Effect of cationic surfactants on structure and morphology of mesostructured MOFs†
Ya-Nan
Guo
,
Yantao
Li
,
Bo
Zhi
,
Daojun
Zhang
,
Yunling
Liu
and
Qisheng
Huo
*
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun. 130012, China. E-mail: huoqisheng@jlu.edu.cn; Fax: 0086-431-85168602; Tel: +86-431-85168602
First published on 11th April 2012
Abstract
Cooperative self-assembly of metal ions, bridging ligands and surfactants is an effective method to prepare mesostructured metal–organic frameworks (MOFs). In this study, we use quaternary ammonium surfactants with different head groups as templates to examine their effects on the structure and morphology of mesostructured MOFs formed by Cu2+ and 5-hydroxy-1,3-benzenedicarboxylic acid. In the presence of low surfactant concentrations, mesostructured MOFs with a variety of morphologies have been synthesized, having disordered, lamellar, p6mm or Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n structure accordingly by increasing surfactant charge density. The particle size of mesostructured MOFs can be controlled from 200 to 600 nm by adjusting the molar ratio of ligand to surfactant. Comparing our experimental results with the synthesis of mesoporous silicas, we find that they follow a similar assembly process and the charge matching between surfactant and MOF framework plays a key role in the formation of mesostructures.
n structure accordingly by increasing surfactant charge density. The particle size of mesostructured MOFs can be controlled from 200 to 600 nm by adjusting the molar ratio of ligand to surfactant. Comparing our experimental results with the synthesis of mesoporous silicas, we find that they follow a similar assembly process and the charge matching between surfactant and MOF framework plays a key role in the formation of mesostructures.
Introduction
Surfactant-based supramolecular templating has attracted great attention for the synthesis of periodic mesoporous materials.1,2 Typical mesoporous silica materials (M41S, SBA, FDU, MSU series, etc.) have been well reported by self-assembly of organic soft-templates and inorganic silicate species over the past two decades.3 Weak noncovalent bonds, for example, electrostatic interactions, hydrogen bonds and van der Waals forces, between the surfactants and inorganic species are important factors in synthesis of mesostructured materials.1 Various mesophases such as 2D hexagonal p6mm,4 3D hexagonal P63/mmc,5 cubic Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m,6Pm
m,6Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n,7Fd
n,7Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m,8Fm
m,8Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m,9Im
m,9Im![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m,10Ia
m,10Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d,11etc. have been synthesized by adjusting these interactions. Recently, a novel mesoporous silica with tri-continuous P63/mcm pore structure has been synthesized,12 by means of a special surfactant that is designed to adjust its interaction with the silicon source. In addition, the self-assembly method is also applied to the syntheses of nanoparticles with controllable size and morphology.13,14 Along with this synthetic approach that is widely accepted and investigated in depth, a liquid-crystal template mechanism15 and a cooperative formation mechanism16 have been used to explain the formation process of mesostructures in the synthesis of mesoporous silica.17 Furthermore, the molecular packing parameter g and charge density matching are used to predict and explain the mesostructures of mesoporous silica and other materials.18,19 Self-assembly of surfactant micelles in the presence of inorganic or organic framework precursors is considered as a common and effective approach for the synthesis of mesostructured materials and now has been applied to synthesize mesoporous non-siliceous materials such as metal oxides,20 non-oxide materials,21 and even polymers and carbons.22 However, few researches focus on the synthesis of mesostructured metal organic frameworks (MOFs) via this assembly method.23
d,11etc. have been synthesized by adjusting these interactions. Recently, a novel mesoporous silica with tri-continuous P63/mcm pore structure has been synthesized,12 by means of a special surfactant that is designed to adjust its interaction with the silicon source. In addition, the self-assembly method is also applied to the syntheses of nanoparticles with controllable size and morphology.13,14 Along with this synthetic approach that is widely accepted and investigated in depth, a liquid-crystal template mechanism15 and a cooperative formation mechanism16 have been used to explain the formation process of mesostructures in the synthesis of mesoporous silica.17 Furthermore, the molecular packing parameter g and charge density matching are used to predict and explain the mesostructures of mesoporous silica and other materials.18,19 Self-assembly of surfactant micelles in the presence of inorganic or organic framework precursors is considered as a common and effective approach for the synthesis of mesostructured materials and now has been applied to synthesize mesoporous non-siliceous materials such as metal oxides,20 non-oxide materials,21 and even polymers and carbons.22 However, few researches focus on the synthesis of mesostructured metal organic frameworks (MOFs) via this assembly method.23
Meanwhile, metal–organic frameworks (MOFs) are widely investigated,24,25 owing to the interest in the creation of novel structures and their potential applications on adsorption and separation,26 gas storage,27 heterogeneous catalysis,28 luminescent properties,29 and magnetic materials.30 Generally, the synthesis of MOFs is straightforward by reaction of metal ions or metal clusters and organic ligands that contain mono-, di-, tri- and tetracarboxylic acids or N, S atoms.31 Moreover, the geometry conformation of ligand and coordination conformation of metal ions have important influence on the structure of MOFs.32
Recently, mesostructured MOFs (meso-MOFs) materials were designed to combine advantages of both mesoporous materials and MOFs materials, which provided a new research direction in materials study. MacLachlan and co-workers prepared metal–organic liquid-crystal-templated mesostructures by tethering alkyl pyrazinium surfactants to Prussian blue in a one-pot synthesis.23,33 Qiu and co-workers designed a supramolecular template strategy to synthesize meso-MOFs by the self-assembly of metal ions and organic ligands in the presence of surfactant micelles.34 Zhao and co-workers used supercritical CO2 as the core of surfactant micelles at high pressure to synthesize MOF nanospheres with well-ordered mesopores in an ionic liquid emulsion system,35 which provided an effective method to construct mesostructures. Zhou and co-workers designed a cooperative template system to prepare mesoporous MOFs by using citric acid as a chelating agent to establish the interaction between framework building-blocks and surfactants.36,37 In our previous work, we designed and prepared meso-MOF via cooperative self-assembly using the cationic surfactant cetyltrimethylammonium chloride (CTAC) as template.38 Soft template self-assembly synthesis approach was successfully introduced into the synthesis of meso-MOFs.
The interfacial charge density matching between the inorganic species and the surfactant head groups affects the structure of the final phase of mesoporous oxide materials.12 However, the self-assembly system for meso-MOFs formation is more complicated, which mainly includes three aspects: (i) coordination mode of metal and ligand varies with the experimental conditions (e.g., ligand structure, solvent polarity, reaction temperature, etc.); (ii) rather than forming mesostructures, metal ions and ligands are more inclined to crystallization; surfactants then can no longer play a template role and only affect the size of crystals;39 (iii) the key interactions between surfactants and frameworks formed by metal ions and ligands in the synthesis of meso-MOFs must be considered.
Here, we study the synthesis of meso-MOFs materials in aqueous solution using cationic surfactants with different charge densities to adjust the interaction between the soft-template and the framework formed by Cu2+ and 5-hydroxy-1,3-benzenedicarboxylic acid (5-OH-BDC). We investigate the surfactant effect on the mesostructure and morphology of products. At low surfactant concentrations, we obtain highly ordered cubic mesophase with the Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n symmetry, 2D p6mm and lamellar MOFs powder materials accordingly by reducing the surfactant charge density. High surfactant concentrations result in the formation of meso-MOF spheres (200–600 nm). Meanwhile, compared to typical mesoporous silica and meso-MOFs, our results show that the charge matching is also a very important factor in the formation of meso-MOFs materials via self-assembly synthesis approach. Ordered mesostructures of MOFs can only be synthesized by the appropriate matching of surfactants and MOFs.
n symmetry, 2D p6mm and lamellar MOFs powder materials accordingly by reducing the surfactant charge density. High surfactant concentrations result in the formation of meso-MOF spheres (200–600 nm). Meanwhile, compared to typical mesoporous silica and meso-MOFs, our results show that the charge matching is also a very important factor in the formation of meso-MOFs materials via self-assembly synthesis approach. Ordered mesostructures of MOFs can only be synthesized by the appropriate matching of surfactants and MOFs.
Experimental section
Chemicals
Cu(NO3)2·3H2O was purchased from Sinopharm Chemical Reagent Co., Ltd. 5-Hydroxy-1,3-benzenedicarboxylic acid (5-OH-BDC) was purchased from Alfa Aesar. N,N-Dimethyl-n-octadecylamine was from TCI (Japan). Cetyl bromide was from Kelong Chemical Reagent Plant (China). Triethylamine was from Tianjin Guangfu Fine Chemical Research Institute. Tripropylamine was from Aladdin Reagent Co., Ltd. n-Hexadecyltrimethylammonium chloride was purchased from HuiShi biochemical reagent Co., Ltd of Shanghai. Hexadecyldimethybenzylammonium chloride and (3-bromopropyl)trimethylammonium bromide were from Nanjing Robiot Co., Ltd. All materials were used as purchased without further purification.Synthesis of surfactants
C16H33(C2H5)3NBr and C16H33(C3H7)3NBr surfactants were synthesized according to previously published procedures.40 Divalent surfactant [C18H37(CH3)2N–(CH2)3–N(CH3)3]Br2 (C18-3-1Br2) was synthesized by the reaction of N,N-dimethyl-n-octadecylamine with (3-bromopropyl)trimethylammonium bromide.5 C18-3-1Cl2 solution was achieved by precipitation–transformation. 1.7 g of silver chloride solid was put into 60 g of 5% C18-3-1Br2 aqueous solution. In dark condition, the mixture was stirred for 3 h then kept static at room temperature for 10 h. After filtration of the precipitate (AgCl and AgBr), 4.2% C18-3-1Cl2 solution was obtained.Synthesis of mesostructured MOFs
The meso-MOFs were prepared through a simple self-assembly method.Synthesis of Cu-(5-OH-BDC)-C18-3-1 meso-MOF powder
Typical synthesis procedure of Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n structured material was as follows: 0.48 g of a 4.2 wt% C18-3-1Cl2 aqueous solution and 8.55 g of H2O were mixed and heated to 40 °C. To this solution, 0.206 g of 5-OH-BDC and 1.36 g of a 20.7 wt% Cu(NO3)2 aqueous solution were added under stirring in turn. Then the mixture was further stirred for 3 h at 40 °C. The reaction mixture molar composition was 0.043 C18-3-1
n structured material was as follows: 0.48 g of a 4.2 wt% C18-3-1Cl2 aqueous solution and 8.55 g of H2O were mixed and heated to 40 °C. To this solution, 0.206 g of 5-OH-BDC and 1.36 g of a 20.7 wt% Cu(NO3)2 aqueous solution were added under stirring in turn. Then the mixture was further stirred for 3 h at 40 °C. The reaction mixture molar composition was 0.043 C18-3-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 Cu2+
1.5 Cu2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.13 (5-OH-BDC)
1.13 (5-OH-BDC)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 555.6 H2O. Finally the mixture was loaded into an autoclave and was heated and kept at 110 °C oven for 24 h. The solid product was filtered off, washed and dried at room temperature. The synthesis of other meso-MOFs was similar to Cu-(5-OH-BDC)-C18-3-1, except that the other surfactants were used as templates instead of C18-3-1 and the mixing process was at room temperature. The reaction mixture molar composition for Cu-(5-OH-BDC)-CTEB-1 was 0.078 CTEB
555.6 H2O. Finally the mixture was loaded into an autoclave and was heated and kept at 110 °C oven for 24 h. The solid product was filtered off, washed and dried at room temperature. The synthesis of other meso-MOFs was similar to Cu-(5-OH-BDC)-C18-3-1, except that the other surfactants were used as templates instead of C18-3-1 and the mixing process was at room temperature. The reaction mixture molar composition for Cu-(5-OH-BDC)-CTEB-1 was 0.078 CTEB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.31 Cu2+
4.31 Cu2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (5-OH-BDC)
1 (5-OH-BDC)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 523.5 H2O. The reaction mixture molar composition for Cu-(5-OH-BDC)-CTEB-2 was 0.14 CTEB
523.5 H2O. The reaction mixture molar composition for Cu-(5-OH-BDC)-CTEB-2 was 0.14 CTEB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.87 Cu2+
2.87 Cu2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (5-OH-BDC)
1 (5-OH-BDC)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 523.5 H2O.
523.5 H2O.
Synthesis of Cu-(5-OH-BDC)-CTAC meso-MOF spheres
The synthesis procedure of meso-MOF spheres was carried out by a procedure similar to that described for Cu-(5-OH-BDC)-C18-3-1 except for the molar ratio of ligand to the surfactant. Typical synthesis of Cu-(5-OH-BDC)-CTAC spheres ( ∼400 nm) was as follows: 4 g of CTAC 8% and 5.87 g of H2O were mixed at room temperature. To this solution, 0.103 g of 5-OH-BDC and 0.68 g of Cu(NO3)2 20.7% aqueous solution were added under stirring in turn. The ratio of 5-OH-BDC to CTAC was 0.566. Then the mixture was further stirred for 3 h at room temperature. Finally the autoclave was heated and kept at 110 °C oven for 24 h. The precipitate was collected by centrifugation, and washed with water three times. 220 nm and 570 nm meso-MOFs spheres were prepared by adjusting the ratio of ligand to surfactant from 0.384 to 0.776.Characterization
The morphologies of the samples were revealed with a JEOL JSM-6700F and Hitachi S-4800 field-emission scanning electron microscope (FE-SEM). Thermal gravimetric analyses (TGA) were performed on a TGA Q500 V20.10 Build 36 thermogravimetric analyzer in air atmospheric environment with a heating rate of 10 °C min−1. The IR spectra were recorded with a Bruker IFS 66 v/S FTIR spectrometer. Powder X–ray diffraction (XRD) data was collected on a Rigaku 2550 diffractometer with Cu-Kα radiation (λ = 1.5418 Å) at 50 kV and 200 mA. CHN elemental analysis data was collected on a Vario MiCRO CUBE elementar.Results and discussion
To investigate the effect of cationic surfactants on the mesostructure and morphology, we chose four alkyl chain quaternary ammonium salts with different charge density as surfactants. Various meso-MOFs with different surfactant/MOFs interface curvature were obtained by the assembly synthetic approach.A variety of mesophases including Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n,5P63/mmc, 5 hexagonal41 and lamellar41 phases are obtained when the dicationic surfactants Cn-s-1 are used to synthesize mesoporous SiO2. To begin with, we chose surfactant C18-3-1 as a template exploration. In our work, the product Cu-(5-OH-BDC)-C18-3-1 with Pm
n,5P63/mmc, 5 hexagonal41 and lamellar41 phases are obtained when the dicationic surfactants Cn-s-1 are used to synthesize mesoporous SiO2. To begin with, we chose surfactant C18-3-1 as a template exploration. In our work, the product Cu-(5-OH-BDC)-C18-3-1 with Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n mesostructure is successfully synthesized using low concentration of surfactant C18-3-1. Fig. 1 shows the powder X–ray diffraction of samples of Cu-(5-OH-BDC)-C18-3-1. The Bragg diffraction peaks are observed at 51.6, 46.5, 43.5, 30.0, 28.8, 27.6, 26.0 Å, respectively, which are indexed in a cubic Pm
n mesostructure is successfully synthesized using low concentration of surfactant C18-3-1. Fig. 1 shows the powder X–ray diffraction of samples of Cu-(5-OH-BDC)-C18-3-1. The Bragg diffraction peaks are observed at 51.6, 46.5, 43.5, 30.0, 28.8, 27.6, 26.0 Å, respectively, which are indexed in a cubic Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n symmetry as 200, 210, 211, 222, 320, 321 and 400 reflections, with unit cell parameter a = 10.3 nm. No other XRD peaks are observed at high angles (2θ > 6°). IR spectroscopy (Fig. 2) indicates the presence of surfactant and the coordination between copper and ligand in Cu-(5-OH-BDC)-C18-3-1. The CH2 groups in alkyl chains of surfactants are identified by the asymmetric stretching band (2920 cm−1) and the symmetric stretching band (2850 cm−1). When we repeatedly washed the sample with deionized water, the relative intensity of C–H stretching vibration peaks in the IR spectrum show no obvious change. There is about 31.2% surfactant in the sample Cu-(5-OH-BDC)-C18-3-1, as calculated according to the CHN data (C 42.8%, H 5.4%, N 6.0%). This indicates that the C–H stretching signals could not come from physically adsorbed surfactant molecules. Comparing with the carboxyl frequencies of the free derivative of 5-OH-BDC at 1680–1715 cm−1, the asymmetric and symmetric vibration bands shift to lower values at 1542 and 1364 cm−1, respectively, which strongly prove the coordination between the carboxyl and metal ions. This IR result for Cu-(5-OH-BDC)-C18-3-1 is the same as for Cu-(5-OH-BDC)-CTAC which we have reported previously.38 In each case the quaternary cationic surfactants are the same type and the interaction between surfactant and MOF should be the same.
n symmetry as 200, 210, 211, 222, 320, 321 and 400 reflections, with unit cell parameter a = 10.3 nm. No other XRD peaks are observed at high angles (2θ > 6°). IR spectroscopy (Fig. 2) indicates the presence of surfactant and the coordination between copper and ligand in Cu-(5-OH-BDC)-C18-3-1. The CH2 groups in alkyl chains of surfactants are identified by the asymmetric stretching band (2920 cm−1) and the symmetric stretching band (2850 cm−1). When we repeatedly washed the sample with deionized water, the relative intensity of C–H stretching vibration peaks in the IR spectrum show no obvious change. There is about 31.2% surfactant in the sample Cu-(5-OH-BDC)-C18-3-1, as calculated according to the CHN data (C 42.8%, H 5.4%, N 6.0%). This indicates that the C–H stretching signals could not come from physically adsorbed surfactant molecules. Comparing with the carboxyl frequencies of the free derivative of 5-OH-BDC at 1680–1715 cm−1, the asymmetric and symmetric vibration bands shift to lower values at 1542 and 1364 cm−1, respectively, which strongly prove the coordination between the carboxyl and metal ions. This IR result for Cu-(5-OH-BDC)-C18-3-1 is the same as for Cu-(5-OH-BDC)-CTAC which we have reported previously.38 In each case the quaternary cationic surfactants are the same type and the interaction between surfactant and MOF should be the same.
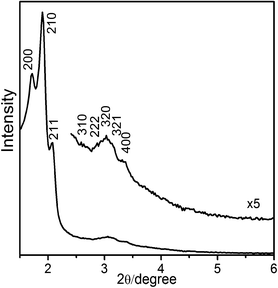 | ||
Fig. 1 XRD pattern of Cu-(5-OH-BDC)-C18-3-1 with Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n mesostructure (ao = 10.3 nm). n mesostructure (ao = 10.3 nm). | ||
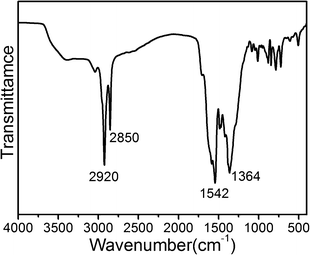 | ||
| Fig. 2 FT-IR spectrum of Cu-(5-OH-BDC)-C18-3-1. | ||
Our synthesis system is acidic and the pH value is 3–4. Comparing our synthesis results with that for mesoporous silica SBA-1 that has the same Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n mesostructure prepared under acidic condition,42,43 we can deduce that the formation of Cu-(5-OH-BDC)-C18-3-1 is similar to that of SBA-1 prepared via the S+X−I+ formation pathway.43 Surfactant C18-3-1 has two quaternary ammonium groups and large head groups (g < 1/3),41 which prefers to form high curvature micelles in aqueous solution and structurally directs the formation of caged mesostructures.44 Therefore, it is reasonable that the self-assembly of Cu2+, 5-OH-BDC and C18-3-1 gives a Pm
n mesostructure prepared under acidic condition,42,43 we can deduce that the formation of Cu-(5-OH-BDC)-C18-3-1 is similar to that of SBA-1 prepared via the S+X−I+ formation pathway.43 Surfactant C18-3-1 has two quaternary ammonium groups and large head groups (g < 1/3),41 which prefers to form high curvature micelles in aqueous solution and structurally directs the formation of caged mesostructures.44 Therefore, it is reasonable that the self-assembly of Cu2+, 5-OH-BDC and C18-3-1 gives a Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n mesostructure in our synthesis system. When the same molarity of CTAB surfactant is used in the same conditions, however, only p6mm meso-MOF is obtained (Fig. S1, ESI†). Compared with the Cu-(5-OH-BDC)-CTAC with p6mm mesostructure we have reported on previously,38 it is obvious that the anion of the surfactant does not affect the formation of the mesostructure. Furthermore, synthesis reaction conditions such as temperature, time and solvent are the same. So the framework composition of Cu-(5-OH-BDC)-C18-3-1 should be the same or similar to that of Cu-(5-OH-BDC)-CTAC.38 The change of charge density of surfactant results in change of meso-MOF product to retain charge matching between surfactant and MOF. Charge matching between the micelles and the MOFs is thus the key factor to form Pm
n mesostructure in our synthesis system. When the same molarity of CTAB surfactant is used in the same conditions, however, only p6mm meso-MOF is obtained (Fig. S1, ESI†). Compared with the Cu-(5-OH-BDC)-CTAC with p6mm mesostructure we have reported on previously,38 it is obvious that the anion of the surfactant does not affect the formation of the mesostructure. Furthermore, synthesis reaction conditions such as temperature, time and solvent are the same. So the framework composition of Cu-(5-OH-BDC)-C18-3-1 should be the same or similar to that of Cu-(5-OH-BDC)-CTAC.38 The change of charge density of surfactant results in change of meso-MOF product to retain charge matching between surfactant and MOF. Charge matching between the micelles and the MOFs is thus the key factor to form Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n meso-MOFs.
n meso-MOFs.
In fact, by examining other synthesis conditions, we find that the synthesis of meso-MOFs systems is very complicated. The nature of the products is sensitive to the reaction parameters. One of the important factors is the molecular structure of the template. The headgroup structure of cationic surfactant19 may affect the formation of meso-MOFs. When C16H33(C2H5)3N+Br− (CTEB) is used as the template, p6mm and lamellar materials are the main products and the powder XRD patterns of Cu-(5-OH-BDC)-CTEB-1 and Cu-(5-OH-BDC)-CTEB-2 are shown in Fig. 3. Furthermore, as the charge density of surfactant decreases, charge matching between the surfactant and MOF is insufficient to generate ordered mesostructures, i.e., the interaction between surfactant and MOFs is too weak to form meso-MOF structures. The large head-group surfactant C16H33(C3H7)3N+Br− (CTPB) gives a disordered material and only one broad XRD peak is observed (Fig. S2, ESI†).
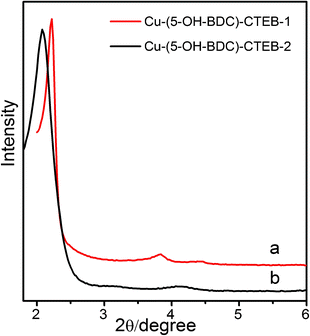 | ||
| Fig. 3 XRD patterns of (a) Cu-(5-OH-BDC)-CTEB-1 and (b) Cu-(5-OH-BDC)-CTEB-2. | ||
Furthermore, we find that the meso-MOFs synthesized by self-assembly approach have different morphologies. Fig. 4 gives a clear observation of the dominant morphology for each sample. It is known that slow growth rate is the fundamental requirement for well-shaped morphology.45 When we reduce the concentration of reactants to slow down the meso-MOF growth rate, the morphology of Cu-(5-OH-BDC)-C18-3-1 is changed from a sphere to cube. However the mesostructure of the product remains the same. Under high-magnification, the square and hexagonal planes of Cu-(5-OH-BDC)-C18-3-1 are clearly shown in Fig. 4a. Thus, it can be seen that dilute condition is conducive to generating regular morphology. In addition, it is noteworthy that meso-MOF of p6mm structure prepared by CTEB has gyroid morphology46 as is shown in Fig. 4b. The possible explanation for this shape is that hexagonal cylindrical micelles–MOF undergo growth and curvature of this hexagonal cylinder.47 However, lamellar mesostructured Cu-(5-OH-BDC)-CTEB-2 has ribbon morphology (Fig. 4c). Although there is a larger difference between the composition of meso-MOF and mesoporous SiO2, they still have similar morphology with the same mesostructure when they are synthesized by soft template self-assembly. Thus we deduce the two materials have similar assembly formation process. Therefore, the charge matching is a major factor in the formation of a given morphology and mesophase.
 | ||
| Fig. 4 SEM images of (a) Cu-(5-OH-BDC)-C18-3-1, (b) Cu-(5-OH-BDC)-CTEB-1 and (c) Cu-(5-OH-BDC)-CTEB-2. | ||
We find that low concentrations of surfactants are conducive to the formation of meso-MOFs powder. However, surfactant in high concentration not only plays a template role, but also affects the size of the product. When increasing the surfactant concentration and adjusting the ratio of ligand and surfactant, we obtain size controlled (200–600 nm) meso-MOF spheres. SEM images and XRD pattern of Cu-(5-OH-BDC)-CTAC spheres (400 nm) are shown in Fig. 5. The particle size of Cu-(5-OH-BDC)-CTAC spheres decreases as the molar ratio of surfactant to ligand increases. There is a broad peak in its XRD pattern, which indicates these meso-MOF spheres have disordered mesostructure. The IR result (Fig. S3. ESI†) is similar to that of meso-MOFs powder discussed above.
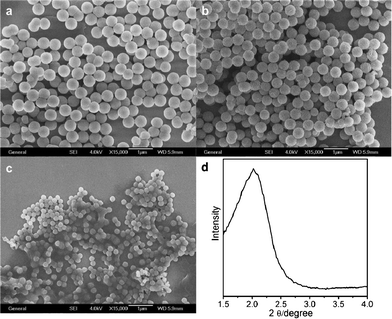 | ||
| Fig. 5 SEM images for Cu-(5-OH-BDC)-CTAC meso-MOFs spheres: (a) ∼570 nm, (b) ∼400 nm, (c) ∼220 nm; (d) XRD pattern of Cu-(5-OH-BDC)-CTAC spheres (400 nm). | ||
On the basis of the above results, we summarize and compare the surfactant effects on silica and MOFs mesostructure in Table 1. These results indicate that with the surfactant charge density increasing from C16H33(C3H7)3N+, C16H33(C2H5)3N+, C16H33(CH3)3N+ to C18-3-1, we could get MOFs with disordered, lamellar, 2D p6mm and the Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n symmetries. We conclude that high charge density is conducive to generating high curvature of the mesostructure. Fig. 6 shows a schematic diagram of the basic units of meso-MOF and SiO2 self-assembly synthesis systems. It is easy to see that the number of Coulomb interaction sites in silica species is more than that for the organic ligand in the same unit length from the diagram. In the synthesis of mesoporous silica, different mesostructures can be obtained by adjusting the charge matching,48 which means the formation of a mesostructure requires a certain matching condition. In our experimental system, ligands lead to limitations in size and geometry of space configuration. When the MOFs resulting from 5-OH-BDC coordinating to copper assemble with the surfactant, the matching between them cannot meet required conditions to generate specific mesostructures. Therefore, when the same surfactant is used as a soft-template only a limited number of mesostructures are obtained in MOFs. Charge density matching42 for mesoporous silica is also applicable to the synthesis of meso-MOFs. However, the mesostructure formation process of meso-MOFs is more complicated. When different ligands and metal ions are chosen to make metal organic coordination polymers, their coordination modes will vary. Consequently, if new synthetic strategies are applied to satisfy the appropriate synthesis conditions, other meso-MOFs will be synthesized.
n symmetries. We conclude that high charge density is conducive to generating high curvature of the mesostructure. Fig. 6 shows a schematic diagram of the basic units of meso-MOF and SiO2 self-assembly synthesis systems. It is easy to see that the number of Coulomb interaction sites in silica species is more than that for the organic ligand in the same unit length from the diagram. In the synthesis of mesoporous silica, different mesostructures can be obtained by adjusting the charge matching,48 which means the formation of a mesostructure requires a certain matching condition. In our experimental system, ligands lead to limitations in size and geometry of space configuration. When the MOFs resulting from 5-OH-BDC coordinating to copper assemble with the surfactant, the matching between them cannot meet required conditions to generate specific mesostructures. Therefore, when the same surfactant is used as a soft-template only a limited number of mesostructures are obtained in MOFs. Charge density matching42 for mesoporous silica is also applicable to the synthesis of meso-MOFs. However, the mesostructure formation process of meso-MOFs is more complicated. When different ligands and metal ions are chosen to make metal organic coordination polymers, their coordination modes will vary. Consequently, if new synthetic strategies are applied to satisfy the appropriate synthesis conditions, other meso-MOFs will be synthesized.
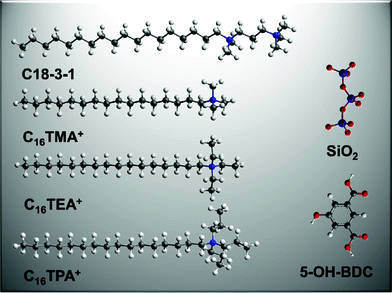 | ||
| Fig. 6 Schematic diagram of the basic units of the self-assembly system. | ||
| Surfactant | meso-Silica | meso-MOFs |
|---|---|---|
| C18-3-1 |
P63/mmc, Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n n |
Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n n |
| C16H33N(CH3)3+ |
p6mm, lamellar, cmm, Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d d |
p6mm |
| C16H33N(C2H5)3+ |
p6mm, Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n n |
p6mm, lamellar |
| C16H33N(C3H7)3+ | p6mm | Disordered |
Conclusions
Four different charge density surfactants are chosen to investigate their effect on meso-MOF formation. Phase changes in meso-MOF are observed if the charge density of surfactant is changed. The obtained meso-MOFs have disordered, lamellar, p6mm and Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n structures accordingly by increasing the charge density of surfactants (from C16H33(C3H7)3N+, C16H33(C2H5)3N+, C16H33(CH3)3N+ to C18-3-1), and these materials have characteristic morphologies. We find that the charge matching is a very important factor in the formation of mesostructures. Ordered mesostructure of MOFs can only be synthesized in the appropriate matching of surfactant and MOF. The morphology and particle size of product are sensitive to the concentration of the surfactant. Comparing with the mesoporous silica synthesis, we find that meso-MOFs formation have a similar assembly process, which provides advantages for further study. However, the relatively complicated formation of meso-MOFs is due to their more complex characteristics. Therefore the synthesis system of meso-MOFs has more variability than that of silica. The research of meso-MOFs has a broad possibility in creating novel mesostructures, investigating unique properties and developing new applications.
n structures accordingly by increasing the charge density of surfactants (from C16H33(C3H7)3N+, C16H33(C2H5)3N+, C16H33(CH3)3N+ to C18-3-1), and these materials have characteristic morphologies. We find that the charge matching is a very important factor in the formation of mesostructures. Ordered mesostructure of MOFs can only be synthesized in the appropriate matching of surfactant and MOF. The morphology and particle size of product are sensitive to the concentration of the surfactant. Comparing with the mesoporous silica synthesis, we find that meso-MOFs formation have a similar assembly process, which provides advantages for further study. However, the relatively complicated formation of meso-MOFs is due to their more complex characteristics. Therefore the synthesis system of meso-MOFs has more variability than that of silica. The research of meso-MOFs has a broad possibility in creating novel mesostructures, investigating unique properties and developing new applications.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant Nos. 21171064 and 21071059).References
- Y. Wan and D. Y. Zhao, Chem. Rev., 2007, 107, 2821–2860 CrossRef CAS.
- C. Gao and S. Che, Adv. Funct. Mater., 2010, 20, 2750–2768 CrossRef CAS.
- Y. Wan, Y. F. Shi and D. Y. Zhao, Chem. Commun., 2007, 897–926 RSC.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548–552 CrossRef CAS.
- Q. Huo, D. I. Margolese and G. D. Stucky, Chem. Mater., 1996, 8, 1147–1160 CrossRef CAS.
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024–6036 CrossRef CAS.
- Q. Huo, D. I. Margolese, U. Ciesla, P. Feng, T. E. Gier, P. Sieger, R. Leon, P. M. Petroff, F. Schuth and G. D. Stucky, Nature, 1994, 368, 317–321 CrossRef CAS.
- S. Shen, Y. Li, Z. Zhang, J. Fan, B. Tu, W. Zhou and D. Zhao, Chem. Commun., 2002, 2212–2213 RSC.
- J. Fan, C. Yu, F. Gao, J. Lei, B. Tian, L. Wang, Q. Luo, B. Tu, W. Zhou and D. Zhao, Angew. Chem., Int. Ed., 2003, 42, 3146–3150 CrossRef CAS.
- C. Yu, B. Tian, J. Fan, G. D. Stucky and D. Zhao, J. Am. Chem. Soc., 2002, 124, 4556–4557 CrossRef CAS.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710–712 CrossRef CAS.
- Y. Han, D. Zhang, L. L. Chng, J. Sun, L. Zhao, X. Zou and J. Y. Ying, Nat. Chem., 2009, 1, 123–127 CrossRef CAS.
- Z. A. Qiao, L. Zhang, M. Y. Guo, Y. L. Liu and Q. S. Huo, Chem. Mater., 2009, 21, 3823–3829 CrossRef CAS.
- B. G. Trewyn, I. I. Slowing, S. Giri, H. T. Chen and V. S. Y. Lin, Acc. Chem. Res., 2007, 40, 846–853 CrossRef CAS.
- G. S. Attard, J. C. Glyde and C. G. Goltner, Nature, 1995, 378, 366–368 CrossRef CAS.
- A. Monnier, F. Schuth, Q. Huo, D. Kumar, D. Margolese, R. S. Maxwell, G. D. Stucky, M. Krishnamurty, P. Petroff, A. Firouzi, M. Janicke and B. F. Chmelka, Science, 1993, 261, 1299–1303 CAS.
- Y. Wan, Y. F. Shi and D. Y. Zhao, Chem. Mater., 2008, 20, 932–945 CrossRef CAS.
- J. N. Israelachvili, D. J. Mitchell and B. W. Ninham, J. Chem. Soc., Faraday Trans., 1976, 72, 1525–1568 RSC.
- S. H. Tolbert, C. C. Landry, G. D. Stucky, B. F. Chmelka, P. Norby, J. C. Hanson and A. Monnier, Chem. Mater., 2001, 13, 2247–2256 CrossRef CAS.
- S. W. Boettcher, J. Fan, C.-K. Tsung, Q. Shi and G. D. Stucky, Acc. Chem. Res., 2007, 40, 784–792 CrossRef CAS.
- Y. F. Shi, Y. Wan and D. Y. Zhao, Chem. Soc. Rev., 2011, 40, 3854–3878 RSC.
- Y. Meng, D. Gu, F. Zhang, Y. Shi, H. Yang, Z. Li, C. Yu, B. Tu and D. Zhao, Angew. Chem., Int. Ed., 2005, 44, 7053–7059 CrossRef CAS.
- X. Roy and M. J. MacLachlan, Chem.–Eur. J., 2009, 15, 6552–6559 CrossRef CAS.
- S. Kitagawa, R. Kitaura and S.-i. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS.
- J. R. Long and O. M. Yaghi, Chem. Soc. Rev., 2009, 38, 1213–1214 RSC.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- L. J. Murray, M. Dinca and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294–1314 RSC.
- L. Ma, C. Abney and W. Lin, Chem. Soc. Rev., 2009, 38, 1248–1256 RSC.
- M. D. Allendorf, C. A. Bauer, R. K. Bhakta and R. J. T. Houk, Chem. Soc. Rev., 2009, 38, 1330–1352 RSC.
- M. Kurmoo, Chem. Soc. Rev., 2009, 38, 1353–1379 RSC.
- A. U. Czaja, N. Trukhan and U. Muller, Chem. Soc. Rev., 2009, 38, 1284–1293 RSC.
- D. Sun, D. J. Collins, Y. Ke, J.-L. Zuo and H.-C. Zhou, Chem.–Eur. J., 2006, 12, 3768–3776 CrossRef CAS.
- X. Roy, L. K. Thompson, N. Coombs and M. J. MacLachlan, Angew. Chem., Int. Ed., 2008, 47, 511–514 CrossRef CAS.
- L.-G. Qiu, T. Xu, Z.-Q. Li, W. Wang, Y. Wu, X. Jiang, X.-Y. Tian and L.-D. Zhang, Angew. Chem., 2008, 120, 9629–9633 CrossRef.
- Y. Zhao, J. Zhang, B. Han, J. Song, J. Li and Q. Wang, Angew. Chem., Int. Ed., 2011, 50, 636–639 CrossRef CAS.
- M. B. Yue, W. Q. Jiao, Y. M. Wang and M. Y. He, Microporous Mesoporous Mater., 2010, 132, 226–231 CrossRef CAS.
- L.-B. Sun, J.-R. Li, J. Park and H.-C. Zhou, J. Am. Chem. Soc., 2012, 134, 126–129 CrossRef CAS.
- Y. Li, D. Zhang, Y.-N. Guo, B. Guan, D. Tang, Y. Liu and Q. Huo, Chem. Commun., 2011, 47, 7809–7811 RSC.
- K. M. L. Taylor, A. Jin and W. Lin, Angew. Chem., 2008, 120, 7836–7839 CrossRef.
- C. A. Bunton, R. Bacaloglu and F. Ortega, J. Phys. Chem., 1989, 93, 1497–1502 CrossRef.
- H. Hagslaett, O. Soederman and B. Joensson, Langmuir, 1994, 10, 2177–2187 CrossRef CAS.
- Q. Huo, D. I. Margolese, U. Ciesla, D. G. Demuth, P. Feng, T. E. Gier, P. Sieger, A. Firouzi and B. F. Chmelka, Chem. Mater., 1994, 6, 1176–1191 CrossRef CAS.
- A. E. Garcia-Bennett, S. Willliamson, P. A. Wright and I. J. Shannon, J. Mater. Chem., 2002, 12, 3533–3540 RSC.
- P. Ziherl and R. D. Kamien, J. Phys. Chem. B, 2001, 105, 10147–10158 CrossRef CAS.
- M.-C. Chao, D.-S. Wang, H.-P. Lin and C.-Y. Mou, J. Mater. Chem., 2003, 13, 2853–2854 RSC.
- H. Yang, N. Coombs and G. A. Ozin, Nature, 1997, 386, 692–695 CrossRef CAS.
- G. A. Ozin, H. Yang, I. Sokolov and N. Coombs, Adv. Mater., 1997, 9, 662–667 CrossRef CAS.
- L. Han, K. Miyasaka, O. Terasaki and S. Che, J. Am. Chem. Soc., 2011, 133, 11524–11533 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra20396k/ |
| This journal is © The Royal Society of Chemistry 2012 |
