A highly regioselective sp3 C–H amination of tertiary amides based on Fe(II) complex catalysts†
Xuerong
Mao
,
Yuanzhao
Wu
,
Xiaoxiang
Jiang
,
Xunhua
Liu
,
Yixiang
Cheng
* and
Chengjian
Zhu
*
Key Lab of Mesoscopic Chemistry of MOE, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093, China. E-mail: yxcheng@nju.edu.cn,; cjzhu@nju.edu.cn; Fax: +86-25-88317761; Tel: +86-25-83686508
First published on 16th May 2012
Abstract
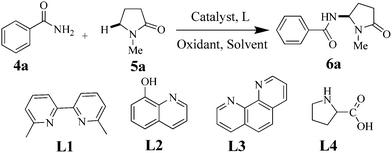
A highly regioselective sp3 C–H amination of aryl amides with N-substituted pyrrolidin-2-ones has been developed with a catalyst Fe(II) complex with TBHP as a benign oxidant. This facile method can offer rapid access to the amination reaction of N-substituted pyrrolidin-2-ones in moderate to excellent yields.
In recent years, heterocyclic compounds containing N, N′-methylenediformamide skeletons have attracted more and more attention because they play very important roles in the pharmaceutical industry (Scheme 1). Lunec and co-workers described compound 1 as small-molecule inhibitors of the MDM2-p53 interaction based on an isoindolinone scaffold.1 Recently, there have been some reports on an isoindolo[2,1-a]quinazoline derivative (compound 2) as a potent inhibitor of TNF-α,2 and benzimidazo[2,1-a]isoindolone (compound 3) showed Batracylin-comparable anti-tumor activity as well as its ability to induce unscheduled DNA synthesis in rat hepatocytes.3
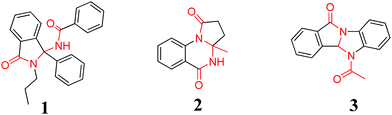 | ||
| Scheme 1 Representative structures of N, N′-methylenediformamide skeleton molecules. | ||
Transition metal-catalyzed C–N formation has represented a powerful and efficient protocol in organic amine chemistry.4 Although many ground-breaking contributions have been devoted to the forging of C–N bonds, such as Buchwald–Hartwig C–N coupling reaction,5N-arylation of amides6 and the Ullmann condensation reaction,7 the prefunctionalized starting materials seriously limit the use of such methods.8 Therefore, a direct C–H bond amination reaction based on transition metal catalysis is highly desirable and of great significance.9 To the best of our knowledge, the work on highly regioselective amination of sp3 C–H bonds remains elusive.10 Up to now, only a few examples for amination methods of sp3 C–H bonds have been reported, however, expensive metal catalysts, such as Ru, Pd and Rh, should be employed.11 Meanwhile, considerable toxicity of those catalysts also limits their applications and possible improvements. Compared with those noble metals, iron catalysis has received considerable attention in modern synthetic chemistry.12 In this paper, we reported a highly Fe(II)-catalyzed regioselective amination of sp3 C–H bonds adjacent to nitrogen atoms under mild conditions.
Initially, we selected benzamide 4a and 5a as the model substrates in the presence of 10 mol% FeCl3 with 1.5 equiv of tertbutyl hydroperoxide (TBHP) as the oxidant and CH3CN as the solvent under air at 80 °C. However, only traces of 6a could be detected (Table 1, entry1). To our delight, 61%, 52% and 64% yields of product 6a could be obtained by using Fe(II) as a catalyst in butanone, acetonitrile and decane, respectively (Table 1, entries 2–4), which also demonstrates that decane is the best solvent for amination reaction of sp3 C–H bonds. It was found that N-(1-methyl-5-oxopyrrolidin-2-yl)benzamide is the main product, but N-((2-oxopyrrolidin-1-yl)methyl)benzamide and N-(1-methyl-2-oxopyrrolidin-3-yl)-benzamide as by-products could not be detected. Meanwhile, if other metal salts were used, such as Fe2(SO4)3, CuCl2, CuBr, CuBr2, Cu(OAc)2, CuSO4, Pd(OAc)2 and Ni(Ac)2, the results showed that these metal salts were proved to be less effective or totally ineffective (see ESI†), indicating that Fe(II) is the best catalyst in this reaction system. Then, some commercially available ligands were used to produce the corresponding Fe(II) complex catalysts (Table 1, entries 13–16), 6,6′-dimethyl-2,2′-bipyridine L1 shows better chemoselectivity and produces higher yields. Moreover, we also sifted out different oxidants; for instance, with O2, PIA, DTBP, H2O2 and MnO2, the desired product 6a was not obtained (entries 5–9). Fortunately, a yield of 84% could be obtained in N2 instead of air if the amination reaction was carried out using 20% Fe(II) and 3.5 equiv TBHP (Table 1, entry 17). Therefore, the optimal conditions for the amination reaction of sp3 C–H bonds were established as L1 (20%), Fe(II) (20%), TBHP (3.5 equiv compared to 4), n-decane (2 mL) in nitrogen atmosphere.
|
|
|||||
|---|---|---|---|---|---|
| Entry | Catalyst | Solvent | Ligand | Oxidant | Yield (%)b |
| a The reaction was carried out with 4a (0.1 mmol), 5a (0.3 mmol), catalyst (0.01 mmol), L (0.01 mmol), oxidant (0.1 mmol) and 2 mL solvent at 90 °C under air for 24 h. b The yield of 6a. c Iodobenzene diacetate. d Di-tert-butylperoxide. e Catalyst (0.005 mmol). f Catalyst (0.015 mmol). g Catalyst (0.02 mmol). h Under nitrogen atmosphere. i 3.5 equiv TBHP (70% in H2O). | |||||
| 1 | FeCl3 | CH3CN | — | TBHP | trace |
| 2 | FeCl2·4H2O | CH3COC2H5 | — | TBHP | 61 |
| 3 | FeCl2·4H2O | CH3CN | — | TBHP | 52 |
| 4 | FeCl2·4H2O | decane | — | TBHP | 64 |
| 5 | FeCl2·4H2O | decane | — | O2 | trace |
| 6 | FeCl2·4H2O | decane | — | PIAc | trace |
| 7 | FeCl2·4H2O | decane | — | DTBP d | 0 |
| 8 | FeCl2·4H2O | decane | — | H2O2 | trace |
| 9 | FeCl2·4H2O | decane | — | MnO2 | trace |
| 10e | FeCl2·4H2O | decane | — | TBHP | 49 |
| 11f | FeCl2·4H2O | decane | — | TBHP | 68 |
| 12g | FeCl2·4H2O | decane | — | TBHP | 72 |
| 13g | FeCl2·4H2O | decane | L1 | TBHP | 79 |
| 14g | FeCl2·4H2O | decane | L2 | TBHP | 65 |
| 15g | FeCl2·4H2O | decane | L3 | TBHP | 73 |
| 16g | FeCl2·4H2O | decane | L4 | TBHP | 52 |
| 17ghi | FeCl2·4H2O | decane | L1 | TBHP | 84 |
| 18 | — | decane | L1 | TBHP | 0 |
| 19 | FeCl2·4H2O | decane | L1 | — | 0 |
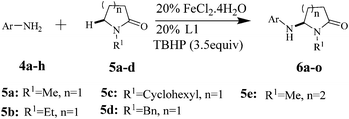
The scope of the Fe(II)-catalyzed amination of sp3 C–H bonds has been explored under the optimized conditions mentioned above. Table 2 lists various yields of the desired amination products. With respect to amide derivatives, both aryl amides and aryl sulfonamides (Table 2, entries 8–9 and 12) can react with N-substituted tert-amides. It was found that amides with electron-donating groups (Table 2, entries 2, 3, 6 and 7) have higher reactivity than those containing electron-deficient groups (Table 2, entries 4 and 5). We also found that the tertiary amides, such as N-substituted cycloamides show better reaction activities than N-substituted piperidin-2-one (Table 2, entry 15). In addition, with N-ethylpyrrolidin-2-ones (Table 2, entries 10–12), moderate yields could be afforded, and only trace products 6m and 6n were obtained owing to steric hindrance (entries 13 and 14). Herein, we chose 1-methylpyrrolidin-2-one as a substrate to explain regioselectivity of the amination reaction. This substrate has three different hydrogen atoms from N-methylene, N-methyl or methylene of the amide group, which are potentially susceptible to the amination reaction. Interestingly, the result indicates that only the product 6a arises from the C–H reaction of the N-methylene group. It can be attributed to the reason that stereoelectronic factor plays an important role in C–H regioselectivity of the amination reaction, that is, a favorable alignment of the C–H bond with the nitrogen lone pair can lead to C–H bond cleavage at the ring.13 It can further be demonstrated as shown in the proposed mechanism where radical intermediate 41 can favorably form intermediate 43 (Scheme 2).
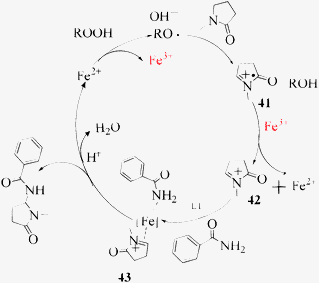 | ||
| Scheme 2 The proposed mechanism of the C–H amination process. | ||
|
|
|||
|---|---|---|---|
| Entry | Time/h | Product | Yield (%) b |
| a Reaction conditions: 4 (0.1 mmol), 5 (0.3 mmol), L1 (20 mmol%), FeCl2·4H2O (20 mmol%), TBHP (3.5 equiv compared to 4), n-decane (2 mL) in nitrogen atmosphere, at 90 °C. b Isolated yield. | |||
| 1 | 4 |
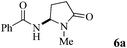
|
84 |
| 2 | 4 |
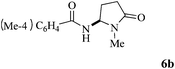
|
87 |
| 3 | 3 |
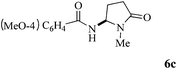
|
89 |
| 4 | 4 |
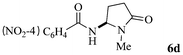
|
63 |
| 5 | 4 |
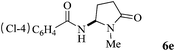
|
62 |
| 6 | 3 |
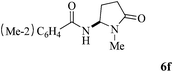
|
74 |
| 7 | 3 |
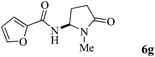
|
82 |
| 8 | 4 |
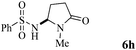
|
71 |
| 9 | 4 |
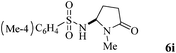
|
69 |
| 10 | 4 |
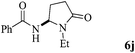
|
60 |
| 11 | 4 |
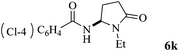
|
58 |
| 12 | 3 |
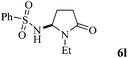
|
56 |
| 13 | 4 |
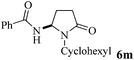
|
28 |
| 14 | 4 |
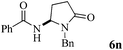
|
37 |
| 15 | 4 |
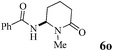
|
32 |
The proposed mechanism of the present C–H amination process is shown in Scheme 2. We found that the reaction rate and yield dramatically decrease under our standard coupling conditions when radical scavenger BHT (2,6-di-tert-buthyl-p-cresol) was added to the amination reaction system of benzamide and N-methylpyrrolidin-2-one. Herein, a free radical reaction process would be involved. According to some relevant reports14 and our experimental results, we think that the tert-butyl radicals could react with N-methyllpyrrolidin-2-one through a single-electron transfer (SET) to afford the more stable radical intermediate 41 while t-BuOOH produces tert-butyl radicals and hydroxyl in the presence of Fe(II) salts. It is probable that the radical cation 41 first reacts with Fe(III) to produce cation 42 which then combines with Fe(II), ligand 6,6′-dimethyl-2,2′-bipyridine L1 and amide to form the complex 43. The amination product could be produced after the removal of the Fe(II) catalyst.
In summary, we have described a direct and regioselective amination reaction based on an efficient and inexpensive Fe(II) complex catalyst with TBHP as a benign oxidant. This reaction also offers a facile method for the construction of C–N bonds.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 20972065, 21074054, 51173078, 21172106) and National Basic Research Program of China (2010CB923303).References
- (a) R. Hardcastle, S. U. Ahmed, H. Atkins, G. Farnie, B. T. Golding, R. J. Griffin, S. Guyenne, C. Hutton, P. Källblad, S. J. Kemp, M. S. Kitching, D. R. Newell, S. Norbedo, J. S. Northen, R. J. Reid, K. Saravanan, H. M. G. Willems and J. Lunec, Bioorg. Med. Chem. Lett., 2005, 15, 1515 CrossRef; (b) I. R. Hardcastle, S. U. Ahmed, H. Atkins, G. Farnie, B. T. Golding, R. J. Griffin, S. Guyenne, C. Hutton, P. Källblad, S. J. Kemp, M. S. Kitching, D. R. Newell, S. Norbedo, J. S. Northen, R. J. Reid, K. Saravanan, H. M. G. Willems and J. Lunec, J. Med. Chem., 2006, 49, 6221 CrossRef.
- (a) K. Pfeffer, Cytokine Growth Factor Rev., 2003, 14, 185 CrossRef CAS; (b) K. S. Kumar, P. M. Kumar, K. A. Kumar, M. Sreenivasulu, A. A. Jafar, D. Rambabu, G. R. Krishna, C. M. Reddy, R. Kapavarapu, K. Shivakumar, K. K. Priya, K. V. L. Parsa and M. Pal, Chem. Commun., 2011, 47, 2010 Search PubMed.
- A. Cul, A. Daïch, B. Decroix, G. Sanz and L. V. Hijfte, Tetrahedron, 2004, 60, 11029 CrossRef CAS.
- (a) A. Ricci, For selected examples Modern Amination Methods, Wiley-VCH, Weinheim, 2000; (b) A. Ricci, Amino Group Chemistry From Synthesis to the Life Sciences, Wiley-VCH, Weinheim, 2008; (c) B. J. Li, S. L. Tian, Z. Fang and Z. J. Shi, Angew. Chem., 2008, 120, 1131 CrossRef; (d) A. Armstrong and J. Collins, Angew. Chem., Int. Ed., 2010, 49, 2282 CrossRef CAS; (e) Z. Wang, Y. M. Zhang, H. Fu, Y. Y. Jiang and Y. F. Zhao, Org. Lett., 2008, 10, 1863 CrossRef CAS; (f) T. Kawano, K. Hirano, T. Satoh and M. Miura, J. Am. Chem. Soc., 2010, 132, 6900 CrossRef CAS; (g) J. F. Hartwig, Nature, 2008, 455, 314 CrossRef CAS; (h) S. M. Guo, B. Qian, Y. J. Xie, C. G. Xia and H. M. Huan, Org. Lett., 2011, 13, 522 CrossRef CAS; (i) S. J. Zhu, J. Dong, S. M. Fu, H. F. Jiang and W. Zeng, Org. Lett., 2011, 13, 4914 CrossRef CAS; (j) S. F. Martin, Pure Appl. Chem., 2009, 81, 195 CrossRef CAS and references therein.
- (a) For selected Hartwig reaction examples: D. S. Surry and S. L. Buchwald, Angew. Chem., Int. Ed., 2008, 47, 6338 CrossRef CAS; (b) J. F. Hartwig, Acc. Chem. Res., 2008, 41, 1534 CrossRef CAS.
- (a) A. Klapars, J. C. Antila, X. Huang and S. L. Buchwald, J. Am. Chem. Soc., 2001, 123, 7727 CrossRef CAS; (b) A. Klapars, X. Huang and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 7421 CrossRef CAS.
- (a) J. Hassan, M. Sevingnon, C. Gozzi, E. Shulz and M. Lemaire, Chem. Rev., 2002, 102, 1359 CrossRef CAS; (b) S. V. Ley and A. W. Thomas, Angew. Chem., 2003, 115, 5558 CrossRef; (c) S. V. Ley and A. W. Thomas, Angew. Chem., Int. Ed., 2003, 42, 5400 CrossRef CAS; (d) J. Y. Kim, S. H. Cho, J. Joseph and S. Chang, Angew. Chem., Int. Ed., 2010, 49, 9899 CrossRef CAS.
- (a) G. S. Kumar, C. U. Maheswari, R. A. Kumar, M. L. Kantam and K. R. Reddy, Angew. Chem., Int. Ed., 2011, 50, 11748 CrossRef CAS; (b) J. Rodolphe, H. Julien, R. Alice, S. K. Julien and B. Olivier, Chem.–Eur. J., 2010, 16, 2654 CrossRef.
- (a) Y. Miyake, K. Nakajima and Y. Nishibayashi, J. Am. Chem. Soc., 2012, 134, 3338 CrossRef CAS; (b) H. M. L. Davies and M. S. Long, Angew. Chem., Int. Ed., 2005, 44, 3518 CrossRef CAS; (c) H. Li, B. J. Li and Z. J. Shi, Catal. Sci. Technol., 2011, 1, 191 RSC.
- F. Collet, R. H. Dodd and P. Dauban, Chem. Commun., 2009, 5061 RSC.
- (a) C. G. Espino and J. Du Bois, Angew. Chem., Int. Ed., 2001, 40, 598 CrossRef CAS; (b) C. G. Espino, P. M. Wehn, J. Chow and J. Du Bois, J. Am. Chem. Soc., 2001, 123, 6935 CrossRef CAS; (c) K. J. Fraunhoffer and M. C. White, J. Am. Chem. Soc., 2007, 129, 7274 CrossRef CAS; (d) J. J. Neumann, S. Rakshit, T. Dröge and F. Glorius, Angew. Chem., Int. Ed., 2009, 48, 6892 CrossRef CAS; (e) C. Zhu and J. R. Falck, Chem. Commun., 2012, 48, 1674 RSC; (f) T. S. Mei, X. S. Wang and J. Q. Yu, J. Am. Chem. Soc., 2009, 131, 10806 CrossRef CAS.
- (a) C. L. Sun, B. J. Li and Z. J. Shi, Chem. Rev., 2011, 111, 1293 CrossRef CAS; (b) Z. P. Li, R. Yu and C. J. Li, Angew. Chem., Int. Ed., 2008, 47, 7497 CrossRef CAS; (c) C. Liu, H. Zhang, W. Shi and A. W. Lei, Chem. Rev., 2011, 111, 1780 CrossRef CAS.
- S. Angioni, D. Ravelli, D. Emma, D. Dondi, M. Fagnoni and A. Albini, Adv. Synth. Catal., 2008, 350, 2209 CrossRef CAS.
- (a) J. Xie, H. M. Li, J. C. Zhou, Y. X. Cheng and C. J. Zhu, Angew. Chem., Int. Ed., 2012, 51, 1252 CrossRef CAS; (b) E. Boess, C. Schmitz and M. Klussmann, J. Am. Chem. Soc., 2012, 134, 5317 CrossRef CAS; (c) Y. M. Zhang, H. Fu, Y. Y. Jiang and Y. F. Zhao, Org. Lett., 2007, 9, 3813 CrossRef CAS; (d) T. Yoshimitsu, Y. Arano, H. Nagaoka and J. Am, J. Am. Chem. Soc., 2005, 127, 11610 CrossRef CAS; (e) Z. P. Li, D. S. Bohle and C. J. Li, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 8928 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, experimental details, NMR spectra, MS spectra of important compounds, and Competition experiment data. See DOI: 10.1039/c2ra20707a |
| This journal is © The Royal Society of Chemistry 2012 |