Shaping up: spontaneous formation of ordered mesoscopic salt bowls†
Katla Sai
Krishna‡
,
Bosukonda V. V. S. Pavan
Kumar
and
Muthusamy
Eswaramoorthy
*
Nanomaterials and Catalysis Laboratory, Chemistry and Physics of Materials Unit and DST Unit on Nanoscience, Jawaharlal Nehru Centre for Advanced Scientific Research, Jakkur P.O., Bangalore, India 560064. E-mail: eswar@jncasr.ac.in; Fax: (+91)80-220-2766; Tel: (+91)80-2208-2870
First published on 10th May 2012
Abstract
The spontaneous formation of hexagonally organized, mesoscopic salt-bowls is reported here by a simple heating of manganous sulphate salt with a polyvinylpyrrolidone polymer. The crystallization of salt around the gas bubble during the decomposition of polymer leaves strikingly beautiful mesoscopic structures.
The synthesis of inorganic materials with complex patterns will have significant implications in separation, catalysis and biomedicine.1 Inorganic biominerals are known to display stunning structural patterns at different length scales, defying their rigid geometric symmetry.2–4 Mimicking such complex forms in inorganic synthesis is still a challenging task, though the guiding principles involved in the formation of many biological minerals more understood now than ever before. Nevertheless, some progress has been made in generating inorganic structures with complex forms and patterns by mimicking the biological design principles.5 Synergistic synthesis, transcriptive synthesis, metamorphic reconstruction and microphase separation mechanisms are usually identified with inorganic morphosynthesis.1,6 For example, the synergistic assembly of organic and inorganic molecules has been the basis for the formation of zeolites and meso-structured materials.7 To take the complexity to higher levels, vesicle-based synthesis, in which nature masters the art of patterning skeletons at multiple length-scales in a spatio–temporal process, has been used to create mesoscale inorganic structures of different shapes and patterns.8 Assembly of inorganic nanoparticles into mesoscale structures was possible by template directed or surfactant/polymer mediated self-assembly process.9–11 Similarly, individual components of micrometer and millimeter sizes have also been tailored to self-assemble (mesoscale self-assembly) into complex 3-D structures through capillary, electrostatic, optical, gravitational or magnetic interactions.11 However, the great challenge in making individual components of different shapes other than spherical and plate like forms limit this strategy in microfabrication.12
Here we report a spontaneous formation of ordered, mesoscale structures made up of inorganic salt bowls. These tiny bowls are generating a lot of interest due to their unique application as ‘containers’ to hold ultra-low volumes.13–20 Arrays of these ultra-low volume containers are applied in molecular biology21 for screening and the bio-sensing of proteins and DNA. The spontaneously formed salt bowls through our simple synthesis are water-soluble and are used as a template to obtain gold bowls.
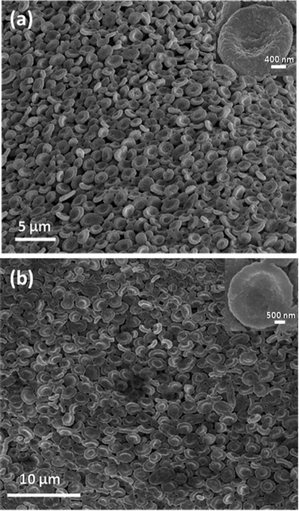
Fig. 1a shows the scanning electron microscope (SEM) image of an array of manganous sulfate salt bowls obtained after calcination of the MnSO4-PVP composite film at 550 °C for 5 h. Since the decomposition temperature of the MnSO4 salt is around 850 °C, MnSO4 doesn't undergo any change, except the loss of water of crystallization from the precursor MnSO4·H2O. Calcination removes the polymer, PVP, leaving the film composed of salt bowls to curl (see ES1†).
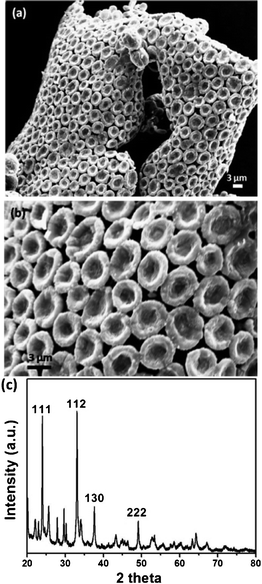 | ||
| Fig. 1 (a) SEM image of an ordered array of MnSO4 bowls obtained after calcination of the MnSO4–PVP composite film at 550 °C for 5 h. (b) Higher magnification SEM image showing the closer-view of the hexagonally-ordered array of MnSO4 bowls. (c) PXRD pattern of the MnSO4 bowls. | ||
The transmission electron microscope (TEM) image shows that each bowl is made up of several smaller particles (ESI, Fig. S2†). The electron diffraction (ED) pattern confirms the polycrystalline nature of a single bowl (ESI, Fig. S3†). The powder X-ray diffraction (PXRD) pattern of the calcined sample confirms that the bowls are made up of crystalline MnSO4 salt (Fig. 1c). The mesoscopic bowls of around 3 to 5 μm are exquisitely arranged in a hexagonal pattern, though in many cases the bowls are not in direct contact with the six neighbouring bowls, as shown in Fig. 1b. In some cases the rims of the bowls are not perfectly circular. The wall thickness of the bowls is in the range of 500–700 nm and the depth of the bowls is about 500 nm.
To understand the formation mechanism of the ionic salt into mesoscopic bowls upon removal of the polymer, calcination of the composite film is carried out at different temperatures. Fig. 2 and 3 show the field-emission scanning electron microscope (FESEM) images of various structures formed during the calcination processes. Calcination at 200 °C for 5 h shows the formation of flakes several microns in size (∼20 to 200 μm) originating from the cracking of a large film which is quite understandable because, at that temperature neither the polymer nor MnSO4 salt decompose. Thermo-gravimetric analysis (TGA) (ESI, Fig. S4†) shows that the degradation of polymer starts at 370 °C and completes at around 470 °C. The composite film calcined at 370 °C for 5 h and showed the signature of ball-like structures embedded within the matrix which probably emerged from the partial crystallization of the MnSO4 salt around the gas bubbles generated by the decomposition of the polymer. At 420 °C for 5 h, further crystallization of salt in association with the removal of the carbonaceous layer leaves the bubbles burst open at the top, leading to the formation of a large number of hemi-spherical structures (ESI, Fig. S5†). TGA shows that only about 50% of the polymer was removed at this temperature. Further heating of this composite to 550 °C for 5 h gives rise to hexagonally organized bowls (Scheme in Fig. 4). There are regions in which the bowls are less organized, probably due to unevenness on the surface of the initial PVP-MnSO4 film (ESI, Fig. S6†).
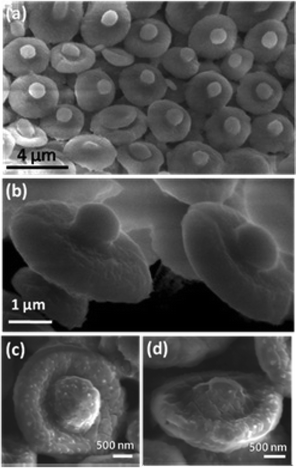 | ||
| Fig. 2 FESEM images of Ball-in-bowl forms of MnSO4 evolved during calcination of the composite film at 470 °C for 5 h. (a) & (c) showing the top-view, (b) & (d) showing the side-view of the bowls. | ||
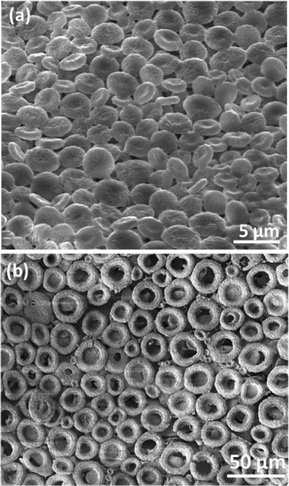 | ||
| Fig. 3 (a) Circular discs with partial dents in their center formed by calcination at 470 °C for 5 h. (b) Ring shaped structures formed at 550 °C for 5 h. | ||
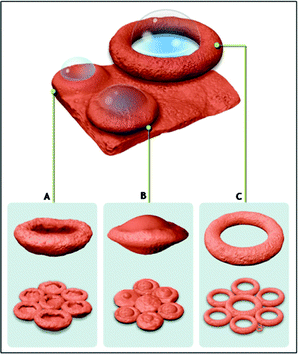 | ||
| Fig. 4 Scheme showing formation process of different MnSO4 morphologies through gas-bubble template method, Scheme A: Bowls, Scheme B: Ball-in-bowl shaped structures, Scheme C: Ring shaped structures (for convenience, all morphologies are shown to be evolved from the same film). | ||
In some cases, the crystallization of the manganous sulphate salt around the bubbles deforms them to oblate spheroids (ESI, Fig. S7†) which are not able to burst open while heating. Instead, these spheroids shrink inwards during polymer removal to form a disc-shaped morphology (Fig. 3a). If the thickness of the spheroid wall is high due to salt crystallization, the buckling will not be complete, leading to ball-in-bowl shaped structures (Fig. 2, Scheme B in Fig. 4 and Fig. S8, ESI†). On the other hand, if the bubbles formed during calcination are larger than the thickness of the film, hexagonally arrayed ring like structures of diameter around 20 microns are obtained (Fig. 3b and Scheme C in Fig. 4).
The effect of solvent on the shape formation is checked by replacing ethanol with water in the synthesis step which results in ring-shaped structures (ESI, Fig. S9†). When the reaction was carried out with more polymer, the MnSO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PVP ratio (wt/wt) was 1
PVP ratio (wt/wt) was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, no bowl formation was observed, instead a flaky solid product was obtained. On the other hand, when the MnSO4:PVP ratio was 1
4, no bowl formation was observed, instead a flaky solid product was obtained. On the other hand, when the MnSO4:PVP ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the bowl morphology was retained, indicating the optimum salt concentration necessary for crystallization to occur around the bubble during shaped structures, but yielding micron-sized irregular structures. Similarly, when the polymer PVP was replaced with polyvinylalcohol (PVA), calcination of the composite at 550 °C for 5 h yielded MnSO4 with large, ring-shaped tubular structures with diameters of 20 to 30 microns (ESI, Fig. S10†). This suggests that the carbonaceous film formed during decomposition of the polymer plays an important role in controlling the bubble formation around which the crystallization of the salt occurs.
1, the bowl morphology was retained, indicating the optimum salt concentration necessary for crystallization to occur around the bubble during shaped structures, but yielding micron-sized irregular structures. Similarly, when the polymer PVP was replaced with polyvinylalcohol (PVA), calcination of the composite at 550 °C for 5 h yielded MnSO4 with large, ring-shaped tubular structures with diameters of 20 to 30 microns (ESI, Fig. S10†). This suggests that the carbonaceous film formed during decomposition of the polymer plays an important role in controlling the bubble formation around which the crystallization of the salt occurs.
Considering the solubility of the MnSO4 salt in water, use of these MnSO4 bowls as water soluble template is carried out for the generation of gold bowls. For this, gold is sputter-coated onto the MnSO4 bowls to form a thick layer (∼60 nm) followed by dipping them in water for 1 h to remove the salt. Fig. 5a shows a FESEM image of the gold coated MnSO4 bowls. The hollow gold bowls (Fig. 5b and Fig. S11, ESI†) formed after the removal of MnSO4 core clearly demonstrate the possibility of using these MnSO4 bowls as water soluble template to prepare other metal and metal oxide bowls.
 | ||
| Fig. 5 (a) Gold-coated MnSO4 bowls (b) Gold bowls formed after the removal of MnSO4 salt core. | ||
In summary, manganous sulphate salt micro-bowls have been successfully synthesized by a gas-bubble template method. These bowls are spontaneously formed as hexagonal arrays during the synthesis stage and can be used as ultra-low volume containers. Further, because of their solubility in water, these bowls can be successfully used as templates to make gold bowls.
References
- S. Mann and G. A. Ozin, Nature, 1996, 382, 313 CrossRef CAS.
- G. A. Ozin, Acc. Chem. Res., 1997, 30, 17 CrossRef CAS.
- S. Mann, Angew. Chem., Int. Ed., 2000, 39, 3392 CrossRef CAS.
- C. Sanchez, H. Arribart and M. G. Guille, Nat. Mater., 2005, 4, 277 CrossRef CAS.
- S. Mann, Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry, Oxford University Press, Oxford, 2001 Search PubMed.
- H. Yang, N. Coombs and G. A. Ozin, Nature, 1997, 386, 692 CrossRef CAS.
- C. T. Kresge, M. Leonowicz, W. J. Roth, J. C. Vartuli and J. C. Beck, Nature, 1992, 359, 710 CrossRef CAS.
- E. Dujardin and S. Mann, Adv. Eng. Mater., 2002, 4, 461 CrossRef CAS.
- M. Li, H. Schnablegger and S. Mann, Nature, 1999, 402, 393 CrossRef CAS.
- H. Cölfen and S. H. Yu, MRS Bull., 2005, 30, 727 CrossRef.
- M. Boncheva and G. M. Whitesides, MRS Bull., 2005, 30, 736 CrossRef CAS.
- G. M. Whitesides and M. Boncheva, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4769 CrossRef CAS.
- K. S. Krishna, U. Mansoori, N. R. Selvi and M. Eswaramoorthy, Angew. Chem., Int. Ed., 2007, 46, 5962 CrossRef CAS.
- J. Dinesh, U. Mansoori, P. Mandal, A. Sundaresan and M. Eswaramoorthy, Angew. Chem., Int. Ed., 2008, 47, 7685 CrossRef.
- N. S. John, N. R. Selvi, M. Mathur, R. Govindarajan and G. U. Kulkarni, J. Phys. Chem. B, 2006, 110, 22975 CrossRef CAS.
- R. J. Jackman, D. C. Duffy, E. Ostuni, N. D. Willmore and G. M. Whitesides, Anal. Chem., 1998, 70, 2280 CrossRef CAS.
- J. E. Barton and T. W. Odom, Nano Lett., 2004, 4, 1525 CrossRef CAS.
- S. H. Im, U. Jeong and Y. Xia, Nat. Mater., 2005, 4, 671 CrossRef.
- X. D. Wang, E. Graugnard, J. S. King, Z. L. Wang and C. J. Summers, Nano Lett., 2004, 4, 2223 CrossRef CAS.
- J. Zhang, S. S. Wang, S. D. Zhang, Q. H. Tao, L. Pan, Z. Y. Wang and Z. P. Zhang, J. Phys. Chem. C, 2011, 115, 20061 CAS.
- Y. Rondelez, G. Tresset, K. V. Tabata, H. Arata, H. Fujita, S. Takeuchi and H. Noji, Nat. Biotechnol., 2005, 23, 361 CrossRef CAS.
Footnotes |
| † Electronic Supplementary Information (ESI) available: Experimental details, TGA and XRD graphs, FESEM images of intermediate structures. See DOI: 10.1039/c2ra20596c/ |
| ‡ Present address: Center for Advanced Microstructures and Devices, Louisiana State University, Baton Rouge, USA 70806 |
| This journal is © The Royal Society of Chemistry 2012 |
