5 Hz). 13C NMR (CDCl3, 75 MHz) δ 180.8, 156.6, 137.8, 131.4, 131.1, 128.6, 127.5, 126.3, 124.2, 37.5, 31.5, 29.8, 28.6, 22.6, 14.1. HRMS Calcd for C15H19OS [M + H]+ 247.1157, found 247.1162.
1). Yield 33 mg (53%). Yellow oil. 1H NMR (CDCl3, 300 MHz) δ 8.48 (d, 1H, J = 7.6 Hz), 7.51 (m, 3H), 6.86 (s, 1H), 2.66 (t, 2H, J = 7.6 Hz), 1.73 (m, 2H), 1.33 (m, 6H), 0.88 (t, 3H, J = 6.5 Hz). 13C NMR (CDCl3, 75 MHz) δ 180.8, 156.6, 137.8, 131.4, 131.1, 128.6, 127.5, 126.3, 124.2, 37.5, 31.5, 29.8, 28.6, 22.6, 14.1. HRMS Calcd for C15H19OS [M + H]+ 247.1157, found 247.1162.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 43 mg (72%). Light yellow solid, mp 115–118 °C (lit.19 114–117 °C). 1H NMR (CDCl3, 300 MHz) δ 8.76 (m, 2H), 7.69 (m, 2H), 7.51 (m, 4H), 7.24 (s, 1H). 13C NMR (CDCl3, 75 MHz) δ 181.4, 159.2, 154.8, 152.9, 136.8, 136.4, 131.2, 129.5, 128.1, 127.0, 123.6, 123.0. ESIMS [M + H]+m/z 240.2.
1). Yield 43 mg (72%). Light yellow solid, mp 115–118 °C (lit.19 114–117 °C). 1H NMR (CDCl3, 300 MHz) δ 8.76 (m, 2H), 7.69 (m, 2H), 7.51 (m, 4H), 7.24 (s, 1H). 13C NMR (CDCl3, 75 MHz) δ 181.4, 159.2, 154.8, 152.9, 136.8, 136.4, 131.2, 129.5, 128.1, 127.0, 123.6, 123.0. ESIMS [M + H]+m/z 240.2.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Yield 65 mg (97%). Light red solid. 1H NMR (CDCl3, 300 MHz) δ 7.90 (m, 1H), 7.82 (m, 2H), 7.74 (s, 1H), 7.45 (m, 4H), 7.02 (m, 2H), 3.93 (s, 3H). 13C NMR (CDCl3, 75 MHz) δ 201.0, 179.5, 158.2, 145.6, 133.5, 131.0, 130.6, 128.5, 127.1, 125.4, 121.1, 116.3, 112.0, 56.0. ESIMS [M + H]+m/z 271.2.
1). Yield 65 mg (97%). Light red solid. 1H NMR (CDCl3, 300 MHz) δ 7.90 (m, 1H), 7.82 (m, 2H), 7.74 (s, 1H), 7.45 (m, 4H), 7.02 (m, 2H), 3.93 (s, 3H). 13C NMR (CDCl3, 75 MHz) δ 201.0, 179.5, 158.2, 145.6, 133.5, 131.0, 130.6, 128.5, 127.1, 125.4, 121.1, 116.3, 112.0, 56.0. ESIMS [M + H]+m/z 271.2.
Acknowledgements
The authors wish to thank the National Natural Science Foundation of China (Grant Nos. 20972083 and 21172128), and the Ministry of Science and Technology of China (Grant No. 2012CB722605) for financial support.References
- H. Nakazumi, T. Ueyama and T. Kitao, J. Heterocycl. Chem., 1985, 22, 1593 CrossRef CAS.
- H. Nakazumi, T. Ueyama and T. Kitao, J. Heterocycl. Chem., 1984, 21, 193 CrossRef CAS.
- J. Couquelet, P. Tronche, P. Niviere and G. Andraud, Trav. Soc. Pharm. Montpellier, 1963, 23, 214 CAS.
- M. H. Holshouser, L. J. Loeffler and I. H. Hall, J. Med. Chem., 1981, 24, 853 CrossRef CAS.
- R. K. Razdan, R. J. Bruni, A. C. Mehta, K. K. Weinhardt and Z. B. Papanastassiou, J. Med. Chem., 1978, 21, 643 CrossRef CAS.
- D. Dhanak, R. M. Keenan, G. Burton, A. Kaura, M. G. Darcy, D. H. Shah, L. H. Ridgers, A. Breen, P. Lavery, D. G. Tew and A. West, Bioorg. Med. Chem. Lett., 1998, 8, 3677 CrossRef CAS.
- (a) F. Bossert, Justus Liebigs. Ann. Chem., 1964, 40, 680 Search PubMed; (b) S. W. Schneller, Adv. Heterocycl. Chem., 1975, 18, 59 CrossRef CAS; (c) H. Nakazumi, S. Wanatabe, T. Kitaguchi and T. Kitao, Bull. Chem. Soc. Jpn., 1990, 63, 847 CrossRef CAS.
- (a) W. E. Truce and D. L. Goldhamer, J. Am. Chem. Soc., 1959, 81, 5795 CrossRef CAS; (b) K. Buggle, J. J. Delahunty, E. M. Philbin and N. D. Ryan, J. Chem. Soc. C, 1971, 3168 RSC.
- (a) B. Willy and T. J. J. Müller, Synlett, 2009, 1255 CAS; (b) B. Willy, W. Frank and T. J. J. Müller, Org. Biomol. Chem., 2010, 8, 90 RSC.
- (a) A. K. Chakraborti, L. Sharma and M. K. Nayak, J. Org. Chem., 2002, 67, 6406 CrossRef CAS; (b) J. A. Dodge, M. G. Stocksdale, K. J. Fahey and C. D. Jones, J. Org. Chem., 1995, 60, 739 CrossRef CAS.
- R. Bernini, S. Cacchi, G. Fabrizi and A. Sferrazzaa, Synthesis, 2009, 1209 CAS.
- C. Lin, T. H. Duh, W. D. Lu, J. L. Lee, C. Y. Lee, C. C. Chen and M. J. Wu, J. Chin. Chem. Soc., 2004, 51, 183 CAS.
- S. Ma, J. Liu, S. Li, B. Chen, J. Cheng, J. Kuang, Y. Liu, B. Wan, Y. Wang, J. Ye, Q. Yu, W. Yuan and S. Yu, Adv. Synth. Catal., 2011, 353, 1005 CrossRef CAS.
- M. R. Detty, J. Am. Chem. Soc., 1983, 105, 883 CrossRef CAS.
- J. I. Lee, Bull. Korean Chem. Soc., 2009, 30, 710 CrossRef CAS.
- D. H. Wadsworth, J. Org. Chem., 1980, 45, 4611 CrossRef CAS.
- R. K. Razdan, J. Med. Chem., 1978, 21, 643 CrossRef CAS.
- F. Bossert, Justus Liebigs Annalen der Chem., 1964, 680, 40 CAS.
- F. C. Fuchs, Molecules, 2009, 14, 3814 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: 1H and 13C NMR spectra of compounds 2a–v and Ia. See DOI: 10.1039/c2ra20897k/ |
| This journal is © The Royal Society of Chemistry 2012 |