Layer-by-layer assembly of Zn(II) and Ni(II) 5,10,15,20-tetra(4-ethynylphenyl)porphyrin multilayers on Au using copper catalyzed azide-alkyne cycloaddition†
Alexandra
Krawicz
a,
Joseph
Palazzo
b,
Gwo-Ching
Wang
b and
Peter H.
Dinolfo
*a
aDepartment of Chemistry and Chemical Biology, Rensselaer Polytechnic Institute, 110 8th Street, Troy, NY 12180, USA. E-mail: dinolp@rpi.edu
bDepartment of Physics, Applied Physics and Astronomy, Rensselaer Polytechnic Institute, 110 8th Street, Troy, NY 12180, USA
First published on 13th June 2012
Abstract
We have developed a versatile layer-by-layer (LbL) fabrication method to assemble porphyrin based multilayer thin-films on electron-beam evaporated Au surfaces utilizing copper(I) catalyzed azide-alkyne cycloaddition (CuAAC) as both a means of anchoring the films to the Au surface and coupling the individual molecular layers together. The molecular based multilayer films are comprised of Zn(II) and Ni(II) 5,10,15,20-tetra(4-ethynylphenyl)porphyrin and a bis-azido linker layer. Herein, we describe the fabrication and characterization of multilayer films on Au surfaces modified with an azido-terminated alkanethiol self assembled monolayer. The absorbance growth trends, as followed by UV-vis absorption, show a consistent linear increase that extends over tens of bilayers. Multilayer film thicknesses were obtained from spectroscopic ellipsometry, using a Cauchy model applied over the transparent range, and resulted in a consistent linear growth trend. Optical constants, index of refraction and extinction coefficients, were then determined using an oscillator model over the entire visible region. The resulting extinction coefficients were consistent with those typical of Zn(II) and Ni(II) porphyrin absorption spectra. The topology of the films and surface roughness was analyzed by tapping mode atomic force microscopy (TM-AFM) and confirmed the continuous nature of the films. X-Ray photoelectron spectroscopy (XPS) was consistent with the expected elemental composition of the porphyrin based films assembled on Au surfaces. Additionally, XPS was used to examine the utility of ethylenediaminetetraacetic acid disodium salt (Na2EDTA) as a Cu chelator to remove adventitious catalyst following multilayer fabrication.
Introduction
The modification of electrode surfaces using molecularly ordered thin films, with tunable electrochemical and photophysical properties, has widespread applications in the field of molecular electronics, and photovoltaics, among others.1–4 One of the most common methods of adding functionality to electrode surfaces is through the use of self assembled monolayers (SAMs).5 Another method is the layer-by-layer (LbL) fabrication technique, which can generate ordered thin films, composed of multiple building blocks, efficiently and inexpensively.6 This methodology has the potential to control the orientation and ordering of the films' components at the molecular level through simple solution deposition techniques. The LbL approach is a convenient and precise technique, which allows for the facile engineering of electronic, photophysical, and chemical properties into the nanostructured films.7,8LbL thin film formation can be accomplished by a series of sequential self-limiting coupling reactions that each deposit a single layer of material on the surface at a time. A wide variety of multilayer thin films have been assembled on different substrate surfaces via this technique using polymer, inorganic and molecular building blocks. There exist a variety of interlayer coupling reactions which have been explored as coupling techniques for the LbL assembly method. Examples include electrostatically assembled polyelectrolytes,8 alpha-zirconium phosphate coupled dyes,9–16 Langmuir–Blodgett films, palladium-pyridyl coordination,17–21 polymeric layers made through siloxane polymerization,22–28 or various other organic reactions.29–34
We have recently developed a molecular LbL thin film fabrication methodology utilizing copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) reactions as both a means to link the layers together and attach them to oxide surfaces.35–37 This technique was used to assemble nanoscale multilayer films of tetraphenyl-Zn(II)-porphyrins and perylenediimide building blocks on several substrates, including SiO2, indium tin oxide (ITO), quartz and glass. The resulting films showed reproducible and linear growth trends for absorbance and thickness over tens of layers.35–37 Discovered in 2001 by Sharpless and coworkers38 and Meldal and coworkers,39 CuAAC has been popularized as a rapid, facile, and robust cycloaddition reaction utilizing inexpensive Cu(I) catalyst. This simple reaction is tolerant of a variety of conditions and other functional groups, and has been used extensively as a surface modification technique on multiple surfaces.40–45 While a few other groups have used this reaction to fabricate triazole-linked polymer based multilayers,46–49 to our knowledge we were the first to report the use of CuAAC to build molecular multilayer films.35–37
In this report, we describe the extension of this LbL multilayer fabrication method to include tetraphenylporphyrin building blocks assembled on electron-beam (e-beam) evaporated Au surfaces that will allow for the examination of charge transport properties via electrochemical methods and scanning probe microscopies.50 E-beam evaporated gold provides an ideal surface for the growth of nanostructured thin films due to its relatively smooth surface and ability to form high-quality mixed azido-alkane SAMs required for CuAAC based multilayer growth.40,41,51,52 Multilayer formation on Au electrode surfaces opens up the possibility of additional characterization methods for nanoscale thin films and provides a reproducible platform to analyze electrochemical electron transfer rates.51 Since our initial report of molecular LbL thin film fabrication on oxide surfaces, others have used CuAAC for LbL generation of phenyl-triazole based molecular wires on Au(111) electrodes.53
We have been particularly interested in the use of porphyrin building blocks in the assembly of these films. The highly tunable electrical and optical properties of porphyrins have led to their use in a wide range of materials chemistry applications.54 Multilayer thin films assembled using porphyrin based molecular building blocks could lead to a wide range of applications including artificial photosynthetic processes,3,55,56 semiconductor sensitization,57–60 and catalysts,42,61–63 among others.64,65 Previously, porphyrin based molecular multilayer films have been assembled in a LbL fashion using a variety of covalent and non-covalent coupling methods including, transition metal coordination,14–16,66–68 electrostatic interactions between electrolytes,69–71 and purely organic linkages.32–34
Herein, we describe the synthesis and characterization of multilayer films of Zn(II) and Ni(II) 5,10,15,20-tetra(4-ethynylphenyl)porphyrin (1 and 2 respectively) assembled by CuAAC on e-beam evaporated Au surfaces. Multilayer growth was monitored by UV-visible spectroscopy, observing an increase in absorbance at the porphyrin Soret and Q-bands with each additional porphyrin layer. Spectroscopic ellipsometry was used to determine the film thickness and optical constants, which are important to predict or understand the nonlinear optical properties of these multilayers. The surface morphology was explored by AFM, commonly employed in the analysis of films composed of porphyrins and phthalocyanines,72 to obtain a representative image of the topology and to inspect the integrity and roughness of the film. X-Ray photoelectron spectroscopy (XPS) was used to analyze the chemical composition, along with determining the left over copper catalyst in the film structures. Porous materials created via CuAAC often contain excess copper ions, but several extraction methods have been employed to remove the adventitious catalyst.73,74 XPS was used herein to determine the amount of copper remaining in the Au supported porphyrin multilayer films before and after treatment with ethylenediaminetetraacetic acid disodium salt (Na2EDTA).
Results and discussion
Multilayer growth
Fig. 1 outlines our methodology for assembling molecular multilayers using CuAAC reactivity. The process relies on two sequential self-limiting CuAAC reactions of a multi-ethynyl functionalized tetraphenylporphyrin (1 or 2) and a multi-azido linker (3 or 4). The fabrication process begins with a mixed azido-alkane SAM formed on an e-beam evaporated Au surface to provide the initial attachment point. The azide-terminated SAM is then reacted with the ethynyl functionalized porphyrin (1 or 2) under CuAAC conditions (step 1, Fig. 1). This step results in a densely packed monolayer of porphyrin attached to the SAM through 1,4-subsituted 1,2,3-triazole linkages and a surface that is now terminated in acetylene groups. After a series of solvent washes to remove unreacted starting material and catalyst, another CuAAC reaction is performed on the surface with a multi-azido linker creating an azide terminated surface (step 2 in Fig. 1). The combination of steps 1 and 2 result in one molecular bilayer added to the surface. The two self-limiting reactions are then repeated sequentially to yield additional molecular bilayers which are covalently attached to the surface via 1,4-subsituted 1,2,3-triazoles. | ||
| Fig. 1 Schematic representation of molecular LbL multilayer growth using CuAAC reactivity on Au surfaces. | ||
Fig. 2 shows the visible absorption spectra taken throughout the fabrication of a multilayer of 1 and 4 on a glass supported 20 nm thick, optically semi-transparent Au surface. With each consecutive CuAAC reaction of 1 with the azide terminated surface there was a consistent increase in absorbance for the Soret peak at ∼440 nm. The refractive index of Au changes dramatically over the visible region; n is ∼1.45 from 400–450 nm, but drops sharply to ∼0.13 in the range of 600–900 nm.75 This drastic difference in refractive index creates artifacts in the absorbance and reflectance spectra of multilayers grown on Au. In the region between 600–900 nm, the Fresnel equations predict that a large percentage of incident light is reflected back from the surface due to the significant difference in the refractive index between water (n = 1.33), in which the sample is placed, and Au (∼0.13). As the multilayer is assembled on the surface, the refractive index rises to that of the film (∼1.5, vide infra) resulting in less reflected light (greater transmission) and the appearance of a negative absorption region. Within that increased transmission range, the appearance of two Q-bands associated with 1 can clearly be seen at 560 and 600 nm. Similar absorptivity changes were observed when linker 3 was incorporated into the films (Figure S1†).
 | ||
| Fig. 2 Top: UV-Vis spectra of multilayers of 1 and 4 assembled on an azide-terminated SAM on optically transparent e-beam evaporated Au (20 nm thickness). The absorption spectra for porphyrin layers are shown. Bottom: Absorbance vs. the number of bilayers at the Soret and Q-bands illustrate the linear dependence for multilayer growth. | ||
Multilayer fabrication was also followed using near-normal specular reflection off of thicker Au surfaces (100 nm). Fig. 3 shows the Fresnel reflectivity for the visible region throughout multilayer growth from 1 through 16 bilayers of 1 and 4. The spectra consistently decrease in reflectivity as each bilayer is added to the Au surface. The spectra show similar porphyrin features as found in the transmission mode measurements (see Fig. 2 above) with decreases in reflectivity at the Soret and Q-band region. The bands are shifted towards longer wavelengths by approximately 10–20 nm due to complications from the Kramers–Kronig effect. The complex index of refraction (ñ) is composed of the real refractive index (n) and the imaginary part (ik) according to the Kramers–Kronig relationship (eqn (1)), where k is the extinction coefficient of the film.76
| ñ(λ) = n(λ) + ik(λ) | (1) |
 | ||
| Fig. 3 Specular reflectivity scans of 1 through 16 bilayers of 1 and 4 on 100 nm thick Au. Only porphyrin layer spectra are shown for clarity. | ||
Due to the high optical density of the porphyrin based multilayers, the reflectivity spectra of these samples show a first-derivative like line shape, with increased reflectance on the high energy side of the absorption features, as predicted by the Kramers–Kronig effect.77 Similar trends in specular reflectivity changes were observed for multilayers formed with 2 and 4 (Figure S2†).
Spectroscopic ellipsometry
Ellipsometry is a non-destructive surface analysis technique that is capable of determining the thickness and optical properties of nanoscale thin films. Spectroscopic ellipsometry measures the change in phase (Δ) and amplitude (Ψ) of elliptically polarized light reflected off of a substrate surface as a function of wavelength (λ). The data for Δ(λ) and Ψ(λ) are then fit to a model describing the complex index of refraction (ñ) and thickness of the material as described above in eqn (1) for the Kramers–Kronig relationship.76 The porphyrin based films described in this study have strong, localized absorptions in the visible region due to the Soret and Q-band transitions, thus somewhat complicating the analysis of the ellipsometry data.Spectroscopic ellipsometry was employed throughout the growth of multilayers on Au substrates to provide information on both the thickness and optical constants of the thin films. Fig. 4 shows the measured Δ(λ) data in the range of 405–742 nm, collected at a 65° angle of incidence, for multilayers of 1 and 4. The Δ(λ) parameters are particularly sensitive to thickness changes of the material.78–80 As additional bilayer reactions are performed on the Au surface, there is a consistent decrease in Δ(λ), especially in the region around 460 nm where the Soret band of 1 is located. This is consistent with the increasing multilayer thickness from 4 to 16 bilayers. Similar trends in Δ(λ) were observed during multilayer growth using the other molecular components outlined in Fig. 1.
 | ||
| Fig. 4 Ellipsometry Δ trend for increasing number of bilayers of 1 and 4 assembled on Au and acquired at a 65° angle of incidence. | ||
To calculate the thickness of the multilayer thin films, we employed the Cauchy dispersion model (eqn (2)), over the non-absorbing region (674–741 nm) of the porphyrin, to describe the refractive index (n) as a function of the wavelength (λ).80
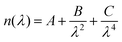 | (2) |
This methodology allows for the straightforward determination of film thickness, without additional fitting parameters required to describe k(λ) for the material. Ellipsometry has been used to find the film thickness of other absorbing materials such as porphyrins,32,81 phthalocyanines,55,72,82,83 LbL assembled polypyridyl–PdCl2 films,21 and even CuAAC coupled dendrimeric films49 using similar methods.
Fig. 5 shows the ellipsometrically determined film thickness versus the number of bilayers added to the Au surface of 1 and 2, with linker 4. The results show the expected linear increase in film thickness versus the number of bilayers, with growth rates of 1.5 and 1.8 nm per bilayer for films comprised of 1 and 4, and 2 and 4 respectively. The film growth rates of multilayers on Au are slightly lower than those of similar multilayers on Si(100) measured by both ellipsometry and X-ray reflectivity.37 There are several possible explanations for the differences in growth rates of these films such as the higher surface roughness of the e-beam evaporated Au as compared to the native oxide of Si(100) substrates. Au substrates generated by e-beam evaporation predominantly form a Au(111) surface, with varying grain sizes of 45–60 nm, depending on evaporation conditions and rates.5,84 Surfaces roughnesses of e-beam evaporated Au also vary somewhat, with typical values of around 2–4.5 nm (rms).85,86 The native oxide or Si(100) substrates on the other hand, show rms roughness values of less than 1 nm, depending on the cleaning methods used.87,88 In addition to differences in surface roughness, the alkane-thiol based SAMs on Au(111) have a higher packing density than alkyl-siloxane SAMs on silicon.5 A dense monolayer of azido-alkane thiol SAM moieties may promote multiple click reactions of one porphyrin with the substrate, in turn changing the angle of growth and reducing the number of available acetylene groups for the next surface reaction. Thus, given the dendritic nature of the molecule building blocks, the multilayer growth scheme outlined in Fig. 1 represents a simplified view of the bonding configurations within the film. We previously compared the X-ray reflectivity determined film thicknesses of similar porphyrin multilayer films assembled on Si(100)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2 substrates with the intermolecular distances obtained from DFT molecular modelling to predict a range of potential growth angles. A molecular growth angle of 45° was calculated for a purely trans-bonding mode (using the 5- and 15-meso-positions on the porphyrin), whereas a 70° angle was calculated for a cis-bonding mode (5- and 10-meso-positions on the porphyrin).37 Polarized GATR (grazing angle attenuated total reflectance) IR spectra of porphyrin multilayers assembled on ITO, a rougher surface than either e-beam evaporated Au or Si(100)
SiO2 substrates with the intermolecular distances obtained from DFT molecular modelling to predict a range of potential growth angles. A molecular growth angle of 45° was calculated for a purely trans-bonding mode (using the 5- and 15-meso-positions on the porphyrin), whereas a 70° angle was calculated for a cis-bonding mode (5- and 10-meso-positions on the porphyrin).37 Polarized GATR (grazing angle attenuated total reflectance) IR spectra of porphyrin multilayers assembled on ITO, a rougher surface than either e-beam evaporated Au or Si(100)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SiO2, yielded a porphyrin orientation of ∼45°, suggesting the trans-bonding mode dominates the growth patterns.36 Nevertheless, the growth patterns of these multilayer films are likely influenced by both the substrate roughness and packing density of azides for the initial surface attachment layer. In this work, the surface density of the azides from the alkanethiol SAMs on e-beam evaporated Au is likely higher than that on oxide surfaces examined previously. Additionally, with the multi-functional porphyrin building blocks employed, it is possible that cross-linking can occur within the multilayer structure, leading to a combination of binding motifs present within the film.
SiO2, yielded a porphyrin orientation of ∼45°, suggesting the trans-bonding mode dominates the growth patterns.36 Nevertheless, the growth patterns of these multilayer films are likely influenced by both the substrate roughness and packing density of azides for the initial surface attachment layer. In this work, the surface density of the azides from the alkanethiol SAMs on e-beam evaporated Au is likely higher than that on oxide surfaces examined previously. Additionally, with the multi-functional porphyrin building blocks employed, it is possible that cross-linking can occur within the multilayer structure, leading to a combination of binding motifs present within the film.
 | ||
| Fig. 5 Plot of film thickness vs. number of bilayers for multilayer films of 1 and 4, and 2 and 4. The error bars were calculated based on standard deviation and are on the scale of the data point size, and therefore not shown. | ||
Optical constants have also been obtained ellipsometrically for absorbing films, containing porphyrins and phthalocyanines, by modeling the dielectric functions of the material with an oscillator model covering the full spectral range.72,82,89 These methods typically use a fixed film thickness obtained from Cauchy modeling in the transparent region, or other methods.89 We performed a full spectroscopic ellipsometry analysis of the thicker multilayer structures. The upper plots in Fig. 6 show representative ellipsometry data (Δ(λ) and Ψ(λ)) and fits for the range of 410.0 to 741.5 nm (44 data points) for multilayers of 1 and 4, and 2 and 4 on Au(111) surfaces. The resulting model for Δ(λ) and Ψ(λ), shown as solid lines, is in excellent agreement with the measured data.
 | ||
| Fig. 6 Spectroscopic ellipsometry data (410.0 to 741.5 nm, 44 data points) and fitting results for multilayers of 1 and 4 (left panels, a–c) and 2 and 4 (right panels, d–f). The top and middle panels show the Δ and Ψ data (open symbols) and fits (solid lines) collected at an incident angle of 55, 60, 65, 70, and 75°. The bottom panel shows the resulting optical constants for the multilayer films as determined by the oscillator model. The index of refraction (n) is shown as a dashed red line and the extinction coefficient (k) as a solid blue line. | ||
The bottom plots show the resulting n(λ) and k(λ) profiles for the multilayer films as derived from the modeled dielectric constants. As can be observed in the lower plots of Fig. 6, the k(λ) profiles closely match those of the absorption profile obtained from UV-vis spectroscopy for the porphyrin multilayers assembled on transparent Au (see Fig. 2). The multilayers containing 1 (left plots) show a Soret absorbance feature at 435 nm and Q-bands at 560 and 610 nm. These are red-shifted relative to the solution spectra of 1 due to aggregation effects within the film.35–37 The k(λ) profile for multilayers containing 2 display Soret and Q-band features at 410 and 530 nm, which are closer to those of the solution spectra of 2. Additionally, the n(λ) spectrum of both porphyrin based multilayers matches that of the specular reflectivity assembled on opaque Au(111) substrates (see Fig. 3 and S2†). The refractive index (n) for the porphyrin multilayer films is in the range of 1.4–1.5 for films of 1 and 4, and 1.45–1.55 for films of 2 and 4 in the non-resonant region (674–741.5 nm).
Although optical constants for phthalocyanine are more commonly reported in the literature than for porphyrin films, the n(λ) spectrum of vacuum evaporated thin films of free base porphyrin was reported to be between 1.2–1.4 (in the range of 4–12 eV) with prominent features between 0.5–4eV.90 Additionally, the k(λ) profile had prominent features that reflected the UV-vis absorption profile.90 The literature value of the extinction coefficient for phthalocyanine films from spectroscopic ellipsometry also shows agreement with the absorption spectra and the refractive index range is comparable to our porphyrin multilayers.91 Others have reported refractive index values for phthalocyanine containing films in the range of 1.6–1.8 in the nonresonant region of the visible spectrum.72
Tapping mode atomic force microscopy
Topography and surface roughness was analyzed with tapping mode atomic force microscopy (TM-AFM) to confirm the continuity and integrity of the film on the Au surfaces. Fig. 7 shows representative 1 μm × 1 μm topography and amplitude TM-AFM images for multilayers comprised of 2 and 4. The amplitude images remove long-range variations and offsets, and therefore are sometimes better for visualization of the surface features.92 The values of root mean squared roughness (rms) for the images in Fig. 7 are 2.1, 2.6 and 3.7 nm for 1, 10 and 20 bilayers of 2 and 4, respectively. The TM-AFM images of the multilayers of 1 and 4 are shown in Fig. S3† and yielded rms values of 2.5, 2.1 and 3.8 for 4, 10 and 16 bilayers, respectively. Larger area scans of 10 μm × 10 μm show continuous multilayer films and comparable roughness values. There was no apparent surface damage after repeated TM-AFM scans of the same area. The rms tends to increase slightly as more layers are deposited, and is similar to observations made for similar multilayers on silicon.37 The roughness of the multilayer films is similar to that of the underlying Au surface,85,86 suggesting that the film morphology is templated somewhat by the underlying substrate.55 | ||
| Fig. 7 TM-AFM images of 1 (left), 10 (middle) and 20 (right) bilayers of multilayers based on 2 and 4. The upper images are topography AFM height profiles and the lower are amplitude scans, a pseudoderivative of the topography data to emphasize the structural characteristics. All TM-AFM images areas are 1 μm × 1 μm. | ||
X-Ray photoelectron spectroscopy and copper removal methods
We employed X-ray photoelectron spectroscopy (XPS) to confirm the surface attachment of the molecular components and to estimate the amount of copper catalyst remaining in the multilayer film. XPS is a common surface characterization technique and has been used to analyze porphyrin films on gold93 and other surfaces.32 Copper contamination was reported by others who used CuAAC reactivity in the assembly of LbL films and the functionalization of acetylene-terminated monolayers.94 In some cases, the remaining copper catalyst was removed by EDTA37 or other chelating agents.73A representative XPS spectrum of a multilayer film consisting of 2 bilayers of 1 and 4 before and after treatment with 0.01 M Na2EDTA is shown in Fig. 8. (The XPS spectra for multilayers of 1 and 3 are shown in Fig. S4.†) The spectrum shows all of the expected atomic peaks for the empirical formula for the 1 and 4 multilayer structure. High resolution spectra of the N 1s peak (Fig. S5†) display a broadened spectrum with binding energies around 398–401 eV due to multiple types of nitrogen atoms in the film, including the porphyrin ring, 1,2,3-triazole and unreacted azide groups. Additionally, high-resolution spectra for the Zn 2p1/2 and 2p3/2 (Fig. S6†) show peaks at binding energies of 1044.6 ± 0.2 and 1021.7 ± 0.2 eV, respectively, in close agreement with values previously reported for other Zn porphyrins.4,32,95–99
 | ||
| Fig. 8 XPS spectra of multilayers of 1 and 4 before (green dashed line) and after (red solid line) treatment with Na2EDTA(aq). | ||
Table 1 contains atomic composition data taken from XPS spectra for 2 bilayers of 1 and 3 and 1 and 4, before and after treatment with Na2EDTA(aq). Table 1 also includes a comparison of the atomic ratios of Cu and N to that of Zn. It is clear from the XPS elemental spectra that some copper catalyst remains in the film following multilayer fabrication. In an attempt to remove the left over Cu, we exposed each film to a 0.01 M Na2EDTA solution (pH = 4.8) following each CuAAC reaction. As can be seen in Table 1, the percentage of Zn did not change significantly after Na2EDTA(aq) treatment and stayed within 0.9 to 1.2%. Meanwhile, the amount of Cu detected was between 1–2.8 times higher with respect to Zn before Na2EDTA(aq) treatment, but dropped to a ratio of 1–1.3 afterwards.
| Atomic peak | 1 and 3 | 1 and 3 Na2EDTA | 1 and 4 | 1 and 4 Na2EDTA | Monolayer of 5 | Monolayer of 5 Na2EDTA |
|---|---|---|---|---|---|---|
| C 1s | 69.3 | 68.9 | 66.3 | 63.2 | 63.8 | 64.0 |
| N 1s | 10.2 | 9.8 | 9.5 | 11.5 | 9.5 | 5.8 |
| O 1s | 8.2 | 7.5 | 7.1 | 6.5 | 0.6 | 3.7 |
| Au 4f7/2 | 8.8 | 11.1 | 12.3 | 16.1 | 22.8 | 23.5 |
| Cu 2p3/2 | 2 | 1.3 | 2.5 | 1.6 | 1.3 | 0.1 |
| Zn 2p3/2 | 1.2 | 1.1 | 0.9 | 1.2 | 1.8 | 1.4 |
| S 2p | 0.3 | 0.3 | 1.4 | <0.1 | 1.3 | 1.5 |
| Ratios of atomic composition to Zn | ||||||
| Cu 2p3/2 | 1.7 | 1.2 | 2.8 | 1.3 | 0.7 | 0.1 |
| Zn 2p3/2 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| N 1s | 8.5 | 8.9 | 10.6 | 9.6 | 5.4 | 4.2 |
While Na2EDTA was not effective in removing all of the CuAAC catalyst from the multilayer films, XPS was able to provide information about the nature of the remaining Cu species. Fig. 9 shows the high-resolution spectra of the Cu 2p3/2 peaks for multilayers of 1 and 3 and 1 and 4 before and after treatment with Na2EDTA(aq). The high-resolution XPS spectra show a broad set of Cu 2p1/2 and 2p3/2 peaks, consistent with multiple Cu species. Deconvolution of the spectra, shown in Fig. S7†, reveal the presence of both Cu(I) and Cu(II) species at binding energies of 932.4 ± 0.2 and 934.4 ± 0.2 eV, respectively.100–102Table 2 contains a comparison of the Cu(II)/Cu(I) ratios, from deconvolution of the Cu 2p3/2 peaks, before and after Na2EDTA(aq) treatment. Following treatment with Na2EDTA, the XPS data shows a marked decrease in the Cu(II) 2p3/2 peaks relative to Cu(I) (see the ESI†), and the disappearance of the Cu(II) satellite peaks at approximate binding energies of 944 and 962 eV. This data suggests that Na2EDTA(aq) is more effective at removing Cu(II) species than Cu(I) from the multilayer films.
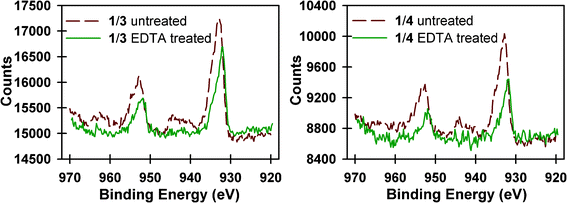 | ||
| Fig. 9 High resolution spectra of the Cu 2p3/2 peak for multilayer films of 2 bilayers of 1 and 3 (left) and 1 and 4 (right) before (dashed red line) and after (solid green line) treatment with Na2EDTA(aq). | ||
| 1 and 4 | 1 and 4 Na2EDTA | 1 and 3 | 1 and 3 Na2EDTA | Monolayer of 5 | Monolayer of 5 Na2EDTA | |
|---|---|---|---|---|---|---|
| Cu(II) | 0.6 | 0.3 | 0.7 | 0.6 | 0.4 | N/A |
| Cu(I) | 1 | 1 | 1 | 1 | 1 | N/A |
We previously found that untreated multilayer films of 1 and 3 assembled on the native oxide of Si(100) substrates had a Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn ratio of 1.36
Zn ratio of 1.36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, whereas films of 1 and 4 had a significantly lower ratio, 0.16
1, whereas films of 1 and 4 had a significantly lower ratio, 0.16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.37 We proposed that the higher amounts of Cu in the multilayers with linker 4 may be a result of the two sulfonates compared to 3 which does not contain any anionic groups. The Na+ peak in the high-resolution XPS spectra was relatively small for the expected atomic composition of the multilayers grown with 4, suggesting that Cu ions exchanged with the sulfonate cations of 4. The comparable amount of Cu present in the multilayers containing linker 3 suggests that there is another functional group, such as unreacted alkynes in 1, that is capable of sequestering Cu. There is a wide range of structurally characterized Cu(I)–alkyne complexes known in the literature.103
1.37 We proposed that the higher amounts of Cu in the multilayers with linker 4 may be a result of the two sulfonates compared to 3 which does not contain any anionic groups. The Na+ peak in the high-resolution XPS spectra was relatively small for the expected atomic composition of the multilayers grown with 4, suggesting that Cu ions exchanged with the sulfonate cations of 4. The comparable amount of Cu present in the multilayers containing linker 3 suggests that there is another functional group, such as unreacted alkynes in 1, that is capable of sequestering Cu. There is a wide range of structurally characterized Cu(I)–alkyne complexes known in the literature.103
We previously reported the structural characterization of multilayer films of 1 and 3 and 1 and 4 on ITO electrode surfaces using grazing-angle attenuated total reflectance FTIR spectroscopy.36 These results, combined with previous thickness determinations,37 established that the porphyrin building blocks are bonded in a trans fashion within the multilayer films, using only two of the available phenyl-ethynyl groups. Thus, the remaining ethynyl groups from 1 could potentially form a Cu(I)–acylide complex, thus sequestering Cu in the film. This is consistent with the observed Cu(I) peaks in the XPS spectra of the multilayers following Na2EDTA(aq) treatment.
To further explore the possibility of Cu(I)–acetylide formation within the multilayer structures comprised of 1, we examined the retention of Cu by a monolayer film of a monoethynyl functionalized porphyrin, Zn(II) 5-(4-ethynylphenyl)-10,15,20-tri-phenyl porphyrin (5). The CuAAC reaction of the single phenyl-ethynyl group with the azido surface would not leave an unreacted alkyne for Cu(I) binding. Comparison of monolayer films of 5 before and after Na2EDTA(aq) showed that the vast majority of Cu(I) and Cu(II) were removed, supporting the assignment of Cu(I)–acetylide binding as the mode of Cu retention in the films (see Fig. S8†). Efforts are currently underway to synthesize trans-di-ethynyl molecules for LbL formation that would limit the number of unreacted functional groups within the films that could sequester excess Cu.
 | ||
| Fig. 10 Structure of Zn(II) 5-(4-ethynylphenyl)-10,15,20-tri-phenyl porphyrin (5). | ||
Conclusions
The UV-vis spectroscopy and spectroscopic ellipsometry results show that the Au(111) surfaces were effectively functionalized with an azide terminated SAM creating a functional platform onto which porphyrins were clicked. Furthermore these azide terminated surfaces enabled the growth of the mixed porphyrin based multilayers as evidenced by the absorbance and film thickness trends. Deviations from linear trends in film thickness may stem from the changing surface properties of the surface onto which the layers are deposited. This technique of thin-film multilayer deposition can control the thickness, bonding architecture, and thus the overall structure and properties of the macroscopic film. The methodology also enables the introduction of different functionalities into the molecular multilayer films.Acknowledgements
This material is based upon work supported by the National Science Foundation under Grant No. 0333314 and Rensselaer Polytechnic Institute.References
- J. S. Lindsey and D. F. Bocian, Acc. Chem. Res., 2011, 44, 638–650 CrossRef CAS.
- B. R. Danger, K. Bedient, M. Maiti, I. J. Burgess and R. P. Steer, J. Phys. Chem. A, 2010, 114, 10960–10968 CrossRef CAS.
- H. Yamada, H. Imahori, Y. Nishimura, I. Yamazaki, T. K. Ahn, S. K. Kim, D. Kim and S. Fukuzumi, J. Am. Chem. Soc., 2003, 125, 9129–9139 CrossRef CAS.
- K. M. Roth, A. A. Yasseri, Z. Liu, R. B. Dabke, V. Malinovskii, K.-H. Schweikart, L. Yu, H. Tiznado, F. Zaera, J. S. Lindsey, W. G. Kuhr and D. F. Bocian, J. Am. Chem. Soc., 2003, 125, 505–517 CrossRef CAS.
- J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Chem. Rev., 2005, 105, 1103–1170 CrossRef CAS.
- K. Ariga, J. P. Hill and Q. M. Ji, Phys. Chem. Chem. Phys., 2007, 9, 2319–2340 RSC.
- Y. Lvov, G. Decher and H. Mohwald, Langmuir, 1993, 9, 481–486 CrossRef CAS.
- G. Decher, Science, 1997, 277, 1232–1237 CrossRef CAS.
- J. Kerimo, D. M. Adams, P. F. Barbara, D. M. Kaschak and T. E. Mallouk, J. Phys. Chem. B, 1998, 102, 9451–9460 CrossRef CAS.
- G. Cao, H. G. Hong and T. E. Mallouk, Acc. Chem. Res., 1992, 25, 420–427 CrossRef CAS.
- H. C. Yang, K. Aoki, H. G. Hong, D. D. Sackett, M. F. Arendt, S. L. Yau, C. M. Bell and T. E. Mallouk, J. Am. Chem. Soc., 1993, 115, 11855–11862 CrossRef CAS.
- H. Lee, L. J. Kepley, H. G. Hong, S. Akhter and T. E. Mallouk, J. Phys. Chem., 1988, 92, 2597–2601 CrossRef CAS.
- H. Lee, L. J. Kepley, H. G. Hong and T. E. Mallouk, J. Am. Chem. Soc., 1988, 110, 618–620 CrossRef CAS.
- A. B. F. Martinson, A. M. Massari, S. J. Lee, R. W. Gurney, K. E. Splan, J. T. Hupp and S. T. Nguyen, J. Electrochem. Soc., 2006, 153, A527–A532 CrossRef CAS.
- K. E. Splan and J. T. Hupp, Langmuir, 2004, 20, 10560–10566 CrossRef CAS.
- A. M. Massari, R. W. Gurney, M. D. Wightman, C. H. K. Huang, S. B. T. Nguyen and J. T. Hupp, Polyhedron, 2003, 22, 3065–3072 CrossRef CAS.
- M. Altman, A. D. Shukla, T. Zubkov, G. Evmenenko, P. Dutta and M. E. van der Boom, J. Am. Chem. Soc., 2006, 128, 7374–7382 CrossRef CAS.
- M. Altman, O. Zenkina, G. Evmenenko, P. Dutta and M. E. van der Boom, J. Am. Chem. Soc., 2008, 130, 5040–5041 CrossRef CAS.
- L. Motiei, M. Feller, G. Evmenenko, P. Dutta and M. E. van der Boom, Chem. Sci., 2012, 3, 66–71 RSC.
- L. Motiei, M. Altman, T. Gupta, F. Lupo, A. Gulino, G. Evmenenko, P. Dutta and M. E. van der Boom, J. Am. Chem. Soc., 2008, 130, 8913–8915 CrossRef CAS.
- L. Motiei, M. Sassi, R. Kaminker, G. Evmenenko, P. Dutta, M. A. Iron and M. E. van der Boom, Langmuir, 2011, 27, 1319–1325 CrossRef CAS.
- G. Evmenenko, M. E. V. D. Boom, J. Kmetko, S. W. Dugan, T. J. Marks and P. Dutta, J. Chem. Phys., 2001, 115, 6722–6727 CrossRef CAS.
- A. Facchetti, A. Abbotto, L. Beverina, M. E. van der Boom, P. Dutta, G. Evmenenko, G. A. Pagani and T. J. Marks, Chem. Mater., 2003, 15, 1064–1072 CrossRef CAS.
- D. Li, M. A. Ratner, T. J. Marks, C. Zhang, J. Yang and G. K. Wong, J. Am. Chem. Soc., 1990, 112, 7389–7390 CrossRef CAS.
- W. Lin, W. Lin, G. K. Wong and T. J. Marks, J. Am. Chem. Soc., 1996, 118, 8034–8042 CrossRef CAS.
- N. Tillman, A. Ulman and T. L. Penner, Langmuir, 1989, 5, 101–111 CrossRef CAS.
- M. E. van der Boom, A. G. Richter, J. E. Malinsky, P. A. Lee, N. R. Armstrong, P. Dutta and T. J. Marks, Chem. Mater., 2000, 13, 15–17 Search PubMed.
- M. E. van der Boom, P. Zhu, G. Evmenenko, J. E. Malinsky, W. Lin, P. Dutta and T. J. Marks, Langmuir, 2002, 18, 3704–3707 CrossRef CAS.
- P. Kohli and G. J. Blanchard, Langmuir, 2000, 16, 4655–4661 CrossRef CAS.
- Y.-h. Li, D. Wang and J. M. Buriak, Langmuir, 2010, 26, 1232–1238 CrossRef CAS.
- I. Schmidt, J. Jiao, P. Thamyongkit, D. S. Sharada, D. F. Bocian and J. S. Lindsey, J. Org. Chem., 2006, 71, 3033–3050 Search PubMed.
- J. Jiao, F. Anariba, H. Tiznado, I. Schmidt, J. S. Lindsey, F. Zaera and D. F. Bocian, J. Am. Chem. Soc., 2006, 128, 6965–6974 CrossRef CAS.
- D. Li, B. I. Swanson, J. M. Robinson and M. A. Hoffbauer, J. Am. Chem. Soc., 1993, 115, 6975–6980 CrossRef CAS.
- D. Li, C. T. Buscher and B. I. Swanson, Chem. Mater., 1994, 6, 803–810 CrossRef CAS.
- P. K. B. Palomaki and P. H. Dinolfo, Langmuir, 2010, 26, 9677–9685 CrossRef CAS.
- P. K. B. Palomaki and P. H. Dinolfo, ACS Appl. Mater. Interfaces, 2011, 3, 4703–4713 Search PubMed.
- P. K. B. Palomaki, A. Krawicz and P. H. Dinolfo, Langmuir, 2011, 27, 4613–4622 CrossRef CAS.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS.
- C. W. Tornoe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057–3064 CrossRef CAS.
- J. P. Collman, N. K. Devaraj and C. E. D. Chidsey, Langmuir, 2004, 20, 1051–1053 CrossRef CAS.
- J. P. Collman, N. K. Devaraj, T. P. A. Eberspacher and C. E. D. Chidsey, Langmuir, 2006, 22, 2457–2464 CrossRef CAS.
- J. P. Collman, N. K. Devaraj, R. A. Decreau, Y. Yang, Y. L. Yan, W. Ebina, T. A. Eberspacher and C. E. D. Chidsey, Science, 2007, 315, 1565–1568 CrossRef CAS.
- S.-Y. Ku, K.-T. Wong and A. J. Bard, J. Am. Chem. Soc., 2008, 130, 2392–2393 CrossRef CAS.
- T. Lummerstorfer and H. Hoffmann, J. Phys. Chem. B, 2004, 108, 3963–3966 CrossRef CAS.
- S. Prakash, T. M. Long, J. C. Selby, J. S. Moore and M. A. Shannon, Anal. Chem., 2006, 79, 1661–1667 Search PubMed.
- D. E. Bergbreiter and B. S. Chance, Macromolecules, 2007, 40, 5337–5343 CrossRef CAS.
- R. Azumi, M. Matsumoto, Y. Kawabata, S. Kuroda, M. Sugi, L. G. King and M. J. Crossley, J. Phys. Chem., 1993, 97, 12862–12869 CrossRef CAS.
- G. Rydzek, J.-S. B. Thomann, N. Ben Ameur, L. C. Jierry, P. Meésini, A. Ponche, C. Contal, A. E. El Haitami, J.-C. Voegel, B. Senger, P. Schaaf, B. t. Frisch and F. Boulmedais, Langmuir, 2009, 26, 2816–2824 Search PubMed.
- R. Vestberg, M. Malkoch, M. Kade, P. Wu, V. V. Fokin, K. B. Sharpless, E. Drockenmuller and C. J. Hawker, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 2835–2846 CrossRef CAS.
- A. L. Eckermann, D. J. Feld, J. A. Shaw and T. J. Meade, Coord. Chem. Rev., 2010, 254, 1769–1802 CrossRef CAS.
- N. K. Devaraj, R. A. Decreau, W. Ebina, J. P. Collman and C. E. D. Chidsey, J. Phys. Chem. B, 2006, 110, 15955–15962 CrossRef CAS.
- N. K. Devaraj, P. H. Dinolfo, C. E. D. Chidsey and J. P. Collman, J. Am. Chem. Soc., 2006, 128, 1794–1795 CrossRef.
- L. Luo and C. D. Frisbie, J. Am. Chem. Soc., 2010, 132, 8854–8855 CrossRef CAS.
- K. Kadish, K. M. Smith and R. Guilard, The Porphyrin Handbook, Academic Press, New York, 1999 Search PubMed.
- F. B. Abdelrazzaq, R. C. Kwong and M. E. Thompson, J. Am. Chem. Soc., 2002, 124, 4796–4803 CrossRef CAS.
- M. R. Wasielewski, Chem. Rev., 1992, 92, 435–461 CrossRef CAS.
- S. Cherian and C. C. Wamser, J. Phys. Chem. B, 2000, 104, 3624–3629 CrossRef CAS.
- F. Odobel, E. Blart, M. Lagree, M. Villieras, H. Boujtita, N. El Murr, S. Caramori and C. Alberto Bignozzi, J. Mater. Chem., 2003, 13, 502–510 RSC.
- Y. Tachibana, S. A. Haque, I. P. Mercer, J. R. Durrant and D. R. Klug, J. Phys. Chem. B, 2000, 104, 1198–1205 CrossRef CAS.
- A. Yella, H.-W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W.-G. Diau, C.-Y. Yeh, S. M. Zakeeruddin and M. Grätzel, Science, 2011, 334, 629–634 CrossRef CAS.
- A. M. D. R. Gonsalves and M. M. Pereira, J. Mol. Catal. A: Chem., 1996, 113, 209–221 CrossRef CAS.
- M. L. Merlau, M. del Pilar Mejia, S. T. Nguyen and J. T. Hupp, Angew. Chem., Int. Ed., 2001, 40, 4239–4242 CrossRef CAS.
- J. Nakazawa, B. J. Smith and T. D. P. Stack, J. Am. Chem. Soc., 2012, 134, 2750–2759 CrossRef CAS.
- C. M. Drain, A. Varotto and I. Radivojevic, Chem. Rev., 2009, 109, 1630–1658 CrossRef CAS.
- M. Jurow, A. E. Schuckman, J. D. Batteas and C. M. Drain, Coord. Chem. Rev., 2010, 254, 2297–2310 CrossRef CAS.
- D.-J. Qian, C. Nakamura and J. Miyake, Chem. Commun., 2001, 2312–2313 RSC.
- H.-T. Chen, B. Liu, H.-T. Wang, Z.-D. Xiao, M. Chen and D.-J. Qian, Mater. Sci. Eng., C, 2007, 27, 639–645 Search PubMed.
- A. Liu, M. Chen and D.-J. Qian, Colloids Surf., A, 2010, 366, 183–190 CrossRef CAS.
- K. Araki, M. J. Wagner and M. S. Wrighton, Langmuir, 1996, 12, 5393–5398 CrossRef CAS.
- G. Bazzan, W. Smith, L. C. Francesconi and C. M. Drain, Langmuir, 2008, 24, 3244–3249 CrossRef CAS.
- H.-L. Wang, Q. Sun, M. Chen, J. Miyake and D.-J. Qian, Langmuir, 2011, 27, 9880–9889 Search PubMed.
- D. Li, M. Lütt, M. R. Fitzsimmons, R. Synowicki, M. E. Hawley and G. W. Brown, J. Am. Chem. Soc., 1998, 120, 8797–8804 CrossRef CAS.
- P. Wu, A. K. Feldman, A. K. Nugent, C. J. Hawker, A. Scheel, B. Voit, J. Pyun, J. M. J. Fréchet, K. B. Sharpless and V. V. Fokin, Angew. Chem., Int. Ed., 2004, 43, 3928–3932 CrossRef CAS.
- M. Wyszogrodzka and R. Haag, Chem.–Eur. J., 2008, 14, 9202–9214 CrossRef CAS.
- L. G. Schulz and F. R. Tangherlini, J. Opt. Soc. Am., 1954, 44, 362–367 Search PubMed.
- N. Pantelić, C. M. Wansapura, W. R. Heineman and C. J. Seliskar, J. Phys. Chem. B, 2005, 109, 13971–13979 CrossRef CAS.
- F. Stern, S. Frederick and T. David, Solid State Physics, Academic Press, 1963, pp. 299–408 Search PubMed.
- M. A. Hempenius, M. Péter, N. S. Robins, E. S. Kooij and G. J. Vancso, Langmuir, 2002, 18, 7629–7634 CrossRef CAS.
- E. S. Kooij, Y. Ma, M. A. Hempenius, G. J. Vancso and B. Poelsema, Langmuir, 2010, 26, 14177–14181 Search PubMed.
- Guide to Using WVASE32 Spectroscopic Ellipsometry Data Acquisition and Analysis Software, J. A. Woollam Co., Inc., 2008 Search PubMed.
- D. M. Kaschak, J. T. Lean, C. C. Waraksa, G. B. Saupe, H. Usami and T. E. Mallouk, J. Am. Chem. Soc., 1999, 121, 3435–3445 CrossRef CAS.
- T. Basova, V. Plyashkevich and A. Hassan, Surf. Sci., 2008, 602, 2368–2372 Search PubMed.
- B. Bräuer, M. Fronk, D. Lehmann, D. R. T. Zahn and G. Salvan, J. Phys. Chem. B, 2009, 113, 14957–14961 CrossRef CAS.
- J. C. Love, D. B. Wolfe, R. Haasch, M. L. Chabinyc, K. E. Paul, G. M. Whitesides and R. G. Nuzzo, J. Am. Chem. Soc., 2003, 125, 2597–2609 CrossRef CAS.
- E. A. Weiss, G. K. Kaufman, J. K. Kriebel, Z. Li, R. Schalek and G. M. Whitesides, Langmuir, 2007, 23, 9686–9694 CrossRef CAS.
- S. Lani, A. Bosseboeuf, B. Belier, C. Clerc, C. Gousset and J. Aubert, Microsyst. Technol., 2006, 12, 1021–1025 Search PubMed.
- D. Resnik, D. Vrtačnik, U. Aljančič and S. Amon, Sens. Actuators, A, 2000, 80, 68–76 Search PubMed.
- H.-W. Park, B.-K. Ju, Y.-H. Lee, In-Byeong Kang, D. S. Noel, R. H. Malcolm, J.-H. Park and M.-H. Oh, Proc. SPIE, 3046, 1997, 328 Search PubMed.
- J. S. Louis, D. Lehmann, M. Friedrich and D. R. T. Zahn, J. Appl. Phys., 2007, 101, 013503-1–013503-7 Search PubMed.
- B. H. Schechtman and W. E. Spicer, J. Mol. Spectrosc., 1970, 33, 28–48 CrossRef CAS.
- A. B. Djurisic, C. Y. Kwong, T. W. Lau, Z. T. Liu, H. S. Kwok, L. S. M. Lam and W. K. Chan, Appl. Opt., 2003, 42, 6382–6386 Search PubMed.
- D. Li, M. Lütt, M. R. Fitzsimmons, R. Synowicki, M. E. Hawley and G. W. Brown, J. Am. Chem. Soc., 1998, 120, 8797–8804 CrossRef CAS.
- Y.-H. Chan, A. E. Schuckman, L. M. Pérez, M. Vinodu, C. M. Drain and J. D. Batteas, J. Phys. Chem. C, 2008, 112, 6110–6118 CrossRef CAS.
- S. Ciampi, T. Böcking, K. A. Kilian, M. James, J. B. Harper and J. J. Gooding, Langmuir, 2007, 23, 9320–9329 CrossRef CAS.
- G. Polzonetti, A. Ferri, M. V. Russo, G. Iucci, S. Licoccia and R. Paolesse, J. Vac. Sci. Technol., A, 1999, 17, 832–839 CrossRef CAS.
- Z. Zhang, R. Hu and Z. Liu, Langmuir, 2000, 16, 1158–1162 CrossRef CAS.
- L. Wei, H. Tiznado, G. Liu, K. Padmaja, J. S. Lindsey, F. Zaera and D. F. Bocian, J. Phys. Chem. B, 2005, 109, 23963–23971 CrossRef CAS.
- A. A. Yasseri, D. Syomin, R. S. Loewe, J. S. Lindsey, F. Zaera and D. F. Bocian, J. Am. Chem. Soc., 2004, 126, 15603–15612 CrossRef CAS.
- A. A. Yasseri, D. Syomin, V. L. Malinovskii, R. S. Loewe, J. S. Lindsey, F. Zaera and D. F. Bocian, J. Am. Chem. Soc., 2004, 126, 11944–11953 CrossRef CAS.
- D. Briggs and M. P. Seah, Practical Surface Analysis, John Wiley & Sons, Chichester, England, 1990 Search PubMed.
- D. C. Frost, A. Ishitani and C. A. McDowell, Mol. Phys., 1972, 24, 861–877 CAS.
- C. Battistoni, G. Mattogno, E. Paparazzo and L. Naldini, Inorg. Chim. Acta, 1985, 102, 1–3 CrossRef CAS.
- M. Meldal and C. W. Tornøe, Chem. Rev., 2008, 108, 2952–3015 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: experimental procedures, visible transmission and specular reflection, TM-AFM images, and survey and high-resolution XPS spectra. See DOI: 10.1039/c2ra20440a |
| This journal is © The Royal Society of Chemistry 2012 |
