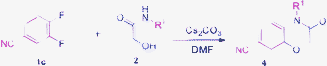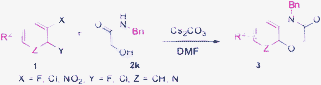DOI:
10.1039/C2RA20334K
(Paper)
RSC Adv., 2012,
2, 7506-7512
A tandem coupling/smiles rearrangement/cyclization approach to 1,4-benzooxazinones or 1,4-pyridooxazinones under mild conditions†
Received
22nd February 2012
, Accepted 12th June 2012
First published on 13th June 2012
Abstract
A facile and efficient approach to 1,4-benzooxazinone or 1,4-pyridooxazinone scaffolds is developed via Smiles rearrangement tandem reaction. 1,4-Benzooxazinones or 1,4-pyridooxazinones with diversity at two substituents on their scaffolds were synthesized conveniently in the presence of cesium carbonate at room temperature in good to excellent yields. The reaction mechanism is assumed by a tandem coupling/Smiles rearrangement/cyclization process.
Introduction
1,4-Benzooxazinones or 1,4-pyridooxazinones are important heterocycle scaffolds and their derivatives have a substantial amount of biological activities, ranging from herbicides and fungicides to therapeutic usable drugs. For example in Fig. 1, compound A, a novel antibacterial or an important antifungal (anti-candida) agent,1 is a potential agent for treating anxiety, depression and negative symptoms in schizophrenia.2 Compound B, isolated from gramineous plants, has been used as plant resistance factors against microbial diseases and insects.3 Compound C, a new analgesic agent,4 is a potential drug for treating heart disease, arrhythmia and myocardial necrosis.5 Compound D, an antithrombotic agent,6 is an attractive target for drug discovery.7,8
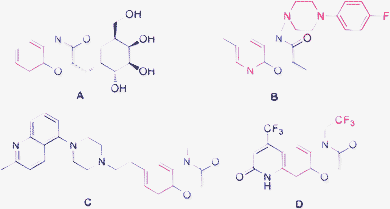 |
| | Fig. 1 Some biologically important compounds containing 1,4-benzooxazinone or 1,4-pyridooxazinone scaffold. | |
The 1,4-benzooxazinone scaffold has been the subject of extensive research in synthetic and medicinal organic chemistry. Three common approaches to 1,4-benzooxazinone scaffold employed 2-nitrophenols, 2-aminophenols or 2-halophenols as the precursors (Fig. 2). The first approach (path A) included three steps of O-alkylation, nitro reduction and intramolecular N-substitution.9 This method employed metal catalyst. In the second approach (path B), 2-aminophenols were treated with ethyl 2-bromoacetate to form 2-amidophenols, which then underwent intramolecular O-alkylation on heating in the presence of a base10 or using a recyclable ionic liquid medium-[omim][BF4] at room temperature.11 In the last approach (path C), Chen group3a developed a CuI catalyzed one-pot synthetic method to construct 1,4-benzooxazinone scaffolds, which started from 2-halophenols, but a costly ligand was required; Zuo group12 used 2-halophenols to synthesize diverse 1,4-benzooxazinones in the presence of a strong base on heating. The above three approaches might suffer from inconvenient operations and a limited number of suitable substrates for diverse synthesis. Therefore, concise and efficient methods to give these heterocyclic motifs are still in demand.
Recently, the tandem reaction for the synthesis of various heterocyclic compounds was reported in our lab.13 In particular, the reaction proceeded via a three-step sequence involving Smiles rearrangement13a to provide the desired products in good yields. Herein, we report a novel and efficient procedure that presents a tandem reaction, involves a broad range of starting materials, and takes place at room temperature. The route of our process, illustrated in Scheme 1, is performed using simple starting materials, such as substituted 1,2-dihalobenzenes or 2-halonitroarenes, 2-hydroxyacetamide to form a 1,4-benzooxazinone or 1,4-pyridooxazinone ring system. The reactions can complete rapidly in the presence of cesium carbonate and DMF as solvent at room temperature.
 |
| | Scheme 1 The synthesis of 1,4-benzooxazinones or 1,4-pyridooxazinones. | |
Results and discussion
In our preliminary experiments, 1,4-difluoro-2-nitrobenzene 1b and N-benzyl-2-hydroxyacetamide 2k were chosen as model substrates for the optimization of the reaction conditions including the bases and solvents. It was interesting to note that the product 3b was obtained in 71% yield after 5 h (Table 1, entry 6). We investigated the effect of solvents, the yield in DMF was acceptable (Table 1, entry 6). We also investigated the effect of the bases. The nature of the base had a pronounced impact on the process, and Cs2CO3 was proved to be more effective than K2CO3 or NaH (Table 1, entries 5–7). On the other hand, When 1b was reacted with 2k in the presence of a strong base such as NaH or t-BuOK, the low yields were obtained with the result of forming a non cyclized product. (Table 1, entries 7–8).
Table 1 Optimization of reaction conditionsa
| Entry |
Base |
Solvent |
Time (h) |
Yieldb (%) |
|
Reaction conditions: 1,4-difluoro-2-nitrobenzene 1b (1 equiv.), N-benzyl-2-hydroxyacetamide 2k (1.2 equiv.), base (3 equiv.), r.t.
Isolated yields.
|
| 1 |
K2CO3 |
THF |
14 |
0 |
| 2 |
Cs2CO3 |
THF |
14 |
43 |
| 3 |
K2CO3 |
CH3CN |
12 |
12 |
| 4 |
Cs2CO3 |
CH3CN |
12 |
63 |
| 5 |
K2CO3 |
DMF |
24 |
56 |
| 6 |
Cs2CO3 |
DMF |
5 |
71 |
| 7 |
NaH |
DMF |
6 |
0 |
| 8 |
t-ButOK |
DMF |
7 |
0 |
| 9 |
DBU |
DMF |
7 |
16 |
In order to examine the diversity of the products, we chose a variety of aliphatic or aromatic 2-hydroxy-acetamides to perform this tandem reaction and the results are summarized in Table 2. According to our observation, the yields of the aliphatic substrates (Table 2, entries 7–11) were higher than for the aromatic substrates (Table 2, entries 1–6). In the reaction of aromatic 2-hydroxy-acetamides as substrates, the electron properties of substituents on the aromatic ring did not play an important role in this procedure.
Table 2 The synthesis of benzo[1,4]oxazin-3(4H)-one derivatives 4a
| Entry |
R1 |
2
|
Time (h) |
Product |
Yield (%)b |
|
Reaction conditions: 3,4-difluorobenzonitrile 1c (1 equiv.), 2-hydroxyacetamide 2 (1.2 equiv.), Cs2CO3 (3 equiv.), r.t.
Isolated yields.
Reaction conducted at 60 °C.
|
| 1 |
4-ClC6H4 |
2a
|
7 |
4a
|
77c |
| 2 |
4-FC6H4 |
2b
|
4.5 |
4b
|
82 |
| 3 |
4-BrC6H4 |
2c
|
6.8 |
4c
|
78c |
| 4 |
4-MeC6H4 |
2d
|
6.5 |
4d
|
64 |
| 5 |
Ph |
2e
|
6.8 |
4e
|
72 |
| 6 |
4-MeOC6H4 |
2f
|
4.5 |
4f
|
88 |
| 7 |
3,4-(MeO)2-C6H3(CH2)2 |
2g
|
2.2 |
4g
|
85 |
| 8 |
Pr |
2h
|
3.3 |
4h
|
85 |
| 9 |
c-Hex |
2i
|
2.8 |
4i
|
92 |
| 10 |
i-Pr |
2j
|
3 |
4j
|
95 |
To extend the substrate scope, we performed the reaction of 3,4-difluorobenzonitrile 1c with N-benzyl-2-hydroxyacetamide 2k in the presence of Cs2CO3 at room temperature for 3.5 h to obtain the desired 4-benzy-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-6-carbonitrile 3c (Table 3, entry 3) in 92% yield. The result encouraged us to synthesize 1,4-benzooxazinone or 1,4-pyrido-oxazinone derivatives from different substrates under the same reaction condition (Table 3). As observed in Table 3, the substrates of 1,2-dihaloarenes or 2-halonitroarenes which have an electron-withdrawing group (Table 3, entries 1–9 and 13–15) or an electron-donating group (Table 3, entries 10–12) provided the desired products 3 in good yields. It was interesting that the symmetric compound 3i was obtained with an acceptable yield (Table 3, entry 9). Moreover, the substrates with a cyano group (Table 3, entries 3–4) could provide higher yields than the ones with nitro group (Table 3, entries 1–2) in a short time.
Table 3 The synthesis of benzo[1,4]oxazin-3(4H)-one derivatives 3a
| Entry |
1
|
Time (h) |
3
|
Yieldb (%) |
|
Reaction conditions : 1 (1 equiv.), N-benzyl-2-hydroxyacetamide 2k (1.2 equiv.), Cs2CO3 (3 equiv.), r.t.
Isolated yields.
Reaction conducted at 80 °C.
|
| 1 |
 1a
1a
|
5 |
 3a
3a
|
75 |
| 2 |
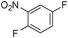 1b
1b
|
5.5 |
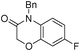 3b
3b
|
71 |
| 3 |
 1c
1c
|
3.5 |
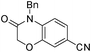 3c
3c
|
92 |
| 4 |
 1d
1d
|
5 |
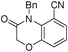 3d
3d
|
79 |
| 5 |
 1e
1e
|
9.5 |
 3e
3e
|
86 |
| 6 |
 1f
1f
|
4 |
 3f
3f
|
86 |
| 7 |
 1g
1g
|
4.8 |
 3g
3g
|
62 |
| 8 |
 1h
1h
|
5.5 |
 3h
3h
|
78 |
| 9 |
 1i
1i
|
4 |
 3i
3i
|
54c |
| 10 |
 1j
1j
|
9.5 |
 3j
3j
|
52c |
| 11 |
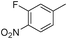 1k
1k
|
7.5 |
 3k
3k
|
51c |
| 12 |
 1l
1l
|
7 |
 3l
3l
|
72 |
| 13 |
 1m
1m
|
3.5 |
 3m
3m
|
68 |
| 14 |
 1n
1n
|
3 |
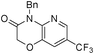 3n
3n
|
73c |
| 15 |
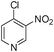 1o
1o
|
6 |
 3o
3o
|
67c |
The formation of 3 is the best explained by a mechanism13a with a three-step sequence in Scheme 2. Firstly, reaction of 1k and 2k generates intermediate 5k for 3 h at room temperature, which can be observed by TLC monitoring. Secondly, the key step of Smiles rearrangement results in the formation of the spiro-type intermediate 7 (Meisenheimer complex). Then, intermediate 8, produced from spiro-type intermediate 7 leads to target compound 3 in the third step. The structure of 3k was confirmed by X-ray analysis (Fig. 3). The intermediate product 5k was also confirmed by NMR and mass spectrometry. The regiochemistry of all of the other compounds is assumed to be the same.
 |
| | Fig. 3 X-ray structure of compound 3k. | |
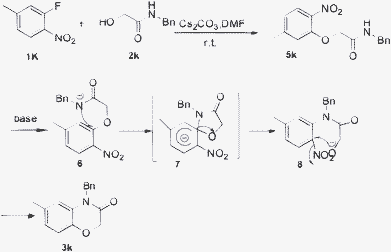 |
| | Scheme 2 The proposed mechanism for the formation of 3k. | |
Conclusions
In line with increasing global environmental safety regulation, we designed a green, novel and efficient method to synthesize a variety of 1,4-benzooxazinones or 1,4-pyridooxazinones via Smiles rearrangement tandem reaction. Various 1,4-benzooxazinones or 1,4-pyridooxazinones with diversity at two substituents on their scaffolds were synthesized in good to excellent yields. To the best of our knowledge, this process is the easiest method for the synthesis of compound 3i successfully. This transition metal-free tandem process has potential applications in the synthesis of biologically and medicinally relevant compounds.
Experimental section
All reagents and solvents were pure analytical-grade materials purchased from commercial sources and were used without further purification, if not stated. All reactions were monitored by thin-layer chromatography (TLC). 1H NMR spectra were recorded on a Bruker Avance 400 or 300 spectrometer at 400 or 300 MHz, using CDCl3 as solvent and tetramethylsilane (TMS) as internal standard. 13C NMR spectra were run in the same instrument at 100 or 75 MHz. Melting points were determined on an XD-4 digital micro melting point apparatus. HRMS spectra were determined on a Q-TOF6510 spectrograph (Agilent).
General procedure for the synthesis of 4-benzyl-7-fluoro-2H-benzo[b][1,4]oxazine-3(4H)-one (3b)
To a 50 mL flask equipped with a magnetic stirrer were added N-benzyl-2-hydroxyacetamide 2k (0.12 g, 0.76 mmol), 1,4-difluoro-2-nitrobenzene 1b (0.1 g, 0.63 mmol) and Cs2CO3 (0.62 g, 1.89 mmol). The tube was evacuated and backfilled with N2 (this procedure was repeated 3 times). DMF (10 mL) was added and the mixture was stirred for 5.5 h at room temperature. After completion of the reaction (monitored by TLC), the residue was poured into brine (100 mL). The aqueous solution was then extracted with dichloromethane (3×20 mL) and the combined organic layers were dried over anhydrous MgSO4. The solvent was removed under vacuum to obtain the crude product. The crude product was purified by flash column chromatography on silica gel (EtOAC/n-hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to afford pure product 3b in 71% yields.
3) to afford pure product 3b in 71% yields.
4-(4-chlorophenyl)-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-carbonitrile (4a)
White solid (77%). mp 178–183 °C. 1H NMR (300 MHz, CDCl3) δ 7.56–7.53 (d, 2H, J = 8.7 Hz), 7.33–7.32 (d, 1H, J = 1.8 Hz) 7.23–7.16 (m, 3H) 6.53–6.50 (d, 1H, J = 8.4 Hz) 4.83 (s, 2H) 13C NMR (75 MHz, CDCl3) δ 163.5, 144.8, 135.6, 134.2, 133.2, 130.7, 129.9, 127.1, 120.7, 117.9, 117.1, 107.5, 67.9. HRMS calcd for C15H9N2O2Cl, 284.0472; found, 284.0476.
4-(4-fluorophenyl)-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-carbonitrile (4b)
White solid (82%). mp 157–164 °C. 1H NMR (400 MHz, CDCl3) δ 7.32 (s, 1H) 7.26–7.25 (d, 2H, J = 5.52 Hz) 7.19–7.17 (d, 2H, J = 7.8 Hz) 7.19–7.17 (d, 1H, J = 7.8 Hz) 6.51–6.49 (d, 1H, J = 8.04 Hz) 4.83 (s, 2H) 13C NMR (100 MHz, CDCl3) δ 164.0, 163.7, 161.5, 144.7, 134.4, 130.5, 130.4, 127.1, 120.7, 118.1, 117.6, 107.4, 67.9. HRMS calcd for C15H9FN2O2, 268.0703; found, 268.0700.
4-(4-bromophenyl)-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-carbonitrile (4c)
Pale yellow solid (78%). mp 176–183 °C. 1H NMR (300 MHz, CDCl3) δ 7.72–7.69 (d, 2H, J = 8.7 Hz) 7.33–7.32 (d, 1H, J = 1.5 Hz) 7.20–7.14 (dd, 3H, J = 1.8 Hz) 6.53–6.50 (d, 1H, J = 8.7 Hz) 4.82 (s, 2H) 13C NMR (75 MHz, CDCl3) δ 163.5, 144.8, 134.1, 133.8, 133.7, 130.3, 127.1, 123.6, 120.7, 117.9, 117.2, 107.6, 67.9. HRMS calcd for C15H9N2O2Br, 327.9935; found, 327.9939.
3-oxo-4-(p-tolyl)-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-carbonitrile (4d)
Pale yellow solid (64%). mp 149–151 °C. 1H NMR (400 MHz, CDCl3) δ 7.37–7.35 (d, 2H, J = 8.08 Hz) 7.31–7.30 (d, 1H, J = 1.72 Hz) 7.16–7.12 (m, 3H) 6.53–6.51 (d, 1H, J = 8.4 Hz) 4.82 (s, 2H) 2.44 (s, 3H) 13C NMR (100 MHz, CDCl3) δ 163.7, 144.7, 139.7, 134.7, 132.0, 131.0, 128.3, 126.9, 120.5, 118.2, 117.3, 107.1, 67.9, 21.3. HRMS calcd for C16H12N2O2, 264.0975; found, 264.0979.
3-oxo-4-phenyl-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-carbonitrile (4e)
White solid (72%). mp 171–177 °C. 1H NMR (400 MHz, CDCl3) δ 7.59–7.51 (m, 3H) 7.32–7.31 (d, 1H, J = 1.72 Hz) 7.274–7.270 (m, 2H) 7.17–7.14 (dd, 1H, J = 1.76, 8.4 Hz) 6.50–6.48 (d, 1H, J = 8.4 Hz) 4.83 (s, 2H) 13C NMR (100 MHz, CDCl3) δ 163.6, 144.7, 134.8, 134.5, 130.4, 129.5, 128.6, 127.0, 120.5, 118.2, 117.3, 107.2, 67.9. HRMS calcd for C15H10N2O2, 250.0815; found, 250.0818.
4-(4-methoxyphenyl)3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-carbonitrile (4f)
Brown solid (88%). mp 149–151 °C. 1H NMR (400 MHz, CDCl3) δ 7.31–7.30 (d, 1H, J = 1.72 Hz) 7.18–7.15 (m, 3H) 7.07–7.05 (dd, 2H, J = 2.16, 6.76 Hz) 6.54–6.52 (d, 1H, J = 8.4 Hz) 4.83 (s, 2H) 3.87 (s, 3H) 13C NMR (100 MHz, CDCl3) δ 163.8, 160.2, 144.7, 134.9, 129.6, 127.0, 126.9, 120.5, 118.2, 117.3, 115.6, 107.1, 67.9, 55.6. HRMS calcd for C16H12N2O3, 280.0921; found, 280.0926.
4-(3,4-dimethoxyphenethyl)-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-carbonitrile (4g)
White solid (85%). mp 162–167 °C. 1H NMR (300 MHz, CDCl3) δ 7.32–7.36 (dd, 1H, J = 1.8, 8.4 Hz), 7.27–7.26 (d, 1H, J = 1.5 Hz) 7.03–7.00 (d, 1H, J = 8.4 Hz) 6.82–6.71 (m, 3H) 4.63 (s, 2H) 4.18–4.13 (dd, 2H, J = 7.8, 9.6 Hz) 3.87–3.86 (d, 6H, J = 1.5 Hz) 2.92–2.87 (dd, 2H, J = 6.3, 7.8 Hz) 13C NMR (75 MHz, CDCl3) δ 163.6, 149.2, 148.1, 145.3, 132.7, 129.9, 127.2, 120.8, 118.1, 115.3, 112.0, 111.5, 106.9, 67.4, 55.9, 42.8, 32.8. HRMS calcd for C19H18N2O4,338.1339; found, 338.1329.
3-oxo-4-propyl-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-carbonitrile (4h)
White solid (85%). mp 131–137 °C. 1H NMR (300 MHz, CDCl3) δ 7.36–7.33 (dd, 1H, J = 2.1, 8.7 Hz) 7.27–7.25 (d, 1H, J = 4.5 Hz) 7.05–7.02 (d, 1H, J = 8.4 Hz) 4.66 (s, 2H) 3.94–3.89 (t, 2H, J = 7.8, 15.3 Hz) 1.76–1.61 (m, 2H) 1.02–0.94 (t, 3H, J = 7.2, 14.7 Hz) 13C NMR (100 MHz, CDCl3) δ 163.6, 145.2, 132.7, 127.2, 120.5, 118.2, 115.3, 106.8, 67.3, 42.8, 20.2, 11.1. HRMS calcd for C12H12N2O2, 216.0972; found, 216.0983.
4-cyclohexyl-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-carbonitrile (4i)
White solid (92%). mp 135–137 °C. 1H NMR (300 MHz, CDCl3) δ 7.35–7.32 (dd, 1H, J = 1.8, 8.4 Hz) 7.27–7.26 (d, 1H, J = 1.8 Hz) 7.24–7.21 (d, 1H, J = 8.4 Hz) 4.53 (s, 2H) 4.28–4.12 (m, 1H) 2.40–2.27 (m, 2H) 1.94–1.72 (m, 5H) 1.47–1.21 (m, 3H) 13C NMR (100 MHz, CDCl3) δ 165.5, 146.5, 134.1, 127.1, 120.8, 118.1, 116.7, 106.8, 69.8, 57.3, 29.3, 26.3, 25.2. HRMS calcd for C15H16N2O2, 256.1285; found, 256.1281.
4-isopropyl-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-carbonitrile (4j)
White solid (95%). mp 136–142 °C. 1H NMR (300 MHz, CDCl3) δ 7.36–7.32 (dd, 1H, J = 2.1, 8.7 Hz) 7.28–7.27 (d, 1H, J = 1.8 Hz) 7.22–7.19 (d, 1H, J = 8.7 Hz) 4.81–4.67 (m, 1H) 4.54 (s, 2H) 1.57–1.55 (d, 6H, J = 6.9 Hz) 13C NMR (100 MHz, CDCl3) δ 165.3, 146.4, 133.5, 127.1, 120.8, 118.1, 116.5, 106.8, 68.3, 47.9, 19.7. HRMS calcd for C12H12N2O2, 216.0952; found, 216.0950.
4-benzyl-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-6-carbonitrile (4k)
White solid (92%). mp 132–136 °C. 1H NMR (400 MHz, CDCl3) δ 7.36–7.21 (m, 5H) 7.26 (s, 1H) 7.19–7.20 (d, 1H, J = 1.8 Hz) 6.94–6.92 (d, 1H, J = 8.4 Hz) 5.18 (s, 2H) 4.79 (s, 2H) 13C NMR (100 MHz, CDCl3) δ 164.1, 145.2, 134.9, 132.8, 129.1, 127.9, 127.2, 126.5, 120.4, 118.1, 116.2, 107.1, 67.4, 45.0. HRMS calcd for C16H12N2O2, 264.0972; found, 264.0973.
Yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.84–7.83 (d, 1H, J = 2.48 Hz) 7.81–7.78 (dd, 1H, J = 2.48, 8.88 Hz) 7.36–7.22 (m, 5H) 6.98–6.95 (d, 1H, J = 8.92 Hz) 5.21 (s, 2H) 4.82 (s, 2H) 13C NMR (100 MHz, CDCl3) δ 164.1, 144.9, 143.5, 134.8, 134.3, 129.1, 127.9, 126.6, 118.7, 115.5, 112.64, 67.4, 45.2. HRMS calcd for C15H12N2O4, 284.0689; found, 284.0694.
4-benzyl-7-fluoro-2H-benzo[b][1,4]oxazine-3(4H)-one (3b)
Yellow solid (71%). mp 71–75 °C. 1H NMR (400 MHz, CDCl3) δ 7.33–7.22 (m, 5H) 6.80–6.76(dd, 1H, J = 5.28, 9 Hz) 6.73–6.70 (dd, 1H, J = 2.76, 8.92 Hz) 6.60–6.55 (m, 1H) 5.12 (s, 2H) 4.71 (s, 2H) 13C NMR (100 MHz, CDCl3) δ 164.0, 160.1, 157.7, 146.3, 135.7, 128.9, 127.6, 125.1, 116.3, 109.3, 105.1, 67.7, 45.1. HRMS calcd for C15H12NFO2, 257.0925; found, 257.0926.
4-benzyl-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazine-8-carbonitrile (3d)
Pale yellow solid (79%). mp 92–96 °C. 1H NMR (400 MHz, CDCl3) δ 7.28–7.27 (d, 1H, J = 1.76 Hz) 7.26–7.22 (m, 5H) 7.21–7.20 (d, 1H, J = 1.8 Hz) 7.04–7.00 (q, 1H, J = 7.92 Hz) 5.71 (s, 2H) 4.67 (s, 2H) 13C NMR (100 MHz, CDCl3) δ 165.6, 148.6, 134.9, 131.4, 129.5, 128.7, 127.7, 127.2, 124.8, 122.0, 117.6, 101.9, 68.4, 45.1. HRMS calcd for C16H12N2O2, 264.0972; found, 264.0978.
4-benzyl-6-(trifluoromethyl)-2H-benzo[b][1,4]oxazin-3(4H)-one (3e)
White solid (54%). mp 132–136 °C. 1H NMR (400 MHz, CDCl3) δ 7.35–7.22 (m, 5H) 7.24 (s, 1H) 7.16–7.14 (d, 1H, J = 8.28 Hz) 6.96–6.94 (d, 1H, J = 8.44 Hz) 5.18 (s, 2H) 4.77 (s, 2H) 13C NMR (100 MHz, CDCl3) δ 164.3, 145.2, 135.3, 131.6, 129.0, 127.8, 126.9, 124.9, 122.3, 119.9, 117.3, 115.8, 67.5, 44.9. HRMS calcd for C16H12NF3O2, 307.0893; found, 307.0886.
Yellow solid (86%). mp 73–75 °C. 1H NMR (400 MHz, CDCl3) δ 7.30–7.24 (m, 5H) 6.99–6.87 (m, 4H) 5.15 (s, 2H) 4.71 (s, 2H) 13C NMR (100 MHz, CDCl3) δ 164.7, 145.4, 135.9, 128.9, 127.5, 126.6, 123.9, 122.8, 116.9, 115.7, 67.7, 44.9. HRMS calcd for C15H13NO2, 239.1019; found, 239.1021.
4-benzyl-5-methyl-2H-benzo[b][1,4]oxazin-3(4H)-one (3g)
Yellow solid (62%). mp 88–97 °C. 1H NMR (400 MHz, CDCl3) δ 7.51–7.50 (d, 2H, J = 1.28 Hz) 7.35–7.22 (m, 3H) 7.00–6.99 (d, 1H, J = 2.24 Hz) 6.88–6.85 (dd, 1H, J = 2.28, 8.6 Hz) 6.79–6.77 (d, 1H, J = 8.68 Hz) 5.14 (s, 2H) 4.73(s, 2H) 13C NMR (100 MHz, CDCl3) δ 164.1, 145.9, 135.5, 128.9, 128.9, 127.7, 127.5, 126.6, 122.7, 117. 4, 116.4, 67.6, 44.9. HRMS calcd for C15H12NClO2, 273.0614; found, 273.0610.
4-benzyl-8-chloro-2H-benzo[b][1,4]oxazin-3(4H)-one (3h)
Yellow oil (78%). 1H NMR (400 MHz, CDCl3) δ 7.21–7.13 (m, 5H) 6.97–6.90 (m, 3H) 5.51 (s, 2H) 4.54(s, 2H) 13C NMR (100 MHz, CDCl3) δ 167.3, 150.9, 136.4, 128.4, 127.7, 127.3, 127.1, 125.9, 125.5, 123.9, 115.8, 69.2, 47.0. HRMS calcd for C15H12NClO2, 273.0629; found, 273.0640.
4,6-dibenzyl-6,8-dihydrobenzo[1,2-b;5,4-b]bis([1,4]oxazine)-3,7(2H,4H)-dione (3i)
Red Brown solid (54%). mp 188–203 °C. 1H NMR (400 MHz, CDCl3) δ 7.29–7.26 (m, 6H) 6.99–6.97 (dd, 4H, J = 3.56, 7.36 Hz) 6.66 (s, 1H) 6.37 (s, 1H) 4.87 (s, 4H) 4.65 (s, 4H) 13C NMR (100 MHz, CDCl3) δ 164.1, 141.5, 135.5, 128.9, 127.7, 126.5, 123.9, 106.1, 103.8, 67.8, 45.2. HRMS calcd for C24H20N2O4, 400.1496; found, 400.1493.
4-benzyl-5-methyl-2H-benzo[b][1,4]oxazin-3(4H)-one (3j)
Yellow solid (51%). mp 86–95 °C. 1H NMR (400 MHz, CDCl3) δ 7.24–7.09 (m, 5H) 7.18–7.16 (d, 1H, J = 2.68 Hz) 7.11–7.09 (d, 1H, J = 7.12 Hz) 6.77–6.75 (q, 1H, J = 5.4 Hz) 5.22 (s, 2H) 4.52 (s, 2H) 2.32 (s, 3H) 13C NMR (100 MHz, CDCl3) δ 168.1, 149.6, 136.7, 129.4, 128.5, 128.4, 127.2, 126.8, 126.4, 124.9, 114.8, 69.2, 48.1, 21.0. HRMS calcd for C16H15NO2, 253.1156; found, 253.1151.
4-benzyl-7-methyl-2H-benzo[b][1,4]oxazin-3(4H)-one (3k)
Orange solid (51%). mp 86–95 °C. 1H NMR (400 MHz, CDCl3) δ 7.34–7.25 (m, 5H) 6.88–6.86 (d, 1H, J = 8.12 Hz) 6.76–6.74 (d, 1H, J = 8.2 Hz) 6.69 (s, 1H) 5.14 (s, 2H) 4.68 (s, 2H) 2.19 (s, 3H) 13C NMR (100 MHz, CDCl3) δ 164.9, 143.2, 136.1, 132.4, 128.9, 128.6, 127.4, 126.6, 124.4, 116.6, 116.2, 67.8, 44.9, 21.1. HRMS calcd for C16H15NO2, 253.1176; found, 253.1187.
Orange solid (72%). mp 137–140 °C. 1H NMR (400 MHz, CDCl3) δ 7.32–7.31 (d, 2H, J = 1.28 Hz) 7.29–7.23 (m, 3H) 6.67–6.65 (d, 1H, J = 8.6 Hz) 6.39–6.38 (d, 1H, J = 2.52 Hz) 6.26–6.23 (dd, 1H, J = 2.52, 8.6 Hz) 5.11 (s, 2H) 4.68 (s, 2H) 4.04 (s, 2H) 13C NMR (100 MHz, CDCl3) δ 163.9, 146.4, 142.9, 136.2, 128.8, 127.3, 126.6, 120.7, 116.5, 109.2, 104.1, 67.8, 44.9. HRMS calcd for C15H14N2O2, 254.1128; found, 254.1127.
1-benzyl-1H-pyrido[2,3-b][1,4]oxazin-2(3H)-one (3m)
Pale yellow solid (52%). mp 101–109 °C. 1H NMR (400 MHz, CDCl3) δ 8.02–7.99 (dd, 1H, J = 1.4, 8.4 Hz) 7.46–7.44 (d, 2H, J = 7.12 Hz) 7.29–7.19 (m, 3H) 7.27–7.26 (d, 1H, J = 1.48 Hz) 6.92–6.89 (q, 1H, J = 1.28 Hz) 5.35 (s, 2H) 4.68 (s, 2H) 13C NMR (100 MHz, CDCl3) δ 164.6, 141.6, 141.1, 140.7, 137.1, 128.6, 128.4, 127.4, 123.5, 119.3, 67.5, 42.7. HRMS calcd for C14H12N2O2, 240.0972; found, 240.0976.
1-benzyl-7-(trifluoromethyl)-1H-pyrido[2,3-b][1,4]oxazin-2(3H)-one (3n)
Yellow oil (73%). 1H NMR (400 MHz, CDCl3) δ 8.30 (s, 1H) 7.44 (s, 1H) 7.41–7.40 (d, 2H, J = 1.48 Hz) 7.31–7.23 (m, 3H) 5.37 (s, 2H) 4.77 (s, 2H) 13C NMR (400 MHz, CDCl3) δ 164.2, 144.1, 140.2, 138.2, 136.5, 128.7, 128.5, 127.7, 124.5, 122.6, 121.8, 67.3, 43.1. HRMS calcd for C15H11N2F3O2, 308.0845; found, 308.0842.
4-benzyl-2H-pyrido[4,3-b][1,4]oxazin-3(4H)-one (3o)
Yellow solid (67%). mp 137–140 °C. 1H NMR (400 MHz, CDCl3) δ 8.27 (s, 1H) 8.11–8.10 (d, 1H, J = 5.4 Hz) 7.36–7.23 (m, 5H) 6.80–6.78 (d, 1H, J = 5.4 Hz) 5.14 (s, 2H) 4.79 (s, 2H) 13C NMR (100 MHz, CDCl3) δ 164.3, 144.6, 141.5, 138.7, 135.1, 134.9, 129.1, 127.9, 126.7, 109.7, 67.5, 44.6. HRMS calcd for C14H12N2O2, 240.0972; found, 240.0979.
N-benzyl-2-(5-methyl-2-nitrophenoxy)acetamide (5k)
Yellow solid (71%). mp 95–99 °C. 1H NMR (300 MHz, CDCl3) δ 7.95–7.91 (d, 1H, J = 4.5 Hz) 7.65 (s, 1H) 7.36–7.27 (m, 5H) 6.94–6.91 (dd, 1H, J = 0.9, 8.4 Hz) 6.84 (s, 1H) 4.66 (s, 2H) 4.59–4.57 (d, 2H, J = 6.3 Hz) 2.44 (s, 3H) 13C NMR (100 MHz, CDCl3) δ 166.7, 151.1, 147.3, 137.6, 136.7, 128.7, 127.7, 127.6, 126.7, 122.5, 115.1, 67.8, 43.1, 21.9. HRMS calcd for C16H16N2O4, 300.1283; found, 300.1276.
Acknowledgements
We are grateful to the National Natural Science Foundation of China (No. 21172131) and State Key Laboratory of Natural and Biomimetic Drugs of Peking University (No. K20090205) for financial support of this research.
References
-
(a) E. Feng, H. Huang, Y. Zhou, D. Ye, H. L. Jiang and H. Liu, J. Org. Chem., 2009, 74, 2846–2849 CrossRef CAS;
(b) R. Fringuelli, D. Pietrella, F. Schiaella, A. Guarraci, S. Perito, F. Bistoni and A. Vecchiarelli, Bioorg. Med. Chem., 2002, 10, 1681–1686 CrossRef CAS;
(c) A. Macchiarulo, G. Costantino, D. Fringuelli, A. Vecchiarelli, F. Schiaella and R. Fringuelli, Bioorg. Med. Chem., 2002, 10, 3415–3423 CrossRef CAS.
-
(a) S. M. Bromidge, B. Bertani, M. Borriello, A. Bozzoli, S. Faedo, M. Gianotti, L. J. Gordon, M. Hill, V. Zucchelli, J. M. Watson and L. Zonzini, Bioorg. Med. Chem. Lett., 2009, 19, 2338–2342 CrossRef CAS;
(b) P. Smid, H. K. A. C. Coolen, H. G. Keizer, R. Hes, J. P. Moes, A. P. Hartog, B. Stork, R. H. Plekkenpol, L. C. Niemann, C. N. J. Stroomer, M. T. M. Tulp, H. H. Stuivenberg, A. C. McCreary, M. B. Hesselink, A. H. J. Herremans and C. G. Kruse, J. Med. Chem., 2005, 48, 6855–6869 CrossRef CAS;
(c) L. A. Vliet, N. Rodenhuis, D. D. Jkstra and H. Wikstrom, J. Med. Chem., 2000, 43, 2871–2882 CrossRef.
-
(a) D. B. Chen, G. D. Shen and W. L. Bao, Org. Biomol. Chem., 2009, 7, 4067–4073 RSC;
(b) M. Z. Huang, K. L. Huang, Y. G. Ren, M. X. Lei, L. Huang, Z. K. Hou, A. P. Liu and X. M. Qu, J. Agric. Food Chem., 2005, 53, 7908–7914 CrossRef CAS.
- L. Savelon, J. G. Bizot-Espiard, D. H. Caignard, B. Pfeiffer, P. Renard, M. C. Viauda and G. Guillaumet, Bioorg. Med. Chem., 1998, 6, 133–142 CrossRef CAS.
-
(a) M. Hori, L. Watanable, H. Ohtaka, K. Harada, J. Maruo, T. Morita, T. Yamamoto and H. Tsutsui, EP Patent Appl. 719766, 1996 Search PubMed;
(b) G. Caliendo, E. Perissutti, V. Santagada, F. Fiorino, B. Severino, R. E. V. Bianca, L. Lippolis, A. Pintoc and R. Sorrentinob, Bioorg. Med. Chem., 2002, 10, 2663–2669 CrossRef CAS;
(c) H. Chiu, Y. C. Lin, C. Y. Cheng, M. C. Tai and H. C. Yu, Bioorg. Med. Chem., 2001, 9, 383–393 CrossRef CAS.
- P. S. Anderluh, M. Anderluh, J. Ilas, J. Mravljak, M. S. Dolenc, M. Stegnar and D. Kikelj, J. Med. Chem., 2005, 48, 3110–3113 CrossRef CAS.
- A. G. Sams, M. Hentzer, G. K. Mikkelsen, K. Larsen, C. Bundgaard, N. Plath, C. T. Christoffersen and B. B. Andersen, J. Med. Chem., 2010, 53, 6386–6397 CrossRef CAS.
- Y. O. Long, R. I. Higuchi, T. R. Caferro, T. L. S. Lau, M. Wu, M. L. Cummings, E. A. Martinborough, K. B. Marschke, W. Y. Chang, F. J. Lopez, D. S. Karanewsky and Z. Lin, Bioorg. Med. Chem. Lett., 2008, 18, 2967–2971 CrossRef CAS.
- A. G. Sams, M. Hentzer, G. K. Mikkelsen, K. Larsen, C. Bundgaard, N. Plath, C. T. Christoffersen and B. B. Andersen, J. Med. Chem., 2010, 53, 6386–6397 CrossRef CAS.
- J. Kang, K. H. Kam, M. J. Ghate, H. Zuo, T. H. Kim, C. R. Reddy, S. Chandrasekhar and D. S. Shina, ARKIVOC, 2008,(xiv), 67–76 CAS.
- A. Sharifi, M. Barazandeh, M. S. Abaee and M. Mirzaei, Tetrahedron Lett., 2010, 51, 1852–1855 CrossRef CAS.
-
(a) H. Zuo, L. J. Meng, M. J. Ghate, K. H. Hwang, Y. K. Cho, S. Chandrasekhar, C. R. Reddy and D. S. Shin, Tetrahedron Lett., 2008, 49, 3827–3830 CrossRef CAS;
(b) J. Kang, K. H. Kam, M. J. Ghate, H. Zuo, T. H. Kim, C. R. Reddy, S. Chandrasekhar and D. S. Shina, ARKIVOC, 2008,(xiv), 67–76 CAS.
-
(a) Y. Liu, C. Chu, A. Huang, C. Zhan, Y. Ma and C. Ma, ACS Comb. Sci., 2011, 13, 547–553 CrossRef CAS;
(b) A. Huang, F. Liu, C. Zhan, Y. Liu and C. Ma, Org. Biomol. Chem., 2011, 9, 7351–7357 RSC;
(c) A. Huang, Z. Qiao, X. Zhang, W. Yu, Q. Zheng, Y. Ma and C. Ma, Tetrahedron, 2012, 68, 906–912 CrossRef CAS;
(d) Y. Liu, Y. Ma, C. Zhan, A. Huang and C. Ma, Synlett, 2012, 23, 255–258 CAS.
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. 


 1a
1a
 3a
3a
 1b
1b
 3b
3b
 1c
1c
 3c
3c
 1d
1d
 3d
3d
 1e
1e
 3e
3e
 1f
1f
 3f
3f
 1g
1g
 3g
3g
 1h
1h
 3h
3h
 1i
1i
 3i
3i
 1j
1j
 3j
3j
 1k
1k
 3k
3k
 1l
1l
 3l
3l
 1m
1m
 3m
3m
 1n
1n
 3n
3n
 1o
1o
 3o
3o


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to afford pure product 3b in 71% yields.
3) to afford pure product 3b in 71% yields.