Copper(II) bromide as a procatalyst for in situ preparation of active Cu nanoparticles for reduction of nitroarenes†
Hari K.
Kadam
and
Santosh G.
Tilve
*
Department of Chemistry, Goa University, Taleigao Plateau, Goa–403206, India. E-mail: stilve@unigoa.ac.in; Fax: +91-832-2452886; Tel: +91-832-6519317
First published on 25th April 2012
Abstract
Copper(II) bromide as a procatalyst for the in situ preparation of active Cu nanoparticles for the efficient reduction of nitroarenes using sodium borohydride is described. During reduction, Cl, I, COOH, aliphatic nitro and OCH2Ph groups remain intact displaying the chemoselectivity of the process. The method is also useful for the reduction of cyano and olefinic compounds. Scalability of the method was demonstrated up to 100 mmol.
Introduction
Aromatic amines are important industrial raw materials for the manufacturing of agrochemicals, dyes, pharmaceuticals, polymers and rubbers.1 Reduction of the corresponding nitroarenes via catalytic hydrogenation using molecular hydrogen and noble metals is a common route employed in most cases.2,3 The other reducing systems include sodium borohydride,3 hydrazine hydrate,4,5 formic acid,6 ammonium formate,7,8 and organosilanes,9 in the presence of a metal or its salt which avoids the problem of handling of flammable hydrogen gas. All these methods have their limitations. For example, catalytic hydrogenation is a well established industrial route but selectivity3 is a major issue. Hydrazine hydrate delivers selectivity but the scale-up, due to its explosive nature and toxicity, needs to be addressed. In view of the limitations of these methods, there is continuous interest in developing newer methods.10–13 Recently, metal nanoparticles have shown great promise in organic catalysis;14–16 particularly biorelevant metals1,4,8,11,17,18 like Fe, Co and Cu are of much interest due to their potentially low toxicity and affordability. Stoichiometric Cu nanoparticles,8 in combination with ammonium formate at 120 °C for 8–12 h, have been demonstrated as an effective system for the chemoselective reduction of nitroarenes. With our interest in nitro reduction for natural product synthesis,19,20 we felt that the use of Cu nanoparticles in combination with sodium borohydride should be able to reduce aromatic nitro compounds at ambient conditions. Thus, we herein demonstrate the use of commercially available copper(II) bromide as a procatalyst which in situ generates highly active Cu nanoparticles for the reduction of nitroarenes to anilines with sodium borohydride in a green solvent, ethanol, at ambient temperature (Scheme 1).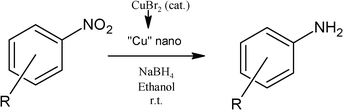 | ||
| Scheme 1 Reduction of nitroarenes. | ||
Results and discussion
Initially, reduction of nitrobenzene with NaBH4 in the presence of commercially available Cu nanoparticles, in a stoichiometric amount, in ethanol at room temperature was attempted. However, complete reduction could not take place even after prolonged reaction time. It is documented that Cu salts on reduction with sodium borohydride result in the formation of Cu nanoparticles.21 We thought of employing such an in situ system for the direct reduction of an aromatic nitro group. Various copper salts18 and FeCl3 were tried by mixing the salts with nitrobenzene in ethanol followed by the addition of sodium borohydride in portions, the results of which are depicted in Table 1.| Entry | Catalyst | % Anilineb | % Nitrobenzeneb |
|---|---|---|---|
| a Reaction conditions: nitrobenzene (1 mmol), catalyst (10 mol%), ethanol (5 mL), NaBH4 (3–5 mmol), r.t., 5 h. b Isolated yield. c Cu nano (1 mmol), 24 h. d CuO (> 1 mmol), NaBH4 (5 mmol), 35 h. e Cu2O (1 mmol), NaBH4 (5 mmol), 24 h, deposited Cu metal seen on the surface of reaction vessel. | |||
| 1 | Cu nanoc | 34 | 40 |
| 2 | Cu-acetate | 12 | 46 |
| 3 | CuCl2 | 22 | 60 |
| 4 | CuBr2 | 90 | 0 |
| 5 | CuI | 41 | 20 |
| 6 | CuOd | 0 | 100 |
| 7 | Cu2Oe | 68 | 18 |
| 8 | FeCl3 | 5 | 70 |
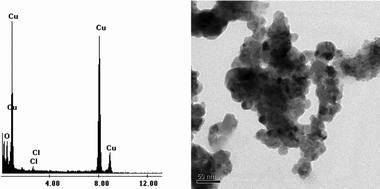
Amongst the catalysts tried, CuBr2 was found to be the catalyst of choice for giving a high yield of aniline within a short period of time (5 h). The formation of Cu nanoparticles in the range 30–40 nm was confirmed by TEM (Fig. 1) images of the recovered catalyst (see ESI†). XRD and EDX studies of the recovered catalyst show the presence of Cu2O along with Cu possibly due to atmospheric oxidation of the catalyst. Also, in the SEM images, micron-sized cubes of Cu2O are observed. To further confirm if there is any role of the oxides during the reduction, the reaction was attempted using CuO and Cu2O. In the case of CuO there was no reduction observed (Entry 6, Table 1) whereas Cu2O (Entry 7, Table 1) showed that reduction does take place, but this may be due to the active copper metal formed which was deposited on the surface of the reaction flask. Other Cu halides and Cu(II) acetate catalyst were also found to catalyze the reaction but to a lesser extent.
Next, various polar protic solvents like water, ethanol, etc. and aprotic solvents like THF were examined (Table 2).
| Entry | Solvent | % Anilineb | % Nitrobenzeneb |
|---|---|---|---|
| a Reaction conditions: nitrobenzene (1 mmol), CuBr2 (10 mol%), solvent (5 mL), NaBH4 (3–5 mmol), r.t., 5 h. b Isolated yield. c 35 h. d NaBH4 (5 mmol). | |||
| 1 | Water | 50 | 15 |
| 2 | Ethanol | 90 | 0 |
| 3 | THF | 30 | 45 |
| 4 | Isopropanolc | 41 | 50 |
| 5 | n-Butanolc | 10 | 72 |
| 6 | t-Butanolc | 15 | 64 |
| 7 | Methanold | 91 | 0 |
The study showed ethanol and methanol to be the best solvents for this catalytic system. Methanol was avoided for further applications due to its harmful effects. Reduction was very slow in other branched and long chain alcohols.
The mole percent of CuBr2 and reaction temperature were then optimized by varying the catalyst amount and temperature (Table 3) and found that 10 mol% of CuBr2 gave the maximum yield (∼90%) at room temperature and increasing the catalyst amount or the temperature did not improve the yield of reduced product. It was expected that the reduction would take place more rapidly at higher temperature. It appears that the rapid decomposition of sodium borohydride at higher temperature might be responsible for the observed results.
| Entry | Mole (%) | % Anilineb | % Nitrobenzeneb |
|---|---|---|---|
| a Reaction conditions: nitrobenzene (1 mmol), CuBr2 (5, 10, 20 mol%), ethanol (5 mL), NaBH4 (3 mmol), r.t. or 60 °C, 5 h. b Isolated yield. | |||
| 1 | 5 | 35 | 52 |
| 2 | 10 | 90 | 0 |
| 3 | 20 | 90 | 0 |
| 4 | 10 (60 °C) | 21 | 36 |
A scalability study with nitrobenzene revealed that reproducible isolated yields were obtained at both 0.1 mmol and 100 mmol scale (90 and 92% respectively.)
The recovered catalyst was again used for 3 consecutive cycles of reduction with nitrobenzene and yields were found to be consistent up to 3 catalytic cycles with increasing duration of reaction, this was expected as Cu nanoparticles tend to oxidise on exposure to air and moisture.
To study the generality and selectivity of this system, various nitroarenes were subjected to reduction (Table 4).
| Entry | Reactant | Product | Time (h) | % Yieldb |
|---|---|---|---|---|
| a Reaction conditions: nitroarenes (1 mmol), CuBr2 (10 mol%), ethanol (5 mL), NaBH4 (3–5 mmol), r.t., 5–12 h. b Isolated yield. c See ref. 8 for spectral data comparison. d See ref. 24. for spectral data comparison. n.r.: no reaction, reactant recovered. | ||||
| 1 |

|
|
5 | 90 |
| 2 |
|
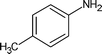
|
5 | 93 |
| 3 |
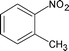
|
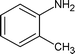
|
5 | 90 |
| 4 |
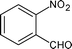
|
|
3 | 85 |
| 5 |
|
|
8 | 78 |
| 6 |
|
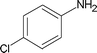
|
4 | 82 |
| 7 |
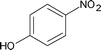
|
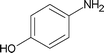
|
3.5 | 88 |
| 8 |

|

|
12 | 85 |
| 9 |

|
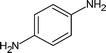
|
14 | 84 |
| 10 |
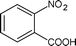
|

|
10 | 86 |
| 11 |

|

|
5 | 92 |
| 12 |
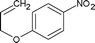
|
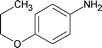
|
12 | 89c |
| 13 |

|
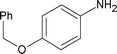
|
12 | 82c |
| 14 |
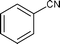
|

|
10 | 88 |
| 15 |
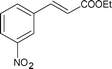
|
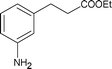
|
9 | 87 |
| 16 |
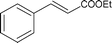
|

|
9 | 91d |
| 17 |
|
— | 12 | n.r. |
| 18 |

|
— | 12 | n.r. |
| 19 |

|
|
0.5 | 96 |
| 20 |

|

|
1 | 95 |
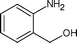
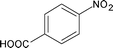
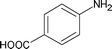
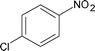
Along with nitrobenzene (Entry 1), p-,o-,m-nitrotoluenes (Entry 2, 3, 11) were readily reduced to p-,o-,m-toluidines. As expected o-nitrobenzaldehyde (Entry 4) rapidly gave o-aminobenzyl alcohol as the sole product. The acid group (Entry 5, 10) was intact and gave the respective aminobenzoic acids after long reaction times. Halogens like chloro and iodo (Entry 6, 18) were retained in the products displaying a selectivity that is usually not obtained when noble metals are used. Functionalities like hydroxyl and amino groups (Entry 7, 9) were not affected in this catalytic system. m-Dinitrobenzene (Entry 8) was completely reduced to m-aminoaniline with 5 eq. of sodium borohydride and no m-nitroaniline was observed during the course of reaction (tlc). This system very well retained the O-benzyl protection during nitro reduction (Entry 13) unlike catalytic hydrogenation, but the allylic double bond (Entry 12) was found to reduce completely. m-Nitroethylcinnamate was reduced to m-aminodihydroethylcinnamate (Entry 15) indicating that conjugated double bonds are also reduced in this system. This was further confirmed by reduction of ethylcinnamate to dihydroethylcinnamate (Entry 16) more efficiently than in an earlier report22 which used an excess of copper salt. Functional group CN (Entry 14) was completely reduced to benzyl amine. Aliphatic nitro group (Entry 17) was unaffected by this catalytic system. Ethyl 3-(3’-aminophenyl)propanoate (Entry 15) obtained in 87% yield using this method forms the building block of 1-pyrazolyl-3-phenylurea-p38-MAP kinase inhibitors (Fig. 2) which are used for the treatment of inflammation and hyperproliferative diseases.23
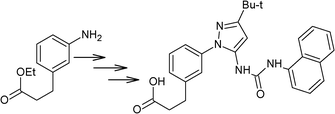 | ||
| Fig. 2 1-Pyrazolyl-3-phenylurea-p38-MAP kinase inhibitor. | ||
To understand the mechanism of the reduction process in this method, some of the known intermediates like nitrosobenzene and azobenzene (Table 4, Entry 19, 20) were subjected to this reduction protocol and were found to reduce completely to aniline as the only product within 0.5 and 1 h respectively. The mechanism (Fig. 3) for the reduction of nitroarenes probably follows both reduction pathways via directly from hydroxyl amine and via azobenzene intermediates. The reduction probably takes place on the active surface of Cu nanoparticles by the liberated hydrogen formed by decomposition of sodium borohydride. The proposed mechanism is further supported by reduction of nitrobenzene using hydrogen gas (60 psi at room temperature) and employing excess active copper (> 1 equiv.) prepared separately from CuBr2 and sodium borohydride.
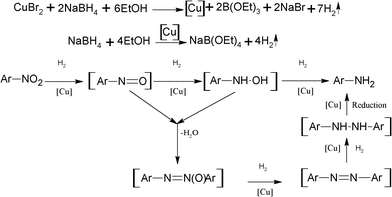 | ||
| Fig. 3 Proposed mechanism. | ||
Conclusions
In conclusion, an efficient method for the reduction of nitroarenes to anilines using copper(II) bromide as a procatalyst for the in situ preparation of active Cu nano using sodium borohydride has been developed. The highlight of the present method is the chemoselective reduction of aromatic nitro groups in the presence of a halo group on the aromatic ring and the inertness of the benzyl ether group during the reduction process. The method is also useful for the reduction of a cyano group and olefin functionality. The experimental simplicity and scalability make the present method an attractive alternative. Further studies for possible asymmetric reduction of trisubstituted olefin will be undertaken soon.Experimental section
General methods
Commercial reagents were purchased from Sigma-Aldrich and used without further purification. Solvents were distilled prior to use. Reactions were monitored by thin layer chromatography (TLC Silica gel 60 F254 purchased from Merck). Column chromatography was performed on silica gel (60–120 mesh). Gas chromatography was done on a Nucon 5765 Gas chromatograph with HP-5 column, N2 as carrier gas and FI detector. 1H NMR (400 MHz) and 13C NMR (100 MHz) were recorded on a Bruker 400 instrument using CDCl3 as the solvent and TMS as an internal standard.Experimental procedure for reduction
Nitrobenzene (1 mmol) and CuBr2 (10 mol%) was dissolved in ethanol (5 mL) and stirred for 2 min and NaBH4 (3–5 mmol) was added slowly (exotherm observed) and stirred at room temperature for 5 h. Reaction was monitored using GC. On completion, the reaction was filtered and solvent was removed using a rotatory evaporator. The crude residue was then partitioned between water (10 mL) and ethylacetate (3 × 15 mL), the combined organic layer was dried over anhydrous sodium sulphate which was filtered off and the solvent was removed using rotatory evaporator to yield the pure product. Products were compared with their authentic samples on TLC, IR, 1H NMR and GC retention time.Acknowledgements
We are pleased to acknowledge financial support from Nano Mission and the Scientific and Engineering Research Council (SERC), Department of Science and Technology (DST) New Delhi, for this investigation. HK is also thankful to UGC & CSIR, New Delhi for the award of Research fellowship.References
- Y. Jang, S. Kim, S. W. Jun, B. H. Kim, S. Hwang, I. K. Song, B. M. Kim and T. Hyeon, Chem. Commun., 2011, 47, 3601–3603 RSC.
- T. Mitsudome, Y. Mikami, M. Matoba, T. Mizugaki, K. Jitsukawa and K. Kaneda, Angew. Chem., Int. Ed., 2012, 51, 136–139 CrossRef CAS.
- (a) H. Blaser, H. Steiner and M. Studer, ChemCatChem, 2009, 1, 210–221 CrossRef CAS; (b) A. Rahman and S.B. Jonnalagadda, Catal. Lett., 2008, 123, 264–268 CrossRef CAS.
- R. V. Jagadeesh, G. Wienhofer, F. A. Westerhaus, A. Surkus, M. Pohl, H. Junge, K. Junge and M. Beller, Chem. Commun., 2011, 47, 10972–10974 RSC.
- Y. Gao, D. Ma, C. Wang, J. Guan and X. Bao, Chem. Commun., 2011, 47, 2432–2434 RSC.
- G. Wienhofer, I. Sorribes, A. Boddien, F. Westerhaus, K. Junge, H. Junge, R. Llusar and M. Beller, J. Am. Chem. Soc., 2011, 133, 12875–12879 CrossRef.
- X. B. Lou, L. He, Y. Qian, Y. M. Liu, Y. Cao and K. N. Fan, Adv. Synth. Catal., 2011, 353, 281–286 CrossRef CAS.
- A. Saha and B. Ranu, J. Org. Chem., 2008, 73, 6867–6870 CrossRef CAS.
- K. Junge, B. Wendt, N. Shaikh and M. Beller, Chem. Commun., 2010, 46, 1769–1771 RSC.
- X. Meng, H. Cheng, S. Fujita, Y. Yu, F. Zhao and M. Arai, Green Chem., 2011, 13, 570–572 RSC.
- L. Pehlivan, E. Metay, S. Laval, W. Dayoub, P. Demonchaux, G. Mignani and M. Lemaire, Tetrahedron, 2011, 67, 1971–1976 CrossRef CAS.
- D. Giomi, R. Alfini and A. Brandi, Tetrahedron, 2011, 67, 167–172 CrossRef CAS.
- R. J. Kalbasi, A.A. Nourbakhsh and F. Babaknezhad, Catal. Commun., 2011, 12, 955–960 CrossRef CAS.
- B. Sreedhar, D. K. Devi and D. Yada, Catal. Commun., 2011, 12, 1009–1014 CrossRef CAS.
- V. Pandarus, R. Ciriminna, F. Beland and M. Pagliaro, Adv. Synth. Catal., 2011, 353, 1306–1316 CrossRef CAS.
- D. He, X. Jiao, P. Jiang, J. Wang and B. Xu, Green Chem., 2012, 14, 111 RSC.
- A. Jagdale and A. Sudalai, Tetrahedron Lett., 2008, 49, 3790–3793 CrossRef CAS.
- For use of Cu and its compounds see (a) K. N. Rao, B. M. Reddy and S. Park, Catal. Commun., 2009, 11, 142–145 CrossRef CAS; (b) S. Yoo and S. Lee, Synlett, 1990, 419–420 CrossRef CAS; (c) S. Ahammed, A. Saha and B. C. Ranu, J. Org. Chem., 2011, 76, 7235 CrossRef CAS; (d) N. Pradhan, A. Pal and T. Pal, Langmuir, 2001, 17, 1800–1802 CrossRef CAS; (e) K. Hanaya, T. Muramatsu and H. Kudo, J. Chem. Soc., Perkin Trans. 1, 1979, 2409–2410 RSC; (f) A. K. Patra, A. Dutta and A. Bhaumik, Catal. Commun., 2010, 11, 651–655 CrossRef CAS; (g) J. Drouin, S. Gauthier, O. Patricola, P. Lanteri and R. Longeray, Synlett, 1993, 791–793 CrossRef CAS; (h) T. Satoh, S. Suzuki, Y. Suzuki, Y. Miyaji and Z. Imai, Tetrahedron Lett., 1969, 10, 4555–4558 CrossRef.
- P. T. Parvatkar, P. S. Parmeswaran and S. G. Tilve, Tetrahedron Lett., 2007, 48, 7870–7872 CrossRef CAS.
- P. T. Parvatkar and S. G. Tilve, Tetrahedron Lett., 2011, 52, 6594–6596 CrossRef CAS.
- A. M. L. Jackelen, M. Jungbauer and G. N. Glavee, Langmuir, 1999, 15, 2322–2326 CrossRef CAS.
- T. Satoh, K. Nanba and S. Suzuki, Chem. Pharm. Bull., 1971, 19, 817–820 CrossRef CAS.
- (a) D. L. Flynn and P. A. Petillo, U.S. Pat. Appl. Publ. 2007, US20070191336 Search PubMed; (b) D. L. Flynn and P. A. Petillo, PCT Int. Appl. 2006, WO2006081034; (c) D. L. Flynn and P. A. Petillo, U.S. Pat. Appl. Publ. 2005, US20050288286 Search PubMed.
- M. Amatore, C. Gosmini and J. Perichon, J. Org. Chem., 2006, 71, 6130–6134 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: spectral data of selected reduction products are available along with XRD, SEM, EDX & TEM images of recovered copper catalyst after nitro reduction. See DOI: 10.1039/c2ra20371e |
| This journal is © The Royal Society of Chemistry 2012 |