Growth of flower-like CdSe dendrites from a Brønsted acid–base ionic liquid precursor†
Jianmin
Ma
a,
Wei
Guo
b,
Xiaochuan
Duan
b,
Taihong
Wang
a,
Wenjun
Zheng
*b and
Liao
Chang
*c
aKey Laboratory for Micro-Nano Optoelectronic Devices of Ministry of Education, State Key Laboratory for Chemo/Biosensing and Chemometrics, Hunan University, Changsha, 410082, P. R. China
bDepartment of Materials Chemistry, Key Laboratory of Advanced Energy Materials Chemistry (MOE), and TKL of Metal and Molecule-Based Material Chemistry, College of Chemistry, Nankai University, Tianjin, 300071 P. R. China. E-mail: zhwj@nankai.edu.cn
cPaleomagnetic Laboratory ‘Fort Hoofddijk’, Department of Earth Sciences, Utrecht University, Budapestlaan 17, 3584 CD Utrecht, The Netherlands. E-mail: L.Chang@uu.nl
First published on 12th June 2012
Abstract
Flower-like CdSe dendrites were prepared from a mixed solution of water, ethanol and ionic liquid based on formic acid and N,N-dimethylformamide. Experimental results show that the growth of flower-like CdSe dendrites is not only determined by the inherent polar structure but also strongly dependent on the type of solvents.
Recently, ionic liquids (ILs) have attracted considerable interest in various fields due to their unique properties.1 More importantly, the properties of ILs could be tuned by changing the structures of their cations and anions, which further promotes the unique features and application of ILs in complex systems.2,3 The collection of these properties has motivated their widespread use in catalysis and organic/inorganic synthesis.4–6 Ionic liquids with designable features offer exciting approaches for achieving desired properties or structures.7–9 Many inorganic nanostructures have been fabricated via various IL-involved processes.10–20 For example, our group synthesized tellurium nanowire bundles using the novel aqueous ionic liquid composed of triethanolamine (TEA) and tetrafluoroboric acid (HBF4) as the reducing agent and structure-directing agent.18 The advantages of ILs for the synthesis of novel nanostructures have been realized. These promising works inspired us to explore a simple IL system for controlled growth of inorganic materials.
Recently, Brønsted acid–base ILs have been subjected to extensive studies because of their simplicity, low viscosity, low cost and reactivity.21–23 Dai et al.24 developed a novel class of proton-transfer ILs whose Brønsted bases are composed of N,N-dimethylformamide (DMF). Considering the solvent characteristics of DMF (e.g., high dielectric constant, low viscosity, wide liquid range, etc.) and chemical reactivity, we aim to synthesize CdSe nanostructures from this protonated DMF-based ionic liquid. Synthesizing CdSe materials is important for many reasons: i) CdSe is one of most attractive semiconductor materials with widely potential applications in fabricating nanoscale electronic, photonic, electromechanical, and biomedical devices;25–28 ii) as an ideal system, CdSe is used to verify the kinetic model of particle growth in solution,29–33 which further directs the synthesis of additional analogue semiconductor materials (e.g., CdS, CdTe, etc.); iii) the wurtzite-structured CdSe crystal consists of nonpolar planes and polar planes,34–36 whose surface energy could be varied by strong electrostatic interactions between the ions of the ionic liquid and the polar surfaces; iv) CdSe can act as a template for directing growth of other materials, such as β-Ag2Se, ZnSe and Cu2Se;37–39 vi) although there are many reports on synthetic methods for CdSe nanostructures (quantum dots,40 nanorods,41 nanowires,42,43 nanobelts,44 dendrites45), it is still meaningful to study novel route to preparation of CdSe nanomaterials for its importance in nanoscience and nanotechnologies. Therefore, it is crucial to develop novel synthetic approaches to control the morphology of CdSe nanostructures.
In this study, we report the synthesis of flower-like CdSe dendrites using a Brønsted acid–base ionic liquid as reducing reagent, solvent, and structure-directing reagent. The formation mechanism of flower-like dendrites is discussed from the point of view of the polar structure of CdSe crystals and the characteristics of the ionic liquid. This reaction system could be further explored to prepare other inorganic materials.
The flower-like CdSe dendrites were synthesized under solvothermal conditions at 150 °C for 24 h, using a mixed solution of water, ethanol, an ionic liquid based on formic acid and N,N-dimethylformamide, cadmium chloride and selenium dioxide as solvents, cadmium and selenium sources, respectively. The crystal structure and phase composition of the CdSe products were first characterized using X-ray diffraction (XRD) analysis. Fig. 1 displays a representative XRD pattern of the as-prepared CdSe samples. The diffraction peaks can be readily indexed to the hexagonal phase of CdSe (JCPDS card, No. 77-2307) with lattice parameters of a = 4.299 Å and c = 7.01 Å. Moreover, the strong and sharp diffraction peak (002) suggests that the crystals grow preferably along the c-axis.46,47 The sharp diffraction peaks also indicate that the products are highly crystalline. In addition, the elemental composition of the flower-like CdSe nanostructures was further investigated by an energy dispersive X-ray (EDX) system attached to the transmission electron microscopy (TEM) (Fig. S1†). In the spectrum, only Cd and Se are observed except for the C and Cu signals that originate from the Cu grid film. Based on the relative areas of the peaks of Cd and Se, the atomic ratio of Cd to Se is calculated to be about 49.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50.4, close to 1
50.4, close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
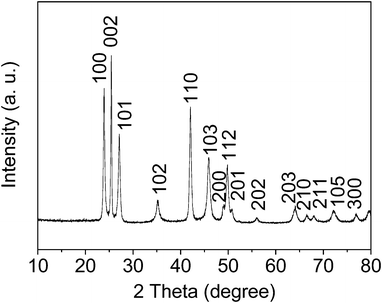 | ||
| Fig. 1 XRD pattern of the as-prepared flower-like CdSe dendrites synthesized under solvothermal conditions with a mixed solution composed of 20 ml ethanol and 5 ml ionic liquid based on formic acid (HCOOH, 88%) and N,N-dimethylformamide (DMF) with the same molar ratio at 150 °C for 24 h. | ||
The morphology and microstructure of the as-synthesized flower-like CdSe nanostructures were further characterized by scanning electron microscopy (SEM) and TEM. Fig. 2a shows the abundance and uniformity of CdSe hierarchical flower-like nanostructures. The high-resolution SEM image shows an individual CdSe microcrystal with a hierarchical flower-like nanostructure (Fig. 2b). Fig. 2c and d show the detailed structures of the part c and d flower-like CdSe dendrite, respectively. The clear lattice fringes in Fig. 2c and d in HR-TEM images indicate the growth of high quality single-crystalline CdSe. Moreover, its single crystallinity was further confirmed by the regular hexagonal patterns in the fast Fourier transform (FFT) of high-resolution TEM images (insets in Fig. 2c and d).
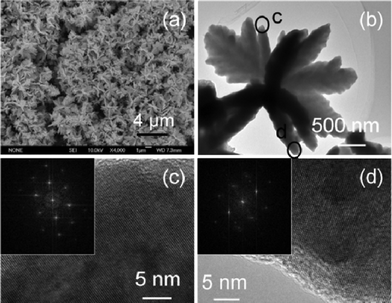 | ||
| Fig. 2 (a) SEM image; (b) TEM image; (c) HR-TEM image of circled c in (b) and FFT pattern (inset in (c)); and (d) HR-TEM image of circled d in (b) and FFT pattern (inset in (d)) of the flower-like CdSe dendrites synthesized in mixed solution composed of 20 ml ethanol and 5 ml ionic liquid based on formic acid (HCOOH, 88%) and N,N-dimethylformamide (DMF) with the same molar ratio at 150 °C for 24 h. | ||
To explain the formation mechanism of flower-like CdSe dendrites, we first consider that the wurtzite-structured CdSe crystal could be structurally described as a number of alternating planes composed of four-fold tetrahedrally-coordinated Se2− and Cd2+ ions stacked alternatively along the c-axis,12 similar to the wurtzite-structured ZnO.48,49 The wurtzite-structured CdSe crystals are composed of nonpolar planes and polar planes.12 In our experiment, the key strategy to control the morphology of CdSe nanomaterials is to vary the surface energy of the polar surfaces by strong electrostatic interactions between the ions of the ionic liquid and the polar surfaces. The orientation growth of the CdSe can be realized by controlling concentration of ionic liquid [DMFH]+ [HCOO]−. With low concentration, the coordination between the Cd2+ ions in the (0001) surface (the Se2− ions in the (0001)) and [HCOO]− ions ([DMFH]+ ions) formed during the processes of nucleation and growth of CdSe. Thus, the CdSe nanocrystals formed first because of the charge interaction between polar planes and moieties of ionic liquid. However, CdSe nanocrystals can further aggregate along the polar directions to form flower-like dendrites by strong electrostatic interactions between the polar surfaces, when moieties of ionic liquid separate from the polar planes of CdSe due to the relatively weak coordinating effects. Dipole–dipole interactions between individual CdSe nanocrystals then induce self-assembly and fusion, producing flower-like dendrites based on the anisotropy in the interactions.50 This is consistent with XRD and HR-TEM results and is further verified from the evolution of flower-like CdSe dendrites formation (Fig. 3). During this process, there is not any solid product during the first four hours, which indicates that the inducing growth of CdSe crystals is long in the present reaction system. After 8 h reaction, there are some dendrites and particles (Fig. 3a). It is well known that the wurtzite-structured CdSe has a preference to form dendrites rather than particles.51,52 When the reaction was terminated after 18 h, the well-defined flower-like dendrites formed and particles completely disappeared. In addition, we found that there is clear difference in the morphologies of the flower-like dendrites (Fig. 3a–c). This indicates that there is an Ostwald ripening process during the growth of flower-like CdSe dendrites. It has been suggested that the formation mechanism of dendrites can be explained by the diffusion-limited aggregation (DLA) model.53 In this model, the randomly moving nuclei formed in such reduction solution can accumulate with each other to form kinetically roughened dendritic structures. Our experimental results seem dissimilar to this model. The reaction would be accelerated with higher concentration of [DMFH]+ [HCOO]−. However, nanoparticles rather than dendrites were produced (Fig. S2†). This may be due to the strong charge interaction between polar planes of CdSe and moieties of ionic liquid, which completely inhibited particle aggregation.
 | ||
| Fig. 3 Evolution process of the as-prepared flower-like CdSe dendrites synthesized under solvothermal conditions with a mixed solution composed of 20 ml ethanol and 5 ml ionic liquid based on formic acid (HCOOH, 88%) and N,N-dimethylformamide (DMF) with the same molar ratio at 150 °C for different times: (a) 8 h; (b) 12 h and (c) 18 h. | ||
In addition to the concentration of ionic liquid, ionic liquid and ethanol also play important roles during the formation process of flower-like CdSe dendrites. When ionic liquid was replaced by the same volume of DMF, irregular microspheres (Fig. S3a†) formed. This implies that the flower-like CdSe dendrites were formed under the combined effect of moieties of ionic liquid [DMFH]+ [HCOO]−. When ethanol (ε = 25.3) was replaced by the same volume of 1-butanol (ε = 17.08), sword-like dendrites (Fig. S3b†) were produced. Irregular aggregates of nanoparticles (Fig. S4†) were formed when ethanol was replaced by the same volume of water (ε = 80.1). These results indicate that the interaction between polar planes of CdSe and moieties of ionic liquid [DMFH]+ [HCOO]− can be altered by changing the polarity of the solvent. In summary, variation in the solvent composition resulted in a series of morphologies with three-dimensional structures.
In summary, novel flower-like CdSe dendrites have been synthesized by optimizing the reaction parameters by a Brønsted acid–base ionic liquid-assisted method. Formation of flower-like dendrites depends on the interaction between the polar structure of CdSe crystals and the ionic liquid. This study suggests that ionic liquid is an important factor in the formation of flower-like CdSe dendrites. Such a Brønsted acid–base ionic liquid method may be extended to synthesize other inorganic materials.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 20971070, 21073095 and 21003041) and MOE (No. RT0927).References
- M. Antonietti, D. B. Kuang, B. Smarsly and Y. Zhou, Angew. Chem., Int. Ed., 2004, 43, 4988 CrossRef CAS.
- L. Y. Xiao, S. J. Zhang and X. M. Lu, J. Chem. Eng. Data, 2007, 52, 596 CrossRef.
- P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley-VCH, Weinheim, 2008 Search PubMed.
- H. Olivier-Bourbigou, L. Magna and D. Morvan, Appl. Catal., A, 2010, 373, 1 CrossRef CAS.
- Z. Ma, J. H. Yu and S. Dai, Adv. Mater., 2010, 22, 261 CrossRef CAS.
- S. Toma, M. Meciarova and R. Sebesta, Eur. J. Org. Chem., 2009, 321 CrossRef CAS.
- J. M. Ma, X. D. Liu, J. B. Lian, X. C. Duan and W. J. Zheng, Cryst. Growth Des., 2010, 10, 2522 CAS.
- J. M. Ma, L. Chang, J. B. Lian, Z. Huang, X. C. Duan, X. D. Liu, P. Peng, T. I. Kim, Z. F. Liu and W. J. Zheng, Chem. Commun., 2010, 46, 7133 RSC.
- J. M. Ma, X. C. Duan, J. B. Lian, T. I. Kim, P. Peng, X. D. Liu, Z. F. Liu, H. B. Li and W. J. Zheng, Chem.–Eur. J., 2010, 16, 13210 CrossRef CAS.
- H. Kaper, F. Endres, I. Djerdj, M. Antonietti, B. M. Smarsly, J. Maier and Y. S. Hu, Small, 2007, 3, 1753 CrossRef CAS.
- B. G. Trewyn, C. M. Whitman and V. S. Y. Lin, Nano Lett., 2004, 4, 2139 CrossRef CAS.
- A. Taubert, Angew. Chem., Int. Ed., 2004, 43, 5380 CrossRef CAS.
- J. M. Zhu, Y. H. Shen, A. J. Xie, L. G. Qiu, Q. Zhang and S. Y. Zhang, J. Phys. Chem. C, 2007, 111, 7629 CAS.
- M. Green, P. Rahman and D. Smyth-Boyle, Chem. Commun., 2007, 574 RSC.
- J. M. Ma, T. H. Wang, X. C. Duan, J. B. Lian, Z. F. Liu and W. J. Zheng, Nanoscale, 2011, 3, 4372 RSC.
- J. M. Ma, Z. F. Liu, J. B. Lian, X. C. Duan, T. I. Kim, P. Peng, X. D. Liu, Q. Chen, G. Yao and W. J. Zheng, CrystEngComm, 2011, 13, 3072 RSC.
- M. A. B. H. Susan, A. Noda, S. Mitsushima and M. Watanabe, Chem. Commun., 2003, 938 RSC.
- J. M. Ma, J. B. Lian, X. C. Duan, Z. F. Liu, P. Peng, X. D. Liu, T. I. Kim and W. J. Zheng, CrystEngComm, 2011, 13, 2774 RSC.
- Y. Zhou and M. Antonietti, Adv. Mater., 2003, 15, 1452 CrossRef CAS.
- N. Zilkova, A. Zukal and J. Cejka, Microporous Mesoporous Mater., 2006, 95, 176 CrossRef CAS.
- M. Yoshizawa, W. Xu and C. A. Angell, J. Am. Chem. Soc., 2003, 125, 15411 CrossRef CAS.
- W. Xu and C. A. Angell, Science, 2003, 302, 422 CrossRef CAS.
- A. Noda, M. A. B. H. Susan, K. Kudo, S. Mitsushima, K. Hayamizu and M. Watanabe, J. Phys. Chem. B, 2003, 107, 4024 CrossRef CAS.
- J. F. Huang, G. A. Baker, H. Luo, K. L. Hong, Q. F. Li, N. J. Bjerrumc and S. Dai, Green Chem., 2006, 8, 599 RSC.
- Y. Cui, Q. Wei, H. K. Park and C. M. Lieber, Science, 2001, 293, 1289 CrossRef CAS.
- Y. Huang, X. Duan, Y. Cui, L. J. Lauhon, K. Kim and C. M. Lieber, Science, 2001, 294, 1313 CrossRef CAS.
- M. S. Gudiksen, L. J. Lauhon, J. F. Wang, D. C. Smith and C. M. Lieber, Nature, 2002, 415, 617 CrossRef CAS.
- X. F. Duan, Y. Huang, R. Agarwal and C. M. Lieber, Nature, 2003, 421, 241 CrossRef CAS.
- X. Peng, J. Wickham and A. P. Alivisatos, J. Am. Chem. Soc., 1998, 120, 5343 CrossRef CAS.
- C. R. Bullen and P. Mulvaney, Nano Lett., 2004, 4, 2303 CrossRef CAS.
- C. Xie, H. Hao, W. Chen and J. Wang, J. Cryst. Growth, 2008, 310, 3504 CrossRef CAS.
- X. C. Duan, X. D. Liu, Q. Chen, H. B. Li, J. Li, X. Hu, Y. Y. Li, J. M. Ma and W. J. Zheng, Dalton Trans., 2011, 40, 1924 RSC.
- L. Manna, E. C. Scher and A. P. Alivisatos, J. Am. Chem. Soc., 2000, 122, 12700 CrossRef CAS.
- C. Ma, Y. Ding, D. Moore, X. D. Wang and Z. L. Wang, J. Am. Chem. Soc., 2004, 126, 708 CrossRef CAS.
- Y. Ding, C. Ma and Z. L. Wang, Adv. Mater., 2004, 16, 1740 CrossRef CAS.
- Z. F. Feng, Q. B. Zhang, L. L. Lin, H. H. Guo, J. Z. Zhou and Z. H. Lin, Chem. Mater., 2010, 22, 2705 CrossRef CAS.
- U. Jeong, J. U. Kim, Y. N. Xia and Z. Y. Li, Nano Lett., 2005, 5, 937 CrossRef CAS.
- D. H. Son, S. M. Hughes, Y. D. Yin and A. P. Alivisatos, Science, 2004, 306, 1009 CrossRef CAS.
- H. B. Li, M. Zanella, A. Genovese, M. Povia, A. Falqui, C. Giannini and L. Manna, Nano Lett., 2011, 11, 4964 CrossRef CAS.
- L. L. Chen, H. Bao, T. Z. Tan, O. V. Prezhdo and X. L. Ruan, J. Phys. Chem. C, 2011, 115, 11400 CAS.
- (a) J. D. Doll, G. Pilania, R. Ramprasad and F. Papadimitrakopoulos, Nano Lett., 2010, 10, 680 CrossRef CAS.
- N. Shpaisman, U. Givan and F. Patolsky, ACS Nano, 2010, 4, 1901 CrossRef CAS.
- Z. F. Feng, Q. B. Zhang, L. L. Lin, H. H. Guo, J. Z. Zhou and Z. H. Lin, Chem. Mater., 2010, 22, 2705 CrossRef CAS.
- C. Liu, P. C. Wu, T. Sun, L. Dai, Y. Ye, R. M. Ma and G. G. Qin, J. Phys. Chem. C, 2009, 113, 14478 CAS.
- X. C. Duan, X. D. Liu, Q. Chen, H. B. Li, J. Li, X. Hu, Y. Y. Li, J. M. Ma and W. J. Zheng, Dalton Trans., 2011, 40, 1924 RSC.
- L. F. Xi and Y. M. Lam, J. Colloid Interface Sci., 2007, 316, 771 CrossRef CAS.
- L. L. Zhao, T. Z. Lu, M. Yosef, M. Steinhart, M. Zacharias, U. Gösele and S. Schlecht, Chem. Mater., 2006, 18, 6094 CrossRef CAS.
- Z. H. Li, Y. X. Luan, Q. Z. Wang, G. S. Zhuang, Y. X. Qi, Y. Wang and C. G. Wang, Chem. Commun., 2009, 6273 RSC.
- X. Zhou, Z. X. Xie, Z. Y. Jiang, Q. Kuang, S. H. Zhang, T. Xu, R. B. Huang and L. S. Zheng, Chem. Commun., 2005, 5572 RSC.
- J. P. Xie, Q. B. Zhang, J. Y. Lee and D. I. C. Wang, J. Phys. Chem. C, 2007, 111, 17158 CAS.
- W. Z. Zhong, Y. Q. Zheng, Z. L. Ding, E. W. Shi and S. K. Hua, J. Synth. Cryst., 2003, 32, 91 CAS.
- H. W. Hou, Q. Yang, C. R. Tan, X. B. Tian and Y. Xie, Mater. Lett., 2005, 59, 3364 CrossRef CAS.
- T. Witten and L. M. Sander, Phys. Rev. Lett., 1981, 47, 1400 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: See DOI: 10.1039/c2ra20315d |
| This journal is © The Royal Society of Chemistry 2012 |
