DOI:
10.1039/C2RA20499A
(Paper)
RSC Adv., 2012,
2, 6773-6783
Received
17th March 2012
, Accepted 26th May 2012
First published on 28th May 2012
Abstract
9,10-Phenanthrenequinone (PQ) reacts with ketones under FeCl3 catalysis to furnish a variety of structurally diverse furan annulated products. While the reaction of PQ with acetone and cyclopentanone furnishes furan annulated ketals, its reaction with ethyl alkyl ketones provides 3-furaldehyde annulated products. In contrast to the reaction of PQ with cyclopentanone, its reaction with cyclohexanone furnishes a tetrahydrobenzofuran annulated secondary alcohol. The reactions of PQ with cycloheptanone and cyclooctanone take a different course to provide 7,8-dihydro-6H-cyclohepta[b]furan and 6,7,8,9-tetrahydrocycloocta[b]furan annulated products, respectively. Mechanistically, the above reactions go through aldol condensation, dehydration and cyclization to form furan-phenanthrene annulated products where in each step FeCl3 catalysis is involved.
1. Introduction
9,10-Phenanthrenequinone (PQ 1) is a fundamental organic molecule widely used in the synthesis of dyes, agrochemicals, preservatives and as a ligand in metal complexes.1 Generally, PQ undergoes two types of reactions, namely (i) two-electron redox reactions with electron donors2 and (ii) aldol condensation with active methylene compounds.3 Of the two, the aldol condensation on one of the ketones followed by dehydration and cyclization provides higher scope for synthetic elaboration into furan, or other such phenanthrenes, annulated with heterocycles exhibiting interesting biological and pharmacological properties.4 Furthermore, some of the furan annulated phenanthrenes show useful photoconducting, optoelectrical and electroluminescence properties.5 In continuation of our studies on the synthesis and structure of polyaryl pyrroles,6 we planned to utilize the 1,2-diketone functional group present in PQ for the synthesis of phenanthrene appended tri- and tetrasubstituted pyrroles. The first step in our strategy for the synthesis of 1,2,4-trisubstituted pyrrole incorporating the phenanthrene motif was aldol condensation of PQ with methyl ketones to provide enediones. Previously, Li and coworkers have carried out FeCl3 mediated aldol condensation of PQ 1 with excess acetone (nearly 30 equivalents) and isolated the anticipated aldol product 2a (Scheme 1).7 However, when we conducted this condensation with a limited amount of acetone (15 equivalents) we isolated unexpected 2,3-dihydroxydihydrofuran annulated phenathrene 3 in addition to the simple aldol condensation product 2a (Scheme 1). Generation of a furan annulated phenanthrene incorporating a 1,2-diol functional group, as seen in 3, is unprecedented. The condensation of PQ 1 with acetone was first reported by Japp and Klingemann over a century ago.8 This reaction yielded 2-methylphenanthro[9,10-b]furan 4 (Scheme 1). On the other hand, relatively recently, Linko and coworkers reported the isolation of propeller shaped bis-tetrahydrofuran annulated phenanthrene 5 from condensation of PQ 1 and acetone on neutral Al2O3 (Scheme 1).9 Intrigued by the isolation of 3, we explored the reaction of PQ with various ketones under FeCl3 catalysis. We now describe the isolation and characterization of several structurally diverse furan annulated phenanthrenes in the reaction of PQ with different ketones in the presence of 6 mol% of FeCl3. FeCl3 is an inexpensive and common Lewis acid catalyst generally employed for a variety of reactions like aldol condensation, Nazarov cyclization, Michael addition, ketal formation, rearrangements, cycloaddition reactions etc.10 Moreover, it is a well-known one-electron oxidant known for effecting phenolic coupling.11 In recent years there have been many reports unravelling the utility of FeCl3 in a wide variety of organic transformations, e.g., oxidative allylation,12 cyanation13etc.
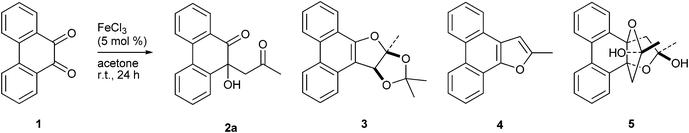 |
| | Scheme 1 FeCl3 mediated condensation of PQ and acetone. | |
2. Results and discussion
2.1. Reaction of PQ 1 with acyclic ketones
The reaction of PQ 1 and acetone 6a (15 equivalents) in the presence of 6 mol% of FeCl3 at rt for 24 h provided a complex mixture of products out of which two crystalline solids, namely PQ-acetone aldol condensation product 2a (14%) and dihydrofuran annulated phenanthrene 3 (26%), could be isolated and characterized (Scheme 2). Notably, this reaction worked only under neat conditions; not in any solvent other than acetone itself. The overall yields of 2a and 3 were lower when the reaction was conducted under high dilution conditions. For example, when 0.48 mmol (100 mg) of PQ 1 in 0.5 mL acetone (about 15 equivalents) was stirred at rt in the presence of 6 mol% of FeCl3, the yield of 3 was 26%, whereas in 3 mL (90 equivalents) of acetone it was only 14%. The reaction did not work when PQ, acetone (5 equivalents) and FeCl3 (6 mol%) were taken in 30 times volume excess of non-polar aprotic solvents like dichloromethane or THF or polar aprotic solvents like DMF. Similarly, the reaction did not work in polar protic solvents like methanol or ethanol. We varied the amount of FeCl3 from 1 mol% to 100 mol% systematically and determined that 6 mol% was the most suitable for the reaction. With a lower amount of FeCl3 (1 mol%), the reaction was very slow; even after 48 h over 80% of PQ remained unreacted. With stoichiometric amounts of FeCl3 the reaction was not clean; several unidentified products along with iron complexes of PQ appeared. Next, we varied the Lewis acid catalysts to find out if the yield of 3 could be improved. We employed 6 mol% of different Lewis acids like iron(III) acetylacetonate, FeSO4, CuSO4, CuBr, ZnCl2, TiCl4, SnCl4, AlCl3, I2 and Et2OBF3 to promote condensation of PQ with acetone. Unfortunately, we did not notice the formation of 2a or 3 in each case, except with SnCl4 which catalyzed the condensation very slowly. Even after 36 h 3 was formed in 16% yield.
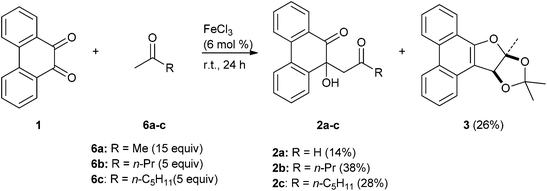 |
| | Scheme 2 FeCl3 mediated condensation of PQ and methyl ketones 6a–c to furnish furan annulated product 3 and aldol condensation products 2a–c. | |
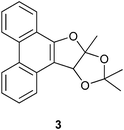
The structure of the novel dihydrofuran annulated phenanthrene 3 was assigned on the basis of spectral and analytical data. The 1H NMR spectrum of 3 displayed three characteristic singlets located at δ 1.90, 1.57 and 1.20 ppm assignable to three methyl groups. The appearance of three singlets for three methyl groups ruled out the possibility of structures 2a, 4 or 5 for the product (see Scheme 1). The 13C NMR spectrum of 3 displayed two quaternary carbons at around δ 115 ppm assignable to two ketal moieties. The structure of 3 was unambiguously confirmed by single crystal X-ray structure analysis.
A possible mechanism for the transformation of PQ and acetone into 3 is given in Scheme 3. Formation of 3 involved two molecules of acetone and one molecule of PQ 1. The first step in the transformation is the condensation of PQ with acetone to form the aldol product 2a. Dehydration of 2a then leads to ene-dione 7. Condensation of ene-dione 7 with a second unit of acetone followed by cyclization furnishes 3. FeCl3 plays the role of a Lewis acid catalyst in promoting each of the three steps, which are thermodynamically controlled.
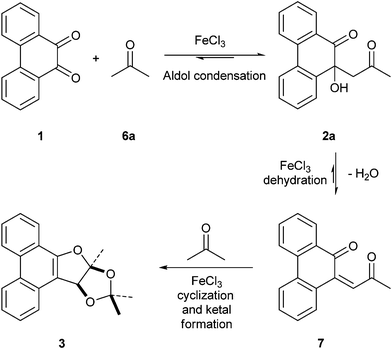 |
| | Scheme 3 Mechanism for the formation of dihydrofuran annulated product 3 from PQ 1 and acetone 6a. | |
To test the generality of the formation of dihydrofuran annulated phenanthrene 3 in the reaction of PQ 1 with methyl ketones, we conducted its reaction with 2-pentanone (n-propyl methyl ketone) 6b and 2-heptanone (n-pentyl methyl ketone) 6c. Both the reactions provided aldol condensation products 2b and 2c, respectively, in moderate yield (Scheme 2). In both the instances we employed 5 equivalents of ketones 6b–c as they have higher boiling points. The reaction course did not change even when 15 equivalents of ketone 6b was employed. Products of the type 3 were not present in the reaction mixture. We believe that, due to steric and entropic reasons, intermediates in the reaction of PQ with methyl ketones 6b and 6c did not proceed all the way to form products of the type 3. Acetophenone did not react with PQ under the present reaction conditions. The FeCl3 catalyzed reaction of PQ with acetone to form the dihydrofuran annulated product 3 appeared to be unique as 1,2-diketones like benzil, isatin or 1,10-phenanthroline-5,6-dione failed to react in a similar fashion.
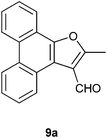
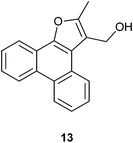
We next conducted the FeCl3 catalyzed reaction of PQ with 2-butanone (ethyl methyl ketone, an example of ethyl alkyl ketone) 8a to realize 2-methylphenanthro[9,10-b]furan-3-carbaldehyde 9a as the only product in 61% yield (Scheme 4). Previously, 9a was isolated by Basavaiah and coworkers from the reaction of PQ with methyl vinyl ketone under Baylis–Hillman reaction conditions.14 Our result is surprising because the final step in the reaction should be FeCl3 mediated, in situ oxidation of the primary alcohol as shown in the proposed mechanism (Scheme 5). The first step is the reaction of the thermodynamically more stable enolate of ethyl methyl ketone with PQ to provide 10 as the aldol product. Dehydration of 10 resulted in enedione 11 as the next intermediate. Cyclization involving enedione and methyl moieties in 11 led to the furan ring as seen in 12. Rearrangement of the tert-alcohol to a primary alcohol resulted in 13. The last step in this remarkable reaction is the FeCl3 catalysed oxidation of the primary alcohol in 13 with atmospheric oxygen to provide the aldehyde 9a. To support the proposed mechanism we reduced the aldehyde 9a back to the alcohol 13 with sodium borohydride and subjected it to FeCl3 catalyzed oxidation in 5 equivalents of ethyl methyl ketone. This reaction provided aldehyde 9a in 95% yield.
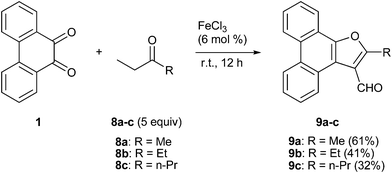 |
| | Scheme 4 FeCl3 catalyzed condensation of PQ 1 with ethyl alkyl ketones 8a–c. | |
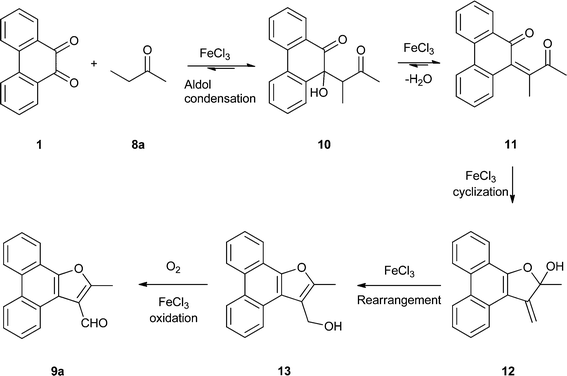 |
| | Scheme 5 Mechanism for the FeCl3 catalyzed reaction of PQ with ethyl methyl ketone 8a to furnish the 3-furaldehyde 9a. | |
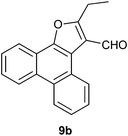
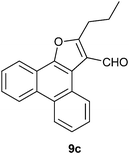
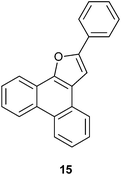
The formation of the 2-alkyl-3-furaldehyde annulated phenanthrenes in the reaction of PQ with ethyl alkyl ketones proved to be general (Scheme 4). When we reacted PQ with two more ethyl ketones 8b–c under FeCl3 catalysis the reactions provided 9b–c in good yield. Spectra of the newly generated aldehydes 9b–c matched well with the parent aldehyde 9a. The product of PQ 1 with propiophenone 14 (an ethyl aryl ketone) was different from other ethyl alkyl ketones. This reaction, conducted at 150 °C, provided 2-phenylphenanthro[9,10-b]furan 15 in low yield as the only product (Scheme 6).15 At rt there was no reaction. We propose that the 2-phenyl-3-furaldehyde derivative that was formed in the reaction underwent iron(III) mediated deformylation to provide 15. There was no reaction between PQ and propanal (an aldehyde with an ethyl chain) or 4-heptanone (a propyl alkyl ketone).
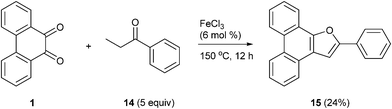 |
| | Scheme 6 Condensation of PQ 1 with propiophenone 14 provides 2-phenylfuran 15 under FeCl3 catalysis. | |
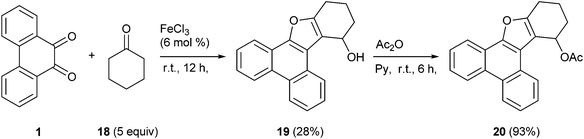
The product formed in the FeCl3 mediated condensation of PQ 1 with cyclohexanone 18 was completely different from that of ketal 17. This remarkable reaction furnished the alcohol 19 as the only product in modest yield (Scheme 8). The structure of 19 was confirmed by analytical, spectroscopic and finally by X-ray single crystal structure analysis. The mechanism for the formation of 19 is given in Scheme 9. The proposed mechanism follows the path taken by ethyl alkyl ketones like 2-butanone (Scheme 5). Unlike the case of 2-butanone condensation with PQ to provide 3-furaldehyde annulated phenanthrene 9a, where final step is the oxidation, in the present case the reaction stopped at the secondary alcohol stage by yielding 19. As given in Scheme 9, the reaction could go through a domino pathway incorporating aldol condensation, dehydration and rearrangement in sequential steps. Acetylation of the alcohol functional group in 19 furnished the acetate 20 whose spectral characteristics matched with those of the parent 19, except the signals which got shifted due to the OAc group.
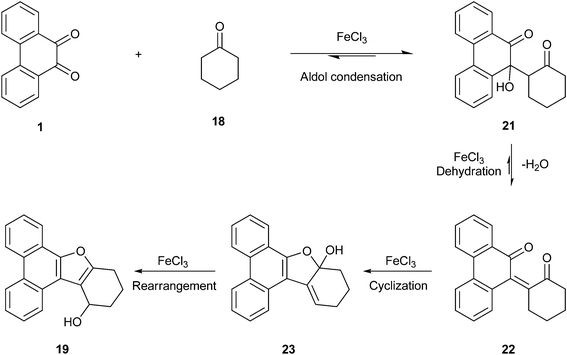 |
| | Scheme 9 Mechanism for the formation of furan annulated product 19 from 9,10-phenanthrenequinone 1 and cyclohexanone 18. | |
The FeCl3 mediated condensation of PQ with cycloheptanone 24a was different from its reaction with cyclohexanone 18. Unlike the reaction of PQ with cyclohexanone where secondary alcohol 19 was isolated, the present reaction furnished the alkene 25a as the only product in good yield (Scheme 10). The reaction of PQ with cycloheptanone went through the same pathway as that of its reaction with cyclohexanone, except that the last step was dehydration to furnish alkene 25a rather than rearrangement to generate an alcohol like 19 (see Scheme 9). The reaction of PQ with cyclooctanone 24b was similar to that of the reaction of PQ with cycloheptanone 24a. This reaction provided the alkene 25b. NMR spectral data of alkenes 25a–b were similar in their features. The structure of 25b was further confirmed on the basis of solving single crystal X-ray diffraction data.
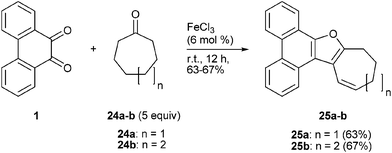 |
| | Scheme 10 FeCl3 catalyzed condensation of PQ 1 with cycloheptanone 24a and cyclooctanone 24b. | |
3.1. Conclusion
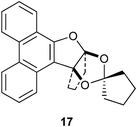
In conclusion, we have shown that PQ 1 reacts with different ketones under FeCl3 catalysis to furnish a variety of furan annulated products. The reaction of PQ with acetone provided a dihyrofuran annulated ketal 3 generated by condensation of two units of acetone with one unit of PQ. Similarly, its reaction with cyclopentanone furnished a propeller shaped ketal 17, which incorporated two units of cyclopentanone and one unit of PQ. The reaction of PQ with longer chain methyl ketones provided only aldol condensation products like 2b–c. The reaction of PQ with ethyl alkyl ketones took an entirely different course to yield 3-furaldehyde annulated products like 9a–c. In this unusual reaction, the last step was FeCl3 mediated oxidation of the secondary alcohol intermediate. In complete contrast to the reaction of PQ with cyclopentanone, its reaction with cyclohexanone furnished the tetrahydrobenzofuran annulated secondary alcohol 19. The products from the reaction of PQ with cycloheptanone or cyclooctanone were different from the products derived from cyclopentanone or cyclohexanone. In these cases, the products were alkenes 25a–b. Thus, we have shown that FeCl3 catalyzed reactions of PQ with ketones provide diverse furan annulated products. Electronic and torsional characteristics of the ketones and the intermediates appear to control the outcome of each of the above reactions.
4. Experimental section
4.1. General
All melting points were uncorrected and were determined using open-ended capillary tubes on a VEEGO VMP-DS instrument. All reactions and chromatographic separations were monitored by thin layer chromatography. Glass plates coated with silica gel (60–120 mesh SRL chemicals) were used for thin layer chromatography. Column chromatography was carried on silica gel (100–200 mesh SRL chemicals) using an increasing percentage of ethyl acetate in hexanes. IR spectra were recorded as KBr pellets on a Nicolet-6700 spectrometer. 1H-NMR (400 MHz), 13C-NMR (100 MHz) and DEPT-135 spectra were recorded for (CDCl3 + CCl4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solutions on a Bruker - 400 spectrometer with tetramethylsilane (TMS) as internal standard; J-values are in Hz. 1H-NMR data are reported as follows: chemical shift (multiplicity: s = singlet, d = doublet, t = triplet, q = quartet and m = multiplet), coupling constant, integrations. 13C NMR spectra were determined with 1H decoupling, 2D NMR spectra (COSY, HSQC and HMBC) for each representative product were recorded to support the assigned structure. High resolution mass spectra were recorded on a Water Q-TOF micro mass spectrometer using the electron spray ionization mode. The X-ray diffraction measurements were carried out at 298 K on an Oxford CrysAlis CCD area detector system equipped with a graphite monochromator and a Mo-Kα fine-focus sealed tube (λ = 0.71073 Å). PQ and all ketones were purchased from Sigma Aldrich chemicals private limited. Ferric chloride was purchased from Qualigens fine chemicals.
1) solutions on a Bruker - 400 spectrometer with tetramethylsilane (TMS) as internal standard; J-values are in Hz. 1H-NMR data are reported as follows: chemical shift (multiplicity: s = singlet, d = doublet, t = triplet, q = quartet and m = multiplet), coupling constant, integrations. 13C NMR spectra were determined with 1H decoupling, 2D NMR spectra (COSY, HSQC and HMBC) for each representative product were recorded to support the assigned structure. High resolution mass spectra were recorded on a Water Q-TOF micro mass spectrometer using the electron spray ionization mode. The X-ray diffraction measurements were carried out at 298 K on an Oxford CrysAlis CCD area detector system equipped with a graphite monochromator and a Mo-Kα fine-focus sealed tube (λ = 0.71073 Å). PQ and all ketones were purchased from Sigma Aldrich chemicals private limited. Ferric chloride was purchased from Qualigens fine chemicals.
4.2. General procedure for iron(III) chloride catalyzed reaction of PQ with ketones
To 9,10-phenanthrenequinone (100 mg, 0.48 mmol) in a covered 10 mL test tube, ketone (2.4 mmol) was added and the mixture was stirred at rt for 5 min. To the resulting mixture, anhydrous FeCl3 (4.7 mg, 6 mol%) was added and stirring continued at rt for 12–24 h. After completion of the reaction (absence of PQ by TLC), excess ketone was evaporated under reduced pressure. The crude product was purified by column chromatography using silica gel (100–200 mesh) and increasing amounts (2 to 10%) of EtOAc in hexanes.
4.2.1. 9a,11,11-Trimethyl-9a,12a-dihydrophenanthro[9',10':4,5]furo[2,3-d][1,3]dioxole 3.
Following the general procedure, the reaction of 9,10-phenanthrenequinone (102.0 mg, 0.49 mmol), propan-2-one (0.5 mL; about 15 equivalents) and iron(III) chloride (5.6 mg, 7.1 mol%) afforded 9a,11,11-trimethyl-9a,12a-dihdrophenanthro[9',10':4,5]furo[2,3-d][1,3]dioxole 3 (Fig. 1) as a white solid in 26% yield; m.p. 170–171 °C; IR (ν): 3072, 2985, 2932, 1610, 1512, 1438, 1231, 1082, 841, 762 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.67 (d, J = 8.3 Hz, 1H), 8.62 (d, J = 8.3 Hz, 1H), 8.16 (dd, J = 8.0, 1.0 Hz, 1H), 7.93 (dd, J = 8.0, 1.0 Hz, 1H), 7.72–7.68 (m, 1H), 7.65–7.60 (m, 2H), 7.54–7.50 (m, 1H), 5.77 (s, 1H), 1.90 (s, 3H), 1.57 (s, 3H), 1.20 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 153.5 (C), 132.9 (C), 129.7 (C), 128.0 (CH), 127.7 (CH), 127.4 (C), 126.8 (CH), 124.4 (CH), 123.5 (CH), 123.4 (CH), 123.33 (CH), 123.29 (CH), 122.0 (C), 117.2 (C), 114.1 (C), 113.6 (C), 85.9 (CH), 28.6 (CH3), 27.4 (CH3), 24.2 (CH3) ppm; HRMS (ESI) calcd for C20H19O3 [M + H]+ 307.1334, found 307.1349.
![ORTEP diagram of 9a,11,11-trimethyl-9a,12a-dihydrophenanthro[9',10':4,5]furo[2,3-d][1,3]dioxole 3 (CCDC 813673) with crystallographic numbering.](/image/article/2012/RA/c2ra20499a/c2ra20499a-f1.gif) |
| | Fig. 1 ORTEP diagram of 9a,11,11-trimethyl-9a,12a-dihydrophenanthro[9',10':4,5]furo[2,3-d][1,3]dioxole 3 (CCDC 813673) with crystallographic numbering. | |
Crystal data for 3.
Empirical formula, C20H18O3; formula weight, 306.34; crystal color, habit: colorless, block; crystal system, orthorhombic; crystal dimensions, 0.55 × 0.35 × 0.3 mm3; lattice parameters, a = 8.1778(2) Å, b = 8.9545(3) Å, c = 21.7298(6) Å; α = 90.00, β = 90.00, γ = 90.00; V = 1591.23 (8) A3; space group, P2 (1); Z = 4; Dc = 1.279 g cm−3; F000 = 648; λ(Mo-Kα) = 0.71073 Å; R (I ≥ 2σ1) = 0.0450, wR2 = 0.1092. Detailed X-ray crystallographic data is available from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK (for compound 3 CCDC 813673†).
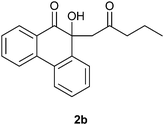
4.2.2 10-Hydroxy-10-(2-oxopentyl)phenanthren-9(10H)-one 2b.
Following the general procedure, the reaction of 9,10-phenanthrenequinone (100.2 mg, 0.48 mmol), pentan-2-one (208.3 mg, 2.41 mmol) and iron(III) chloride (5.8 mg, 7.7 mol %) afforded 10-hydroxy-10-(2-oxopentyl)phenanthren-9(10H)-one 2b (Fig. 2) as a white solid in 38% yield; m.p. 86–87 °C; IR (ν): 3486, 2960, 1694, 765 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.93 (dd, J = 7.6, 1.3 Hz, 1H), 7.88 (d, J = 7.9 Hz, 1H), 7.79–7.66 (m, 3H), 7.45–7.37 (m, 3H), 4.45 (s, 1H), 2.95 (d, J = 14.5 Hz, 1H), 2.75 (d, J = 14.5 Hz, 1H), 2.34–2.25 (m, 2H), 1.51–1.43 (m, 2H), 0.87–0.84 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 207.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 202.4 (C
O), 202.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 139.9 (C), 136.9 (C), 134.8 (CH), 129.5 (C), 129.4 (CH + C), 128.8 (CH), 128.7 (CH), 127.9 (CH), 126.2 (CH), 124.5 (CH), 123.1 (CH), 78.5 (C), 55.0 (CH2), 46.9 (CH2), 16.8 (CH2), 13.7 (CH3) ppm; HRMS (ESI) calcd for C19H18O3Na [M + Na]+ 317.1154, found 317.1142.
O), 139.9 (C), 136.9 (C), 134.8 (CH), 129.5 (C), 129.4 (CH + C), 128.8 (CH), 128.7 (CH), 127.9 (CH), 126.2 (CH), 124.5 (CH), 123.1 (CH), 78.5 (C), 55.0 (CH2), 46.9 (CH2), 16.8 (CH2), 13.7 (CH3) ppm; HRMS (ESI) calcd for C19H18O3Na [M + Na]+ 317.1154, found 317.1142.
 |
| | Fig. 2 ORTEP diagram of 10-hydroxy-10-(2-oxopentyl)phenanthren-9(10H)-one 2b (CCDC 797133) with crystallographic numbering. | |
Crystal data for 2b.
Empirical formula, C19H18O3; formula weight, 294.33; crystal color, habit: colorless, block; crystal system, triclinic; crystal dimensions, 0.38 × 0.36 × 0.36 mm3; lattice parameters, a = 8.2005(8) Å, b = 9.5269(16) Å, c = 11.4959(15) Å; α = 65.961 (14), β = 85.022 (9), γ = 66.268 (12); V = 747.7 (2) A3; space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ; Z = 2; Dc = 1.307 g cm−3; F000 = 312; λ(Mo-Kα) = 0.7107 Å; R (I ≥ 2σ1) = 0.0785, wR2 = 0.1102. Detailed X-ray crystallographic data is available from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK (for compound 2b CCDC 797133†).
; Z = 2; Dc = 1.307 g cm−3; F000 = 312; λ(Mo-Kα) = 0.7107 Å; R (I ≥ 2σ1) = 0.0785, wR2 = 0.1102. Detailed X-ray crystallographic data is available from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK (for compound 2b CCDC 797133†).
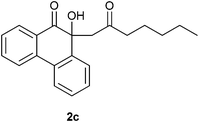
4.2.3. 10-Hydroxy-10-(2-oxoheptyl)phenanthren-9(10H)-one 2c.
Following the general procedure, the reaction of 9,10-phenanthrenequinone (101.2 mg, 0.48 mmol), heptan-2-one (281.0 mg, 2.46 mmol) and iron(III) chloride (5.1 mg, 6.8 mol%) afforded 10-hydroxy-10-(2-oxoheptyl)phenanthren-9(10H)-one 2c as a yellow solid in 28% yield; m.p. 85–86 °C; IR (ν): 3482, 2941, 1694, 765 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.94 (dd, J = 7.6, 1.2 Hz, 1H), 7.88 (d, J = 8.0 Hz, 1H), 7.79–7.66 (m, 3H), 7.45–7.37 (m, 3H), 4.44 (s, 1H), 2.95 (d, J = 14.5 Hz, 1H), 2.75 (d, J = 14.5 Hz, 1H), 2.33–2.29 (m, 2H), 1.45–1.43 (m, 2H), 1.26–1.17 (m, 4H), 0.87–0.84 (t, J = 7.1 Hz, 3H); 13C NMR (100 MHz, CDCl3) 207.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 202.2 (C
O), 202.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 139.8 (C), 136.7 (C), 134.5 (CH), 129.4 (C), 129.3 (C), 129.2 (CH), 128.7 (CH), 128.5 (CH), 127.8 (CH), 126.0 (CH), 124.3 (CH), 122.9 (CH), 78.4 (C), 54.9 (CH2), 44.8 (CH2), 31.2 (CH2), 22.8 (CH2), 22.4 (CH2), 13.9 (CH3) ppm; HRMS (ESI) calcd for C21H22O3Na [M + Na]+ 345.1467, found 345.1466.
O), 139.8 (C), 136.7 (C), 134.5 (CH), 129.4 (C), 129.3 (C), 129.2 (CH), 128.7 (CH), 128.5 (CH), 127.8 (CH), 126.0 (CH), 124.3 (CH), 122.9 (CH), 78.4 (C), 54.9 (CH2), 44.8 (CH2), 31.2 (CH2), 22.8 (CH2), 22.4 (CH2), 13.9 (CH3) ppm; HRMS (ESI) calcd for C21H22O3Na [M + Na]+ 345.1467, found 345.1466.
4.2.4. 2-Methylphenanthro[9,10-b]furan-3-carbaldehyde 9a.
Following the general procedure, the reaction of 9,10-phenanthrenequinone (102.0 mg, 0.49 mmol), butan-2-one (181.0 mg, 2.51 mmol) and iron(III) chloride (4.9 mg, 6.6 mol %) afforded 2-methylphenanthro[9,10-b]furan-3-carbaldehyde 9a as a white solid in 61% yield; m.p. 180–181 °C (reported m.p. 183 °C);14 IR (ν): 3075, 2923, 1684, 753, 720 cm−1; 1H NMR (400 MHz, CDCl3) δ 10.24 (s, 1H), 9.24 (dd, J = 8.3, 1.4 Hz, 1H), 8.64–8.60 (m, 2H), 8.16–8.14 (m, 1H), 7.67–7.60 (m, 4H), 2.76 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 184.7 (CH), 166.8 (C), 148.7 (C), 129.6 (C), 128.7 (C), 128.2 (CH), 127.31 (CH), 127.29 (C), 127.1 (CH), 126.7 (CH), 125.9 (CH), 123.3 (CH), 123.2 (CH), 121.2 (C), 120.9 (C), 120.6 (CH), 117.4 (C), 13.6 (CH3) ppm; HRMS (ESI) calcd for C18H12O2Na [M + Na]+ 283.0735, found 283.0734.
4.2.5. (2-Methylphenanthro[9,10-b]furan-3-yl)methanol 13.
2-Methylphenanthro[9,10-b]furan-3-carbaldehyde 8a (22.6 mg, 0.086 mmol) was dissolved in methanol (0.5 mL) and to this solution sodium borohydride (12 mg, 0.31 mmol) was added at 0 °C for 10 min. Then the temperature was raised to rt and stirred for 2 h. After completion of the reaction (TLC), MeOH was evaporated under reduced pressure. Then the crude product was extracted using dichloromethane (DCM; 20 mL). The DCM layer was washed with water (2 × 20 mL), brine solution (2 × 20 mL) and then dried over anhydrous sodium sulphate. Removal of the solvent under reduced pressure afforded (2-methylphenanthro[9,10-b]furan-3-yl)methanol 13 as a white solid in 81% yield; m.p. 194–195 °C; IR (ν): 3318, 3076, 2922, 2857, 1671, 1445, 1256, 1101, 974, 808, 749 cm−1; 1H NMR (400 MHz, DMSO) δ 8.77(dd, J = 8.1, 1.5 Hz, 2H), 8.57 (dd, J = 8.0, 1.1 Hz, 1H), 8.23 (dd, J = 8.0, 1.2 Hz, 1H), 7.68–7.59 (m, 4H), 4.83 (s, 2H), 2.61 (s, 3H); 13C NMR (100 MHz, DMSO) 150.9 (C), 146.8 (C), 127.9 (C), 127.5 (C), 127.4 (C), 126.62 (CH), 126.56 (CH), 125.2 (C), 125.1 (CH), 124.5 (CH), 123.3 (CH), 123.1 (CH), 121.4 (C), 119.6 (C), 119.5 (CH), 117.3 (C), 54.1 (CH2), 11.7 (CH3) ppm.
4.2.6. 2-Ethylphenanthro[9,10-b]furan-3-carbaldehyde 9b.
Following the general procedure, the reaction of 9,10-phenanthrenequinone (100.9 mg, 0.48 mmol), pentan-3-one (212.8 mg, 2.4 mmol) and iron(III) chloride (6.6 mg, 8.8 mol%) afforded 2-ethylphenanthro[9,10-b]furan-3-carbaldehyde 9b as a yellow solid in 41% yield; m.p. 134–136 °C (reported m.p. 136–138 °C);14 IR (ν): 3081, 2979, 2929, 2851, 2741, 1684, 1447, 868, 757 cm−1; 1H NMR (400 MHz, CDCl3) δ 10.29 (s, 1H), 9.26 (dd, J = 8.1, 1.1 Hz, 1H), 8.60–8.57 (m, 2H), 8.19–8.16 (m, 1H), 7.68–7.57 (m, 4H), 3.18 (q, 2H), 1.49 (t, J = 7.6 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 184.1 (CH), 171.1 (C), 148.5 (C), 129.6 (C), 128.6 (C), 128.1 (CH), 127.3 (C), 127.1 (CH), 126.8 (CH), 126.4 (CH), 125.7 (CH), 123.2 (CH), 123.0 (CH), 121.2 (C), 120.5 (CH), 119.9 (C), 117.3 (C), 21.0 (CH2), 13.2 (CH3) ppm; HRMS (ESI) calcd for C19H14O2Na [M + Na]+ 297.0891, found 297.0896.
4.2.7. 2-Propylphenanthro[9,10-b]furan-3-carbaldehyde 9c.
Following the general procedure, the reaction of 9,10-phenanthrenequinone (100.2 mg, 0.48 mmol), hexan-3-one (246.3 mg, 2.46 mmol) and iron(III) chloride (6.3 mg, 8.0 mol%) afforded 2-propylphenanthro[9,10-b]furan-3-carbaldehyde 9c as a yellow solid in 32% yield; m.p. 168–169 °C; IR (ν): 3082, 2958, 2924, 2865, 1686, 755, 721 cm−1; 1H NMR (400 MHz, CDCl3) δ 10.36 (s, 1H), 9.35 (dd, J = 8.1, 1.2 Hz, 1H), 8.67–8.65 (m, 2H), 8.27–8.25 (m, 1H), 7.71–7.60 (m, 4H), 3.20 (t, J = 7.5 Hz, 2H), 2.01–1.92(m, 2H), 1.11 (t, J = 7.4 Hz, 3H); 13C NMR (100 MHz, CDCl3) δ 184.4 (CH), 170.4 (C), 149.0 (C), 129.9 (C), 128.9 (C), 128.5 (CH), 127.6 (C), 127.5 (CH), 127.1 (CH), 126.7 (CH), 126.1 (CH), 123.5 (CH), 123.3 (CH), 121.5 (C), 121.1 (C), 120.8 (CH), 117.7 (C), 29.6 (CH2), 22.6 (CH2), 14.1 (CH3) ppm; HRMS (ESI) calcd for C20H16O2Na [M + Na]+ 289.1229, found 289.1226.
4.2.8. 2-Phenylphenanthro[9,10-b]furan 15.
9,10-Phenanthrenequinone (100.2 mg, 0.48 mmol) was taken in a 10 mL test tube and propiophenone (148.2 mg, 2.44 mmol) was added under a nitrogen atmosphere and was stirred at rt for 10 min. Then, to the reaction mixture, iron(III) chloride (6.8 mg, 8.7 mol%) was added and stirred at rt for 10 min. (At rt 78.2 mg of solid is formed which is not soluble in ethyl acetate, dichloromethane, dimethylsulfoxide, acetonitrile, methanol and water). Then, the temperature was raised to 150 °C and stirred for 12 h. After completion of the reaction (TLC), the crude product was purified by column chromatography using silica gel (100–200 mesh) and 2% EtOAc in hexanes as the eluent to afford 2-phenylphenanthro[9,10-b]furan 15 as a white solid in 24% yield; m.p. 167–168 °C (reported m.p. 170 °C);15 IR (ν): 3058, 3023, 1606, 1517, 1452, 755, 690 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.69 (d, J = 7.9 Hz, 2H), 8.42 (dd, J = 7.8, 1.0 Hz, 1H), 8.15 (dd, J = 7.4, 1.6 Hz, 1H), 7.97 (dd, J = 8.4, 1.2 Hz, 2H), 7.70–7.59 (m, 4H), 7.52–7.47 (m, 3H), 7.39–7.35 (m, 1H); 13C NMR (100 MHz, CDCl3) 155.2 (C), 149.0 (C), 130.9 (C), 129.3 (C), 129.0 (CH), 128.4 (C), 128.2 (CH), 127.5 (C), 127.2 (CH), 127.1 (CH), 125.9 (CH), 125.4 (CH), 124.7 (CH), 124.1 (CH), 123.8 (CH), 123.6 (CH), 122.5 (C), 122.1 (C), 120.8 (CH), 101.4 (CH) ppm; HRMS (ESI) calcd for C22H15O [M + H]+ 295.1120, found 295.1110.
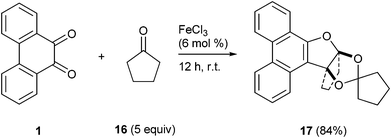
4.2.9. (9aSR,12aSR)-11,12-Dihydro-10H-spiro[9a,12a (epoxymethanooxy)cyclopenta[b]phenanthro[9,10-d]furan-14,1'-cyclopentane] 17.
Following the general procedure, the reaction of 9,10-phenanthrenequinone (100.2 mg, 0.48 mmol), cyclopentanone (196.5 mg, 2.33 mmol), and iron(III) chloride (5.3 mg, 7.1 mol%) afforded (9aS,12aS)-11,12-dihydro-10H-spiro[9a,12a-(epoxymethanooxy)cyclopenta[b]phenanthro [9,10-d] furan-14,1'-cyclopentane] 17 (Fig. 3) as a white solid in 84% yield; m.p. 153–154 °C; IR (ν): 3066, 2963, 1606, 1512, 1433, 1344, 1106, 1010, 949, 761 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.67 (d, J = 8.2 Hz, 1H), 8.63 (d, J = 8.2 Hz, 1H), 8.17 (td, J = 7.7, 1.0 Hz, 1H), 7.95 (dd, J = 7.9, 1.0 Hz, 1H), 7.69–7.59 (m, 3H), 7.55–7.52, (m, 1H), 2.74–2.70 (m, 1H), 2.58–2.55 (m, 1H), 2.09–1.92 (m, 6H), 1.66–1.54 (m, 5H), 1.21–1.18 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 153.1 (C), 132.4 (C), 128.8 (C), 127.5 (CH), 127.43 (CH), 127.39 (C), 126.6 (CH), 125.8 (C), 124.3 (CH), 124.1 (C), 123.4 (CH), 123.3 (CH), 123.0 (CH), 122.9 (CH), 122.0 (C), 115.9 (C), 98.3 (C), 38.0 (CH2), 37.5 (CH2), 37.4 (CH2), 36.8 (CH2), 25.2 (CH2), 24.6 (CH2), 22.5 (CH2) ppm; HRMS (ESI) calcd for C24H23O3 [M + H]+ 359.1647, found 359.1647.
![ORTEP diagram of (9aS,12aS)-11,12-dihydro-10H-spiro[9a,12a-(epoxymethanooxy)cyclopenta[b]phenanthro[9,10-d]furan-14,1'-cyclopentane] 17 (CCDC 805644) with crystallographic numbering.](/image/article/2012/RA/c2ra20499a/c2ra20499a-f3.gif) |
| | Fig. 3 ORTEP diagram of (9aS,12aS)-11,12-dihydro-10H-spiro[9a,12a-(epoxymethanooxy)cyclopenta[b]phenanthro[9,10-d]furan-14,1'-cyclopentane] 17 (CCDC 805644†) with crystallographic numbering. | |
Crystal data for 17.
Empirical formula, C24H22O3; formula weight, 358.42; crystal color, habit: colorless, block; crystal system, monoclinic; crystal dimensions, 0.25 × 0.18 × 0.14 mm3; lattice parameters, a = 11.6236(6) Å, b = 10.7231(7) Å, c = 14.8535(10) Å; α = 90.00, β = 101.277 (5), γ = 90.00; V = 1815.61 (19) A3; space group, P2 (1)/c; Z = 4; Dc = 1.311 g cm−3; F000 = 760; λ(Mo-Kα) = 0.71073 Å; R (I ≥ 2σ1) = 0.1234, wR2 = 0.2153. Detailed X-ray crystallographic data is available from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK (for compound 17 CCDC 805644†).
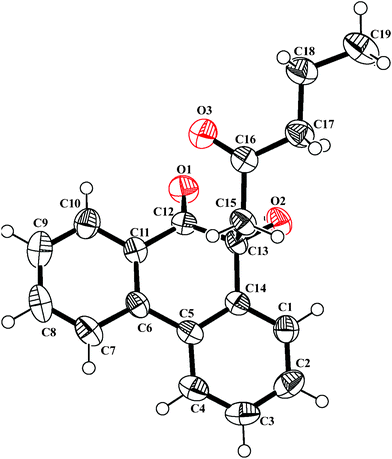
4.2.10. 10,11,12,13-Tetrahydrophenanthro[9,10-b]benzofuran-13-ol 19.
Following the general procedure, the reaction of 9,10-phenanthrenequinone (100.8 mg, 0.48 mmol), cyclohexanone (231.2 mg, 2.35 mmol) and iron(III) chloride (4.9 mg, 6.6 mol%) afforded 10,11,12,13-tetrahydrophenanthro[9,10-b]benzofuran-13-ol 19 (Fig. 4) as a white solid in 28% yield; m.p. 124–125 °C; IR (ν): 3372, 3063, 2934, 1616, 1582, 1440, 1065, 940, 752 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.68 (t, J = 8.1 Hz, 2H), 8.47 (d, J = 8.1 Hz, 1H), 8.26 (d, J = 8.0 Hz, 1H), 7.67–7.59 (m, 4 H) 5.35 (s, 1H), 2.89–2.78 (m, 2H), 2.18–1.92 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 155.2 (C), 148.2 (C), 128.6 (C), 128.2 (C), 127.6 (C), 127.1 (CH), 126.9 (CH), 125.5 (CH), 124.91 (CH), 124.88 (CH), 123.5 (CH), 123.3 (CH), 122.3 (C), 120.2 (CH), 118.5 (C), 117.1 (C), 63.2 (CH), 32.4 (CH2), 23.8 (CH2), 17.7 (CH2) ppm; HRMS (ESI) calcd for C20H16O2Na [M + Na]+ 311.1048, found 311.1058.
![ORTEP diagram of 10,11,12,13-tetrahydrophenanthro[9,10-b]benzofuran-13-ol 19 (CCDC 811040) with crystallographic numbering.](/image/article/2012/RA/c2ra20499a/c2ra20499a-f4.gif) |
| | Fig. 4 ORTEP diagram of 10,11,12,13-tetrahydrophenanthro[9,10-b]benzofuran-13-ol 19 (CCDC 811040†) with crystallographic numbering. | |
Crystal data for 19.
Empirical formula, C20H16O2; formula weight, 288.33; crystal color, habit: colorless, block; crystal system, trigonal; crystal dimensions, 0.54 × 0.48 × 0.42 mm3; lattice parameters, a = 17.7499(6) Å, b = 17.7499(6) Å, c = 24.5070(8) Å; α = 90.00, β = 90.00, γ = 120.00; V = 6686.7(4) A3; space group, R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ; Z = 18; Dcalcd = 1.289 g cm−3; F000 = 2736; λ(Mo-Kα) = 0.71073 Å; R (I ≥ 2σ1) = 0.0529, wR2 = 0.1257. Detailed X-ray crystallographic data is available from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK (for compound 19 CCDC 811040†).
; Z = 18; Dcalcd = 1.289 g cm−3; F000 = 2736; λ(Mo-Kα) = 0.71073 Å; R (I ≥ 2σ1) = 0.0529, wR2 = 0.1257. Detailed X-ray crystallographic data is available from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK (for compound 19 CCDC 811040†).
4.2.11. 10,11,12,13-Tetrahydrophenanthro[9,10-b]benzofuran-13-yl acetate 20.
To the solution of 10,11,12,13-tetrahydrophenanthro[9,10-b]benzofuran-13-ol 19 (31.2 mg, 0.110 mmol) in pyridine (0.5 mL), acetic anhydride (36.3 mg, 0.355 mmol) was added and stirred at rt for 10 min. Then the temperature was raised to 80 °C and stirred for 10 h. After completion of the reaction (TLC), the crude product was extracted using dichloromethane (20 mL). The organic layer was washed with 0.01 N HCl (2 × 20 mL), brine solution (2 × 20 mL) and then dried over anhydrous sodium sulphate. The crude product was purified on column chromatography by using silica (100–200 mesh) and 2% EtOAc in hexanes as the eluent to afford 10,11,12,13-tetrahydrophenanthro[9,10-b]benzofuran-13-yl acetate 20 as a white solid in 76% yield; m.p. 160–161 °C; IR (ν): 3094, 2930, 2847, 1729, 1447, 1377, 1242, 950, 755 cm−1; 1H NMR (400 MHZ, CDCl3) δ 8.66–8.63 (m, 2H), 8.26 (dd, J = 8.1, 1.2 Hz, 1H), 7.91 (dd, J = 7.9, 1.3 Hz, 1H), 7.64–7.54 (m, 4H), 6.54–6.52 (m, 1H), 2.99–2.83 (m, 2H), 2.31–2.25 (m, 2H), 2.12 (s, 3H), 2.05–2.01 (m, 2H; 13C NMR (100 MHZ, CDCl3) δ 170.7 (C), 156.2 (C), 148.4 (C), 128.7 (C), 128.30 (C), 127.6 (C), 127.1 (CH), 126.8 (CH), 125.5 (CH), 124.9 (CH), 123.8 (CH), 123.6 (CH), 123.26 (CH), 122.24 (C), 120.3 (CH), 118.2 (C), 113.5 (C), 66.0 (CH), 29.3 (CH2), 23.6 (CH2), 21.3 (CH2), 18.0 (CH3) ppm.
4.2.12. 11,12-Dihydro-10H-cyclohepta[d]phenanthro[9,10-b]furan 24a.
Following the general procedure, the reaction of 9,10-phenanthrenequinone (99.8 mg, 0.46 mmol), cycloheptanone (271.2 mg, 2.42 mmol) and iron(III) chloride (5.1 mg, 6.6 mol%) afforded 11,12-dihydro-10H-cyclohepta[d]phenanthro[9,10-b]furan 24a as a white solid in 63% yield; m.p. 105–106 °C; IR (ν): 3050, 2925, 1619, 1575, 1507, 1440,1332, 1251, 1157, 1132, 1071, 1038, 946, 856, 756, 716, 624 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.71 (dd, J = 8.1, 0.7 Hz, 1H), 8.65 (d, J = 8.2 Hz, 1H), 8.47 (d, J = 8.0 Hz, 1H), 8.28 (dd, J = 7.9, 1.0 Hz, 1H), 7.63–7.55 (m, 4H), 7.12–7.09 (t, J = 11.5 Hz, 1H), 6.05–6.03 (m, 1H), 3.27 (t, J = 6.2 Hz, 2H), 2.57–2.53 (m, 2H), 2.12–2.09 (m, 2H); 13C NMR (100 MHz, CDCl3) 155.9 (C), 148.0 (C), 130.5 (CH), 129.1 (C), 128.81 (C), 128.75 (CH), 127 0 (CH), 126.9 (CH), 125.6 (CH), 124.6 (CH), 124.1 (CH), 123.9 (CH), 123.4 (CH), 122.4 (C), 120.5 (CH), 120.5 (CH), 118.5 (C), 116.6 (C), 30.3 (CH2), 30.1 (CH2), 23.3 (CH2) ppm; HRMS (ESI) calcd for C21H17O [M + H]+ 289.1279, found 289.1290.
4.2.13. (Z)-10,11,12,13-Tetrahydrocycloocta[d]phenanthro[9,10-b]furan 24b.
Following the general procedure, the reaction of 9,10-phenanthrenequinone (100.3 mg, 0.48 mmol), cyclooctanone (305.6 mg, 2.42 mmol) and iron(III) chloride (6.8 mg, 8.7 mol %) afforded (Z)-10,11,12,13-tetrahydrocycloocta[d]phenanthro[9,10-b]furan 24b (Fig. 5) as a white solid in 67% yield; m.p. 160–161 °C; IR (ν): 3058, 3030, 1609, 1574, 1509, 1446, 1119, 1037, 946, 893, 802, 756, 717 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.70–8.65 (m, 2H), 8.37 (dd, J = 8.0, 1.4 Hz, 1H), 8.30 (dd, J = 7.7, 1.2 Hz, 1H), 7.66–7.55 (m, 4H), 6.94–6.91 (d, J = 10.7 Hz, 1H), 6.07–6.05 (m, 1H), 3.03(s, 2H), 2.32(s, 2H), 1.89(s, 1H), 1.69(s, 1H); 13C NMR (100 MHz, CDCl3) 154.0 (C), 147.7 (C), 131.9 (CH), 128.9 (C), 128.8 (C), 128.5 (CH), 127.0 (CH), 126.9 (CH), 125.5 (CH), 124.7 (CH), 124.5 (CH), 123.7 (CH), 123.5 (CH), 122.6 (C), 122.4 (CH), 120.6 (CH), 119.6 (C), 115.1 (C), 28.1 (CH2), 27.4 (CH2), 26.4 (CH2), 22.7 (CH2) ppm; HRMS (ESI) calcd for C22H19O2 [M + H]+ 299.1430, found 299.1425.
![ORTEP diagram of (Z)-10,11,12,13-tetrahydrocycloocta[d]phenanthro[9,10-b]furan 24b (CCDC 805643) with crystallographic numbering.](/image/article/2012/RA/c2ra20499a/c2ra20499a-f5.gif) |
| | Fig. 5 ORTEP diagram of (Z)-10,11,12,13-tetrahydrocycloocta[d]phenanthro[9,10-b]furan 24b (CCDC 805643†) with crystallographic numbering. | |
Crystal data for 24b.
Empirical formula, C22H18O; formula weight, 298.36; crystal color, habit: colorless, block; crystal system, monoclinic; crystal dimensions, 0.16 × 0.08 × 0.08 mm3; lattice parameters, a = 13.049(3) Å, b = 5.3590(14) Å, c = 21.620(5) Å; α = 90.00, β = 94.35 (2), γ = 90.00; V = 1507.6 (6) A3; space group, P2 (1)/c; Z = 4; Dc = 1.315 g cm−3; F000 = 632; λ(Mo-Kα) = 0.71073 Å; R (I ≥ 2σ1) = 0.2916, wR2 = 0.1183. Detailed X-ray crystallographic data is available from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK (for compound 24b CCDC 805643†).
Acknowledgements
H. S. P. R thanks UGC, UGC-SAP and DST-FIST for financial assistance. V. S. thanks UGC for fellowship. We thank CIF, Pondicherry University and IISc, Bangalore, IIT, Madras and Waters India Pvt Ltd, Bangalore for recording spectra.
References
-
(a) I. Maeda, H. Mitsui and T. Nakamura, Jpn. Kokai Tokkyo Koho., 1985 Search PubMed JP 60233029 A 1985119; CA: 1986
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 148580 CAN104: 148580;
(b) J. Guo and Y. Xue, Taiyuan Ligong Daxue Xuebao, 2001, 32, 140–144 CAS CA: 2001
148580 CAN104: 148580;
(b) J. Guo and Y. Xue, Taiyuan Ligong Daxue Xuebao, 2001, 32, 140–144 CAS CA: 2001![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 332054 CAN135: 305448;
(c)
http://www.chemicalland21.com/specialtychem/finechem/PHENANTHRAQUINONE.htm.
.
332054 CAN135: 305448;
(c)
http://www.chemicalland21.com/specialtychem/finechem/PHENANTHRAQUINONE.htm.
.
-
(a) K. Taguchi, S. Fujii, S. Yamano, A. K. Cho, S. Kamisuki, Y. Nakai, F. Sugawara, J. R. Froines and Y. Kumagai, Free Radical Biol. Med., 2007, 43, 789–799 CrossRef CAS;
(b) P. Milko and J. Roithova, Inorg. Chem., 2009, 48, 11734–11742 CrossRef CAS.
-
(a) F. R. Japp and F. W. Streatfeild, J. Chem. Soc. Trans., 1882, 41, 270–277 RSC;
(b) A. C. Cope, L. Field, D. W. H. MacDowell and M. E. Wright, J. Am. Chem. Soc., 1956, 78, 2547–2551 CrossRef CAS;
(c) R. A. Pascal Jr, D. V. Engen, B. Kahr and W. D. McMillan, J. Org. Chem., 1988, 53, 1687–1689 CrossRef.
-
(a) Y. W. Leong, C. C. Kang, L. J. Harrison and A. D. Powell, Phytochemistry, 1997, 44, 157–165 CrossRef CAS;
(b) P. L. Majumder, S. Banerjee and S. Sen, Phytochemistry, 1996, 42, 847–852 CrossRef CAS;
(c) K. P. Adam and H. Becker, Phytochemistry, 1994, 35, 139 CrossRef CAS;
(d) A. S. Kende and D. P. Curran, J. Am. Chem. Soc., 1979, 101, 1857–1864 CrossRef CAS;
(e) B. P. Hudson and J. K. Barton, J. Am. Chem. Soc., 1998, 120, 6877–6888 CrossRef CAS;
(f) S. Kumar, J. Org. Chem., 1997, 62, 8535–8539 CrossRef CAS;
(g) B. K. Banik, F. Becker and I. Banik, Bioorg. Med. Chem., 2004, 12, 2523–2528 CrossRef CAS;
(h) J. M. Schmidt, J. Mercure, G. B. Tremblay, M. Page, A. Kalbakji, M. Feher, R. Dunn-Dufault, M. G. Peter and P. R. Redden, J. Med. Chem., 2003, 46, 1408–1418 CrossRef CAS;
(i) B. P. Hudson and J. K. Barton, J. Am. Chem. Soc., 1998, 120, 6877–6888 CrossRef CAS;
(j) S. Kumar, J. Org. Chem., 1997, 62, 8535–8539 CrossRef CAS.
-
(a) B. H. Novak and T. D. Lash, J. Org. Chem., 1998, 63, 3998–4010 CrossRef CAS;
(b) F. Tanaka, N. Mase and C. F. Barbas, J. Am. Chem. Soc., 2004, 126, 3692–3693 CrossRef CAS.
-
(a) H. S. P. Rao and S. Jothilingam, Tetrahedron Lett., 2001, 42, 6595–6597 CrossRef;
(b) H. S. P. Rao, S. Jothilingam and H. W. Scheeren, Tetrahedron, 2004, 60, 1625–1630 CrossRef CAS;
(c) H. S. P. Rao, B. K. Gorityala and K. Vasantham, Ind. J. Chem. Section B, 2007, 46B, 1470–1474 CAS.
- H. Li, W. Li and Z. Li, Chem. Commun., 2009, 3264–3266 RSC.
- F. Japp and F. Klingemann, Ber. Dtsch. Chem. Ges., 1888, 21, 2932 CrossRef.
- R. V. Linko, V. K. Belsky, A. V. Varlamov, B. E. Zaitsev and A. Chernyshev, Russ. Chem. Bull., 2001, 50, 1625–1629 CrossRef CAS.
-
(a) A. A. O. Sarhan and C. Bolm, Chem. Soc. Rev., 2009, 38, 2730–2744 RSC;
(b) C. Bolm, J. Legros, J. Le Paih and L. Zani, Chem. Rev., 2004, 4, 6217–6254 CrossRef;
(c) L. A. Paquette and A. D. White, in, Encyclopedia of Reagents for Organic Synthesis, 1995, 4, 2875–2883 Search PubMed.
- K. Wang, M. Lu, A. Yu, X. Zhu and Q. Wang, J. Org. Chem., 2009, 74, 935–938 CrossRef CAS.
- G. Kumaraswamy, A. N. Murthy and A. Pitchaiah, J. Org. Chem., 2010, 75, 3916–3919 CrossRef CAS.
- W. Han and A. R. Ofial, Chem. Commun., 2009, 5024–5026 RSC.
-
(a) D. Basavaiah, S. Roy and U. Das, Tetrahedron, 2010, 66, 5612–5622 CrossRef CAS;
(b) D. Basavaiah, B. Sreenivasulu and J. S. Rao, Tetrahedron Lett., 2001, 42, 1147–1149 CrossRef CAS.
-
(a) D. T. Anderson and W. M. Horspool, J. Chem. Soc., Chem. Commun., 1971, 615 RSC;
(b) D. T. Anderson and W. M. Horspool, J. Chem. Soc., Perkin Trans. 1, 1972, 536–539 RSC;
(c) D. T. Anderson and W. M. Horspool, J. Photochem., 1984, 27, 124–125 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. 







![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solutions on a Bruker - 400 spectrometer with tetramethylsilane (TMS) as internal standard; J-values are in Hz. 1H-NMR data are reported as follows: chemical shift (multiplicity: s = singlet, d = doublet, t = triplet, q = quartet and m = multiplet), coupling constant, integrations. 13C NMR spectra were determined with 1H decoupling, 2D NMR spectra (COSY, HSQC and HMBC) for each representative product were recorded to support the assigned structure. High resolution mass spectra were recorded on a Water Q-TOF micro mass spectrometer using the electron spray ionization mode. The X-ray diffraction measurements were carried out at 298 K on an Oxford CrysAlis CCD area detector system equipped with a graphite monochromator and a Mo-Kα fine-focus sealed tube (λ = 0.71073 Å). PQ and all ketones were purchased from Sigma Aldrich chemicals private limited. Ferric chloride was purchased from Qualigens fine chemicals.
1) solutions on a Bruker - 400 spectrometer with tetramethylsilane (TMS) as internal standard; J-values are in Hz. 1H-NMR data are reported as follows: chemical shift (multiplicity: s = singlet, d = doublet, t = triplet, q = quartet and m = multiplet), coupling constant, integrations. 13C NMR spectra were determined with 1H decoupling, 2D NMR spectra (COSY, HSQC and HMBC) for each representative product were recorded to support the assigned structure. High resolution mass spectra were recorded on a Water Q-TOF micro mass spectrometer using the electron spray ionization mode. The X-ray diffraction measurements were carried out at 298 K on an Oxford CrysAlis CCD area detector system equipped with a graphite monochromator and a Mo-Kα fine-focus sealed tube (λ = 0.71073 Å). PQ and all ketones were purchased from Sigma Aldrich chemicals private limited. Ferric chloride was purchased from Qualigens fine chemicals.
![ORTEP diagram of 9a,11,11-trimethyl-9a,12a-dihydrophenanthro[9',10':4,5]furo[2,3-d][1,3]dioxole 3 (CCDC 813673) with crystallographic numbering.](/image/article/2012/RA/c2ra20499a/c2ra20499a-f1.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 202.4 (C
O), 202.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 139.9 (C), 136.9 (C), 134.8 (CH), 129.5 (C), 129.4 (CH + C), 128.8 (CH), 128.7 (CH), 127.9 (CH), 126.2 (CH), 124.5 (CH), 123.1 (CH), 78.5 (C), 55.0 (CH2), 46.9 (CH2), 16.8 (CH2), 13.7 (CH3) ppm; HRMS (ESI) calcd for C19H18O3Na [M + Na]+ 317.1154, found 317.1142.
O), 139.9 (C), 136.9 (C), 134.8 (CH), 129.5 (C), 129.4 (CH + C), 128.8 (CH), 128.7 (CH), 127.9 (CH), 126.2 (CH), 124.5 (CH), 123.1 (CH), 78.5 (C), 55.0 (CH2), 46.9 (CH2), 16.8 (CH2), 13.7 (CH3) ppm; HRMS (ESI) calcd for C19H18O3Na [M + Na]+ 317.1154, found 317.1142.

![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ; Z = 2; Dc = 1.307 g cm−3; F000 = 312; λ(Mo-Kα) = 0.7107 Å; R (I ≥ 2σ1) = 0.0785, wR2 = 0.1102. Detailed X-ray crystallographic data is available from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK (for compound 2b CCDC 797133†).
; Z = 2; Dc = 1.307 g cm−3; F000 = 312; λ(Mo-Kα) = 0.7107 Å; R (I ≥ 2σ1) = 0.0785, wR2 = 0.1102. Detailed X-ray crystallographic data is available from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK (for compound 2b CCDC 797133†).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 202.2 (C
O), 202.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 139.8 (C), 136.7 (C), 134.5 (CH), 129.4 (C), 129.3 (C), 129.2 (CH), 128.7 (CH), 128.5 (CH), 127.8 (CH), 126.0 (CH), 124.3 (CH), 122.9 (CH), 78.4 (C), 54.9 (CH2), 44.8 (CH2), 31.2 (CH2), 22.8 (CH2), 22.4 (CH2), 13.9 (CH3) ppm; HRMS (ESI) calcd for C21H22O3Na [M + Na]+ 345.1467, found 345.1466.
O), 139.8 (C), 136.7 (C), 134.5 (CH), 129.4 (C), 129.3 (C), 129.2 (CH), 128.7 (CH), 128.5 (CH), 127.8 (CH), 126.0 (CH), 124.3 (CH), 122.9 (CH), 78.4 (C), 54.9 (CH2), 44.8 (CH2), 31.2 (CH2), 22.8 (CH2), 22.4 (CH2), 13.9 (CH3) ppm; HRMS (ESI) calcd for C21H22O3Na [M + Na]+ 345.1467, found 345.1466.![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ; Z = 18; Dcalcd = 1.289 g cm−3; F000 = 2736; λ(Mo-Kα) = 0.71073 Å; R (I ≥ 2σ1) = 0.0529, wR2 = 0.1257. Detailed X-ray crystallographic data is available from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK (for compound 19 CCDC 811040†).
; Z = 18; Dcalcd = 1.289 g cm−3; F000 = 2736; λ(Mo-Kα) = 0.71073 Å; R (I ≥ 2σ1) = 0.0529, wR2 = 0.1257. Detailed X-ray crystallographic data is available from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK (for compound 19 CCDC 811040†).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 148580 CAN104: 148580;
(b) J. Guo and Y. Xue, Taiyuan Ligong Daxue Xuebao, 2001, 32, 140–144 CAS CA: 2001
148580 CAN104: 148580;
(b) J. Guo and Y. Xue, Taiyuan Ligong Daxue Xuebao, 2001, 32, 140–144 CAS CA: 2001![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 332054 CAN135: 305448;
(c)
http://www.chemicalland21.com/specialtychem/finechem/PHENANTHRAQUINONE.htm.
.
332054 CAN135: 305448;
(c)
http://www.chemicalland21.com/specialtychem/finechem/PHENANTHRAQUINONE.htm.
.
![ORTEP diagram of (9aS,12aS)-11,12-dihydro-10H-spiro[9a,12a-(epoxymethanooxy)cyclopenta[b]phenanthro[9,10-d]furan-14,1'-cyclopentane] 17 (CCDC 805644) with crystallographic numbering.](/image/article/2012/RA/c2ra20499a/c2ra20499a-f3.gif)
![ORTEP diagram of 10,11,12,13-tetrahydrophenanthro[9,10-b]benzofuran-13-ol 19 (CCDC 811040) with crystallographic numbering.](/image/article/2012/RA/c2ra20499a/c2ra20499a-f4.gif)
![ORTEP diagram of (Z)-10,11,12,13-tetrahydrocycloocta[d]phenanthro[9,10-b]furan 24b (CCDC 805643) with crystallographic numbering.](/image/article/2012/RA/c2ra20499a/c2ra20499a-f5.gif)