Polyacrylic acid polymer modulates the UCST-type phase behavior of ionic liquid and water†
Awanish
Kumar
,
P. Madhusudhana
Reddy
and
Pannuru
Venkatesu
*
Department of Chemistry, University of Delhi, Delhi, 110 007. E-mail: venkatesup@hotmail.com; pvenkatesu@chemistry.du.ac.in; Fax: +91-11-2766 6605; Tel: +91-11-27666646-142
First published on 25th May 2012
Abstract
Upper critical solution temperature (UCST)-type phase behavior was observed in the critical mixture of water (W) and hydrophobic ionic liquid, 1-hexyl-3-methylimidazolium tetrafluoroborate (IL), by refractive index and fluorescence measurements. The phase separation temperature depends on the composition of IL as well as water. Furthermore, for the first time, the behavior of high molecular weight polymer polyacrylic acid, PAA (Mw = 450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1), as an impurity in the UCST mixture was investigated by simultaneous refractive index (n) measurements for both the coexisting phases of ILW. The refractive index in each phase of ILW as well as three different PAA concentrations (C = 0.5, 1.0, and 1.5 mg cm−3) in the near critical composition of ILW have been measured at temperatures below the system's upper critical point. We observed that the polymer significantly affected on the critical region of ILW. The phase-transition region of coexisting phases of ILW significantly shifts down with an increase in the concentrations of PAA. The Tc values decreases linearly from 58.256 (free of PAA) to 55.168 °C (PAA in ILW) with increasing PAA concentrations in ILW. This indicates that the polymer chain entangles with the coexisting phases, thereby the polymer monomers strongly interact with neighbor solvent particles. At temperatures T close enough to Tc, the critical exponent (β) values were obtained from the measured n values of both the coexisting liquid phases and was found to increase from 0.332 to 0.379, when the PAA concentration changes from 0.5 to 1.5 mg cm−3. The obtained 3D Ising values are modified in the presence of PAA and obviously belong to the renormalized class. These values are higher than that of 0.326 ± 0.002 of pure ILW, which is compatible with the 3D Ising value β = 0.325. In addition, we have performed the fluorescence measurement for the determination of Tc for ILW and in the presence of PAA as an impurity. The observed increase in the fluorescence intensities with temperature predicts unambiguously the formation of the solvation structure at Tc for the critical mixture in presence of the polymer.
000 g mol−1), as an impurity in the UCST mixture was investigated by simultaneous refractive index (n) measurements for both the coexisting phases of ILW. The refractive index in each phase of ILW as well as three different PAA concentrations (C = 0.5, 1.0, and 1.5 mg cm−3) in the near critical composition of ILW have been measured at temperatures below the system's upper critical point. We observed that the polymer significantly affected on the critical region of ILW. The phase-transition region of coexisting phases of ILW significantly shifts down with an increase in the concentrations of PAA. The Tc values decreases linearly from 58.256 (free of PAA) to 55.168 °C (PAA in ILW) with increasing PAA concentrations in ILW. This indicates that the polymer chain entangles with the coexisting phases, thereby the polymer monomers strongly interact with neighbor solvent particles. At temperatures T close enough to Tc, the critical exponent (β) values were obtained from the measured n values of both the coexisting liquid phases and was found to increase from 0.332 to 0.379, when the PAA concentration changes from 0.5 to 1.5 mg cm−3. The obtained 3D Ising values are modified in the presence of PAA and obviously belong to the renormalized class. These values are higher than that of 0.326 ± 0.002 of pure ILW, which is compatible with the 3D Ising value β = 0.325. In addition, we have performed the fluorescence measurement for the determination of Tc for ILW and in the presence of PAA as an impurity. The observed increase in the fluorescence intensities with temperature predicts unambiguously the formation of the solvation structure at Tc for the critical mixture in presence of the polymer.
I. Introduction
The mutual miscibility of liquid–liquid mixtures has been well characterized by a critical phenomenon. The temperature where phase separation occurs for liquid mixtures is attributed by a critical temperature (Tc). Liquid–liquid extraction has often been a favored choice for the development of separation processes. In this context, ionic liquids (ILs), a class of environmental friendly solvents and greener substitutes of volatile organic compounds, are used in a wide range of industrial and useful applications.1–6 The overall properties of ILs results from the composite properties of the cations and the anions that are super acidic, basic, hydrophilic, water miscible, water immiscible, and hydrophobic. The anion is currently used to control the water miscibility,7 but the cation can also influence the hydrophobicity or hydrogen bonding ability.8Liquid–liquid two phase systems formed by IL and W (water) find several useful ways of application in the design of fluid separation processes and purification of various biomolecules.9,10 A detailed knowledge of the behavior of mixtures of ILs + water is certainly paramount both from industrial and fundamental research perspectives.11 The presence of upper critical solution temperature (UCST)12–15 and lower critical solution temperature (LCST)16 have been confirmed for mixtures of ILs and water. Liquid–liquid equilibrium diagrams were determined for IL + water systems using the family of ILs 1-alkyl-3-methylimidazolium tetrafluoroborate. The systems present a UCST, which increases with the increase in the alkyl chain of the cation.13 On the other hand for hydrophobic ILs that are useful for applications of ILW two-phase systems, the upper critical points seem to be located near or above 100 °C.15 The most popular room temperature hydrophobic IL contains a 1-alkyl-3-methylimidazolium cation and the anion BF4−.17,18 The design of safe and environmentally benign separation processes has an increasingly important role in the development of clean manufacturing processes and in the remediation of sites contaminated by an older generation of manufacturing technologies.19 Despite this need, the necessary experimental data for many properties of ILs and their mixtures with water are scarce, particularly the explanation of three-dimensional (3D) Ising values, and often inconsistent between the various sources.
In other words, for the polymer/IL solutions, both UCST20 and LCST21–24 phase behavior have been reported in the open literature, and the outcome of these studies has received much attention. Interestingly, the miscibility of a polymer and IL often depends on the type of polymer as well as on the type of IL in the respective system. In this context, Watanabe's group25–29 have studied the thermal response of a series of polymers dissolved in 1-alkyl-3-methyl imidazolium based ILs. The use of ILs in such biphasic systems necessitates an appreciation of their effect on reactivity but the forces that govern solubility and solvation of the polymers in ILs is at its early stage of investigation. In this regard, Strehmel et al.30 noted that ILs are able to dissolve monomers with widely differing polarities, with their ionic characteristics enabling favorable interactions with highly polar monomers and the aromatic and alkyl groups present in the cation interacting favorably with weakly-polar monomers. Thus, compared with molecular solvents, ILs combine strong Coulomb interactions and many other weak interactions, including hydrogen bonding, cation–π interactions, van der Waals interactions and so on.31 Thus, it would be expected that there exists H-bonds between the protons of the IL and an electron donating group of the polymer, which appears to be the driving force for the solubility of the polymers in ILs.
The advantage of studying critical mixtures is that it provides universal quantities as the critical exponent (β), which essentially, describes the shape of the coexisting curve. In the universality class, systems have the same set of critical exponents β in power laws describing the asymptotic behavior of different properties.32 For binary-mixed solvent systems, the Tc and β are known to be very sensitive to the addition of impurities.32–35 These impurities behave as a third component in the coexisting liquid phases. In this perspective, the effects of long-chain polymer in binary-critical solvents have been well characterized and have great fundamental practical importance.36–38 To our knowledge, however, the details of the impurity-effect in phase separation of water mixture with IL remain unexplored and absolutely absent in the literature. This is because of the limited individual studies that have been conducted with few ILs with little variations of anion, alkyl chain length or additional substituent groups on the IL.
For the first time, we have performed precise measurements of the critical temperature (Tc) using fluorescence spectroscopy and determination of the coexistence curve for the mixture of 1-hexyl-3-methylimidazolium tetrafluoroborate and water (ILW) near its consolute point using refractive index measurements. This reveals that the fluorescence observations can provide promising results in investigating the phase transitions in critical mixtures. We observed that the ILW critical mixture exhibits UCST at 58.256 °C under atmospheric pressure. Moreover, it is interesting that, based on the observed data and upon power scaling laws, we found that the ILW critical mixture used in our study is a member of the 3D Ising criticality class.
A second scope of the work has been to explore the impurity effect as a function of concentrations of high molecular weight polymer, polyacrylic acid (PAA, Mw = 450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) on the ILW critical mixture. The basic chemical structure of PAA along with 1-hexyl-3-methylimidazolium tetrafluoroborate (IL) is represented in Fig. 1. Herein, we report the results of a coexistence curve study of PAA in the proximity of the consolute point of the UCST of IL + W at atmospheric pressure and as a function of the temperature and under three different PAA concentrations. We have detected a difference in the critical temperature Tc when the PAA was added; the shift of Tc and β is dependent on the concentration of the polymer added to the critical mixture. Moreover, β values calculated for the PAA in ILW show that the polymer-containing ILW critical mixtures do not belong to the universality class of the 3D Ising model and obviously belong to the renormalized class.
000 g mol−1) on the ILW critical mixture. The basic chemical structure of PAA along with 1-hexyl-3-methylimidazolium tetrafluoroborate (IL) is represented in Fig. 1. Herein, we report the results of a coexistence curve study of PAA in the proximity of the consolute point of the UCST of IL + W at atmospheric pressure and as a function of the temperature and under three different PAA concentrations. We have detected a difference in the critical temperature Tc when the PAA was added; the shift of Tc and β is dependent on the concentration of the polymer added to the critical mixture. Moreover, β values calculated for the PAA in ILW show that the polymer-containing ILW critical mixtures do not belong to the universality class of the 3D Ising model and obviously belong to the renormalized class.
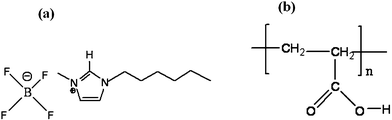 | ||
| Fig. 1 Schematic representation of the structures of (a) 1-hexyl-3-methylimidazolium tetrafluoroborate and (b) polyacrylic acid. | ||
II. Materials and methods
Materials
1-Hexyl-3-methyl imidazolium tetrafluoroborate and polyacrylic acid (PAA), Mw = 450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 (with a nominal Tg 105 °C) were purchased from Sigma Aldrich, USA and used as obtained. Ultra pure water at 18.3 Ω cm resistivity (from Ultra 370 Series, Rions, New Delhi, India) was used for sample preparation.
000 g mol−1 (with a nominal Tg 105 °C) were purchased from Sigma Aldrich, USA and used as obtained. Ultra pure water at 18.3 Ω cm resistivity (from Ultra 370 Series, Rions, New Delhi, India) was used for sample preparation.
Refractive index measurements for the coexisting liquid phases of ILW
The samples were carefully prepared by mass using a Mettler Toledo balance with a precision of ±0.0001 g. Refractive index measurements have been accomplished with a home-built refractometer and a detailed description of the procedure is elucidated elsewhere.36,37 A stirrer was provided to maintain the temperature homogeneity throughout the water bath. The n value of each separate phase was measured simultaneously with having an accuracy of ±0.0004. Visual detection of the solution was employed to determine Tc on the temperature–composition phase diagram corresponding to the coexisting equilibrium at atmospheric pressure. Complete determination of the coexistence curve is a time-consuming process, establishing a precise equilibrium transition temperature can take hours for each data point. Prior to use, precautions were taken during sample transferring into the sample cell to avoid air bubbles. Moreover, no shift of the Tc values was observed during the measurements by checking the Tc values before and after the refractive index measurements. The cell containing the critical mixture was immersed in the isolated water bath where it could be seen through the walls of the water bath, made of 2-cm-thick transparent plexiglass. The temperature of the water bath was controlled up to ±0.002 °C by using a Eurotherm temperature controller (Eurotherm 3500 Series) attached with a RTD probe sensor. The schematic representation of the complete experimental setup is represented in Fig. IS (see supplementary information†). For a sample of pure ILW, we prepared a critical sample from 59.03 g of water and 40.97 g of IL with a UCST of 58.256 ± 0.002 °C using an equal volume criterion, which is in good agreement with the literature values of 58.16 °C reported by Wagner et al.14The solubility of the polymer PAA was checked separately with water and 1-hexyl-3-methyl imidazolium tetrafluoroborate. The measured amount of the polymer (0.5, 1.0 and 1.5 mg cm−3 of PAA) was found to be soluble in both IL as well as in water. PAA was then dissolved with continuous stirring to the ILW critical mixture overnight. In the neighborhood of the critical point, large density fluctuations (concentration fluctuations in a binary mixture) produce large local fluctuations in the refractive index of an ILW critical mixture causing visible light to be scattered strongly, a phenomenon called critical opalescence. As a result, the mixture becomes phase separated when the temperature T is close to the Tc of the mixture. The detailed setup includes a sample cell on a submersible magnetic stirrer clamped with a sample holder. It should be emphasized that the solution turbidity increases with increasing concentrations (above 1.5 mg ml−1) of PAA.
Fluorescence measurements for the coexisting liquid phases of ILW
For fluorescence measurements, the choice of the fluorescent probe was done with care. Nile red was chosen as the fluorescent probe since it is soluble in ILs. 2 × 10−4 g cm−3 of Nile red was dissolved in the bulk solution of IL (10 ml) and then this solution was again used for preparing critical mixture of ILW. Steady-state fluorescence measurements were conducted with a Cary Eclipse Spectrofluorimeter (Varian optical spectroscopy instruments, Mulgrave, Victoria, Australia) equipped with thermostated cell holders and the temperature was kept constant by a circulating water bath using a Peltier device (accuracy of ±0.01 °C) attached to the sample holder of the fluorimeter. The excitation wavelength was set at 555 nm in order to calculate the contribution of Nile red to the overall fluorescence emission. The experiments were performed at 65–50 °C by using a 1 cm sealed cell and both excitation and emission slit width were set at 5 nm, and corrected for background signal.The temperature of the fluorescence cuvette chamber was first raised above the Tc at which a homogeneous mixture of ILW was formed. The steady-state scan was taken for the mixture at that temperature. Then the temperature was step lowered with an increment of 0.5 °C and a scan was taken after a time interval of 2 h. We observed that in the ILW system the diffusion of the components is fast however; we allow a great time interval to achieve complete equilibrium of the molecules of each of the component in each phase. A similar procedure was applied to the rest of the samples of various concentrations of PAA in ILW. The schematic representation of the fluorescence measurements is provided in Scheme 1.
 | ||
| Scheme 1 Schematic representation of the fluorescent intensity decrease with change in temperature. The blue, violet and pink-colored sphere represents the water, IB, and Nile red molecules, respectively. | ||
III. Results
Phase transition and the coexistence curve of ILW
At room temperature the IL forms a two phase system with W. The IL-rich phase (i.e. the lower phase) possesses higher density (1.149 g cm−3) and refractive index (n = 1.430) than the W-rich phase (upper phase). The phase determination of the ILW critical mixture was determined by equilibrating the binary mixture at a given temperature. Upon slow heating this system (by a step of 0.001 °C), a phase boundary was observed in the middle of the vial indicating that the mixture was close to critical point. The position of the meniscus in the centre at 1 m°C below Tc indicated that the volumes of the coexisting liquid phases were equal to the naked eye, a feature of criticality. The composition of this sample is 6.65 mol% of IL. A one-phase system with an upper critical consolute point at 58.256 ± 0.002 °C is formed, which coincides well with the value of 58.16 °C observed by Wagner et al.14 Additionally, the experimental refractive index (n) values of the pure ILW coexisting liquid phases were reproducible and are summarized in Fig. 2 as a function of temperature and also provided in Table IS.† | ||
| Fig. 2 Coexistence curves of the temperature dependence of the refractive index measurements of ILW (■) with different concentrations of PAA (0.5 (•), 1.0 (▾), and 1.5 (☆) mg ml−1, respectively). | ||
The singular behavior in the vicinity of the critical point is characterized by a set of critical exponents. Obviously, β is a tool to classify different types of phase transitions, or to sort them into different universality classes. Our experimental observations indicate that β is universal, independent of the material, and to some extent of the nature of the transition. In order to quantify the experimental results, in the vicinity of the critical point, we obtained β, from the refractive index measurements of two coexisting phases with the relative distance from the Tc, defined by the relation (n1 − n2) ∝ (Tc − T)β, with n1 and n2 being the individual refractive indices of the coexisting phases of ILW. Surprisingly, we found a β value of 0.326 ± 0.002 for a pure ILW system, which is consistent with the 3D Ising value of 0.325. This is quite interesting as for the first time we report that the system composed of critical mixture of a hydrophobic IL (1-hexyl-3-methyl imidazolium tetrafluoroborate) and water can be characterized by an Ising 3D model.
Phase transition and critical behavior of ILW in the presence of PAA as an impurity
To achieve more insight into the temperature dependent miscibility of ILW, we additionally studied the effect of high molecular weight polymer polyacrylic acid (PAA) as a third component in the critical mixture of the same ILW. The measured n values of both coexisting liquid phases are included in Fig. 2 and also in Table IS† as a function of temperature (T) upon approaching the Tc for various concentrations of PAA in ILW. It is interesting to probe the change of the Tc due to the addition of PAA in ILW. The entire shape of the coexistence curves for PAA in the ILW system is similar to those for pure ILW. Fig. 2 shows that the coexistence curves noticeably decreased with increasing concentrations of PAA in the ILW mixture. As is evident from Fig. 2 the n values of the IL-rich phase significantly increase with increasing concentrations of PAA as compared to the water-rich phase. This variation induces a modification of the phase transition region affected by the addition of PAA to an ILW mixture, and presumably PAA is preferentially adsorbed to a larger extent by the IL than the water molecules. These variations in n values indicate that the interaction of the polymer chain with solvent molecules is enhanced in the presence of ionic IL. This may be attributed to the ionic interactions of the electrolytic polymer PAA with IL. The Tc of PAA at three different concentrations (0.5, 1.0, and1.5 mg ml−1) in ILW was experimentally located at 57.126 ± 0.002, 56.084 ± 0.002 and 55.168 ± 0.002 °C, respectively and is represented in Fig. 3. Moreover, looking at the data in Fig. 3, it is observed that as the concentration of PAA increases in ILW, the Tc values decrease linearly which indicates that the polymer favors the mixing of the solvent molecules. | ||
| Fig. 3 PAA effect on critical temperature, Tc, as a function of PAA concentration in ILW. | ||
In the critical phenomena, the value of β for PAA in ILW mixture was obtained from the plot of the difference in refractive index of both coexisting phases (Δn) against relative distance from the Tc (ΔT) by extracting the slope, which is shown in Fig. 4. Moreover, there is an increase in the β value from 0.326 ± 0.002 for pure (ILW) to 0.332 ± 0.004, 0.367 ± 0.005, and 0.379 ± 0.005 for PAA concentration at 0.5, 1.0, and 1.5 mg cm−3, respectively (Fig. 5). This indicates that the large PAA molecules in our ILW sample may induce an indirect interaction between the water molecules and the IL molecules as well as intrachain interactions between the monomers of PAA. Because of the large spatial extent of the macromolecules, such an indirect interaction will enable a water or IL molecule to interact with a large number of other water and IL molecules. Hence, β increases towards the higher mean-field value when the PAA macromolecules are added to the binary mixture ILW. Since a universality class in critical phenomenon is characterized by a set of critical exponents; different values of β imply that the binary mixture ILW with dissolved PAA macromolecules may belong to a different universality class than that (3D Ising) of the pure ILW. Interestingly, the observed β values for PAA in ILW have a fully normalized critical exponent, which is consistent with the theory of Broseta and Leibler.39
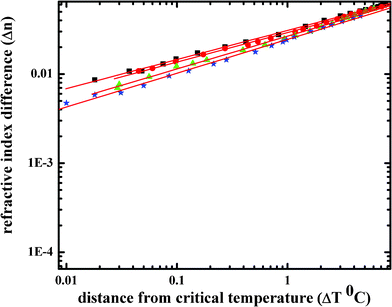 | ||
| Fig. 4 Logarithmic representation of the refractive index difference Δn between the coexisting phases with respect to the distance from the critical temperature ΔT of ILW (■) with different concentrations of PAA (0.5 (●), 1.0 (▼), and 1.5 (☆) mg ml−1, respectively). For the sake of simplicity and clarity, we did not show the fits and error bars for PAA in ILW system. | ||
 | ||
| Fig. 5 PAA effect on critical exponent, β as a function of PAA concentration in ILW. The errors indicate the standard deviation. | ||
Our previous results36,37 together with those of To40 explicitly elucidated that β is different for critical mixtures with and without polymer, and this finding is true whether the binary mixture has an upper critical point or a lower critical point. A similar observation has been seen in the coexistence curve of polystyrene (PS II, Mw = 7.19 × 105) in polystyrene (PS I, Mw = 1.72 × 104) + methylcyclohexane (MC) by using the refractive index measurements by Dobashi et al.19 According to their study, the β of PS I+MC changes from 0.335 ± 0.001 to 0.384 ± 0.004 when PS II was added to PS I+MC. On the basis of these studies, we noticed that the β is closely related to the behavior of the critical point and the third component, such as a polymer, enhances the critical exponent of the binary critical mixture. On the basis of our results, it is clear that PAA is able to enhance the β value of the ILW mixture. In contrast, it appears that the β value of binary critical mixture with ionic salt is the same as that for a pure mixture without ionic salt.41–46
Phase transition of ILW and PAA in ILW monitored by fluorescence
The Tc determination for ILW was obtained from fluorescence measurements. Obviously, the fluorescence results are in good agreement with the n measurements. Since, Nile red is poorly soluble in water; an insignificant overall solvation capacity of pure water exists for these solutes.47 On the other hand, the probe exhibits high solubility in pure IL and in ILW mixtures at high temperature where a single homogeneous phase is obtained. Therefore, we slowly decrease the temperature from a higher value (where single phase is obtained) to lower values, so that the changes in the probe emission intensities could be monitored with decrease in temperature. It is very informative to note that the fluorescence maximum (λmax) for Nile red remained constant (∼657.94 nm) throughout the experiment. These observations suggest the solubility of the Nile red molecule; that most of the sites of the Nile red molecules are solvated by the IL molecules. However, the presence of water molecules significantly modifies the electronic energy by interacting with specific sites of the probe. The amino and the carbonyl groups of the solvatochromic dye should specifically interact strongly with water as well, and due to the change in polarity we observe a high intensity value in the homogeneous region. Simultaneously, we found that as the Tc is approached, the intensity of the fluorescent probe is decreased. This seems to be the diminishing effects of the W interactions around the Nile red molecule at Tc. There may be very complicated interactions between the ILW and PAA in this region and the physics is unclear.40,47 At this moment the plausible explanation based on the experimental results is that the hydrogen-bond between water and the probe becomes weaker as the Tc is approached, and that this effect would also reduce the water concentration around the probe molecule. However, the solvation layer by IL around the probe should exist because of the solubility of the probe in IL. According to Gutkowski et al.47 these differences in the solvation shell are promoted by short range interactions between moieties with different electron densities in the probe molecules and mixtures of solvent molecules with different polarities. Since, the intensity of a fluoroprobe depends on the amount present, therefore, at Tc we can assume that the phase separation has started and the fluoroprobe is separating from the aqueous rich phase along with the IL molecules. The intensity decrease is sharp because of the movement of the molecules of IL and water. This indicates that the mixing of the IL and water is completely thermally controlled and interactions of any type occurring in between the neighboring IL and W molecules are minimal. We noted from fluorescence analysis that at temperatures below Tc the intensity is at a constant value for ILW mixtures which suggests that the separation of two phases has been achieved completely.The fluorescence intensity measurement results obtained for ILW + 0.5 − 1.0 mg cm−3 PAA are collected in Fig. 6 (b and c) and are also very interesting, coinciding very well with the observed Tc value by refractive index measurements. The fluorescence analysis of 1.5 mg cm−3 of polymer in ILW was affected by the aggregation of the polymer throughout the experimental procedure. This may be interpreted in a way that, with increasing concentration of PAA, many-body effects appear because of hydrodynamic, electrostatic and other specific interactions between macromolecule chains. This makes the entire problem nonlinear in respect to the polyelectrolyte concentration, making the interpretation and the inspection of the critical opalescence rather unpredictable. However, the Tc for PAA in ILW as measured by intensity measurements, are located at 58.5, 57.0 and 56.5 °C for 0, 0.5 and 1.0 mg cm−3 of the polymer, respectively. The results clearly indicate the influence of the polymer on the Tc of the ILW critical mixture. It is the effect of polymer conformation that increases the diffusion in the IL as well as W phase, thereby contributing to a decrease in the UCST curve.
 | ||
| Fig. 6 Determination of the critical temperature (Tc) of ILW system through fluorescence intensity measurements in presence of: (a) 0 mg ml−1 PAA; (b) 0.5 mg ml−1 PAA; and (c) 1.0 mg ml−1 of PAA. | ||
Moreover, on comparing Fig. 6 (a–c), we observed that the emission intensity of the probe increases sharply in the presence of PAA (Fig. 6 b and c). This enhancement is observed only when the local environment around the probe is changed; probably due to the solvation effects in the critical mixture while approaching the Tc.47 The introduction of a hydrophilic polymer PAA in ILW causes PAA to be solvated preferentially by the W and IL molecules. Thus, the formation of a new solvation layer on the PAA surface disturbs the environment of the probe in ILW, thereby enhancing the emission intensities.
IV. Discussion
PAA acts as a large macroion that can help balance ionic charges in solutions and their power to stabilize the formula depends considerably on their conformation in the system, which is controlled by the interactions with the solvents.48,49 The structure of PAA has found wide applications in industry because of its ability to form various kinds of hydrogen bonds via carboxyl acid (–COOH) and hydroxyl (–OH) groups with water.50,51 Thus, the addition of small amount of PAA as an impurity can provide electrostatic and hydrogen bonding, making the interactions more complex and leading to phase instability as well as enhancing the flow properties. Scheme 2 shows the various H-bonding possibilities of the polymer PAA with water. The absorbance of water and IL molecules by the PAA chain is mainly due to the stronger electron acceptors, –COOH of PAA, thereby water strongly forms hydrogen bonds with nonbonding oxygen (–O–) orbitals in PAA. This hydrogen-bonding requirement is easily satisfied by water without significant perturbation of the structure of the PAA. Water spirals are formed in such a way that more water molecules accumulate on the cavities surrounding the oxygen atoms of the PAA. A lack of significant perturbation in the structure and the fact that the PAA chain is largely accommodated by the water structure minimizes hydrophobic interactions with and within the polymer chains.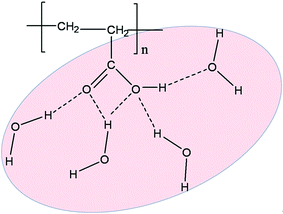 | ||
| Scheme 2 Schematic representation of the interaction of the PAA monomers with water molecules, through H-bonding. | ||
ILs are good solvents for a wide range of polymer molecules.52 For this reason, it seems to be very important to study the structural behavior of polymer-containing ILs for the case of good polymer solubility in ILs. The factors responsible for polymer solubility in ILs are complex and not readily predicted.53 There has been little fundamental understanding of how the structure and chemical composition of ILs govern polymer solubility.54 The study of the ordering of macromolecules in ILs is useful in the understanding of polymer-containing systems where the ILs are used as a medium. Understanding the forces governing the solubility and solvation of polymers in ILs are still at the early stage of development. In the present paper, our special interest is how the structural properties of the polymer modify the solubility behaviors of ILW and, in contrast, depend on the polymer concentration when both IL and water are good solvent for the polymer.
Compared to molecular solvents, ILs combine strong Coulomb interactions and many other weak interactions, including hydrogen bonding, cation–π interaction, van der Waals interactions and so on. Interestingly, the general principle of “like-dissolves-like” is not applied to polymer–IL systems.55 Sashina and Novoselov56 proposed a scheme for interaction of cellulose fragment with an imidazolium-based IL. They proposed that the anion forms hydrogen bonds with the proton donor centers of the polymer links, while bulky cations in the vicinity hinder sterically a reconstruction of hydrogen bonds between the polymer chains destroyed due to solvation. Molecular dynamics simulations57 of PEO in alkyl imidazolium hexafluorophosphate showed that the distance between the oxygen atoms of the PEO chains and the imidazolium cations is slightly shorter than the anion–cation distance, which is likely to be due to the formation of hydrogen bonds. On the basis of these experimental and computational results, it is reasonable to infer that in our system of ILW + PAA, there are strong interactions between the components, including hydrogen bonding between the oxygen atom of the PAA chain and the H atoms of the imidazolium cations. In this context, Noack et al.58 noticed that in the case of imidazolium based ILs the C4 and C5 atoms appear to be almost neutral while the C2 atom exclusively possesses a positive charge owing to the electron deficit in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond. Moreover, they showed that the large positive charge at the C2–H unit and the repulsive interactions due to the C
N bond. Moreover, they showed that the large positive charge at the C2–H unit and the repulsive interactions due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond explain the observed higher acidity of C2–H, respectively. Therefore, it is likely that the H-bond between the H atom on the C2 position and the O atom of PAA plays the most important role in determining the decrease in the UCST of the ILW critical system. However, other H atoms, such as the H atoms in the side chain of the imidazolium ring, may play a role in the formation of H-bonds. Apart from all these, the terminal –OH group in the PAA chain is likely to form hydrogen-bonds with BF4− ion. Due to these hydrogen-bond interactions, solvation around the PAA chain would take place, which must be the origin of the solvophilicity of the PAA chain by the IL. Scheme 3 represents the probable mechanism of the interaction of the IL with the PAA monomers in the polymers chain.
C bond explain the observed higher acidity of C2–H, respectively. Therefore, it is likely that the H-bond between the H atom on the C2 position and the O atom of PAA plays the most important role in determining the decrease in the UCST of the ILW critical system. However, other H atoms, such as the H atoms in the side chain of the imidazolium ring, may play a role in the formation of H-bonds. Apart from all these, the terminal –OH group in the PAA chain is likely to form hydrogen-bonds with BF4− ion. Due to these hydrogen-bond interactions, solvation around the PAA chain would take place, which must be the origin of the solvophilicity of the PAA chain by the IL. Scheme 3 represents the probable mechanism of the interaction of the IL with the PAA monomers in the polymers chain.
 | ||
| Scheme 3 Schematic representation of the interaction of the PAA monomers with IL molecules, through H-bonding. | ||
The prediction of the polymer conformation close to the critical point of the solvents is difficult because most of the polymers become insoluble due to a diverging interaction parameter.59 It is evident, however, from experimental as well as theoretical calculations that at temperatures sufficiently close to Tc the polymer will be completely immersed in the better solvent, in the state of the extended conformational form. Dynamic light scattering measurements in solutions of PAA in water and 2,6-lutidine show evidence of partial chain collapse close to the critical point of the solvents.60 Later, experiments in the same system using fluorescence correlation spectroscopy have confirmed chain reexpansion at the critical point.61 We expect that the PAA molecule in our ILW critical mixture is in the expanded conformation which may induce an indirect interaction between the water molecules and the IL molecules. When PAA is added at a small concentration of ILW, PAA preferably adsorbs to molecules of solvents and thus changes occur on the particle surface. Since, PAA does not have any bulky side groups, local steric and electrostatic hindrances are expected to be minimal and the chain flexibility and mobility can help to accommodate the PAA with W and IL molecules and continuous structural rearrangement with water. Thus, the hydrophilic character of PAA is supported by its solubility in both solvents and their adsorption on PAA surface. Moreover, because of extended conformations and minimal interactions, more and more W and IL molecules are accommodated at the PAA surface; thereby inducing an indirect interactions between large number of W and IL molecules (Scheme 4). The dashed red lines in Scheme 4 point toward the probable H-bonding interactions within the water, IL and monomers of the PAA chains due to solvation at the polymer surface. Hence, β increases towards the renormalized value when PAA is added to the critical mixture of ILW. Since the PAA is well solvated with both the phases we infer, according to Timmermans rules, when the third component is equally solvated in both coexisting liquid phases this leads to a decrease in UCST.62
 | ||
| Scheme 4 Schematic representation of the interaction of the PAA with the IL-rich and water-rich phase, respectively. The dashed red lines indicate the probable H-bonding interactions within the water, IL and monomers of the PAA chains. It is the increase in the intermolecular H-interactions due to the polymer that increases the diffusion between IL and water. | ||
V. Conclusions
Our results explicitly support the fact that ILs represent a novel class of solvents and may now also be considered as a novel medium for liquid–liquid extraction. Limited experience with room temperature ILs based on methylimidazolium cation and tetrafluoroborate anion suggests that such systems may be easily adapted to conventional liquid–liquid extraction practice. We performed experiments describing the role of critical exponents, and critical temperatures of 1-hexyl-3-methyl imidazolium tetrafluoroborate and water in the presence of a high molecular weight of polyacrylic acid (PAA). Our results reveal that the phase transition, and critical temperatures shift down with increasing PAA concentration in IL + W. In the phase-separated region, the critical exponents of PAA in ILW are higher (0.332–0.379) than that (0.326 ± 0.002) of pure ILW. It seems that polyacrylic acid polymer modulates the UCST-type phase behavior of the Ising universality class of ionic liquid and water. We observed that β values may not be universal in binary mixtures containing high molecular weight polymer. These interesting biphasic systems continue to be actively studied in our laboratories for the development of novel, ‘clean’ separation technologies. Regarding polymers as an impurity in critical mixture systems, their partition problems can be much more complicated than might be expected for simple two phase systems. In this regard, the idea of fluorescence investigation for phase transition in critical liquid mixtures can bring much better results. However, more information is needed to identify the effect of polymers on the critical phenomena of IL (varying cation and anion) and water and is the subject of our on-going work.Acknowledgements
Financial support from the Department of Science and Technology (DST) [SR/SI/PC-54/2008] and University Grant Commission (UGC) [37-404/209 (SR)], New Delhi is gratefully acknowledged.References
- T. Welton, Chem. Rev., 1999, 99, 2071–2084 CrossRef CAS.
- V. Govinda, P. Attri, P. Venkatesu and P. Venkateswarlu, Fluid Phase Equilib., 2011, 304, 35–43 CrossRef CAS.
- P. Attri, P. Venkatesu and A. Kumar, J. Phys. Chem. B, 2010, 114, 13415–13425 CrossRef CAS.
- P. Attri, P. M. Reddy, P. Venkatesu, A. Kumar and T. Hoffman, J. Phys. Chem. B, 2010, 114, 6126–6133 CrossRef CAS.
- P. M. Reddy and P. Venkatesu, J. Phys. Chem. B, 2011, 115, 4752–4757 CrossRef CAS.
- T. Vasantha, P. Attri, P. Venkatesu and R. S. Rama Devi, J. Chem. Thermodyn., 2012, 45, 122–136 CrossRef CAS.
- 2nd Mercosur Congress on Chemical Engineering; 4th Mercosur Congress on Process Systems Engineering, M. G. Freire, P. J. Carvalho, A. J. Queimada, L. M. N. B. F. Santos, I. M. Marrucho, J. A. P. Coutinho.
- T. Erdmenger, J. Vitz, F. Wiesbrock and U. S. Schubert, J. Mater. Chem., 2008, 18, 5267–5276 RSC.
- A. M. Scurto, Sudhir N. V. K. Aki and J. F. Brennecke, Chem. Commun., 2003, 572–573 RSC.
- T. Kakiuchi, Anal. Sci., 2008, 24, 1221–1230 CrossRef CAS.
- L. P. N. Rebelo, N. Visak, Z. P. Visak, M. Nunes da Ponte, J. Szydlowski, C. A. Cerderina, J. Troncoso, L. Romani, J. M. S. S. Esperanca, H. J. R. Guedes and H. C. de Sousa, Green Chem., 2004, 6, 369–381 RSC.
- J. L. Anthony, E. G. Maginn and J. F. Brennecke, J. Phys. Chem. B, 2001, 105, 10942–10949 CrossRef CAS.
- F. M. Maia, O. Rodriguez and E. A. Macedo, Fluid Phase Equilib., 2010, 296, 184–191 CrossRef CAS.
- M. Wagner, O. Stanga and W. Schroer, Phys. Chem. Chem. Phys., 2003, 5, 3943–3950 RSC.
- P. Nockemann, K. Binnemans, B. Thijs, T. N. Parac-Vogt, K. Merz, A. Mudring, P. C. Menon, R. N. Rajesh, G. Cordoyiannis, J. Thoen, J. Leys and C. Glorieux, J. Phys. Chem. B, 2009, 113, 1429–1437 CrossRef CAS.
- H. Ohno and K. Fukumoto, Acc. Chem. Res., 2007, 40, 1122–1129 CrossRef CAS.
- Y. Wang, H. Li and S. Han, J. Phys. Chem. B, 2006, 110, 24646–24651 CrossRef CAS.
- B. L. Bhargava, Y. Yasaka and M. L. Klein, Chem. Commun., 2011, 47, 6228–6241 RSC.
- T. Dobashi, M. Nakata and M. Kaneko, J. Chem. Phys., 1984, 80, 948–953 CrossRef CAS.
- T. Ueki and M. Watanabe, Chem. Lett., 2006, 35, 964–965 CrossRef CAS.
- Hau-Nan Lee and T. P. Lodge, J. Phys. Chem. Lett., 2010, 1, 1962–1966 CrossRef CAS.
- T. P. Lodge and Hau-Nan Lee, J. Phys. Chem. Lett., 2011, 115, 1971–1984 Search PubMed.
- K. Fujii, T. Ueki, K. Niitsuma, T. Matsunaga, M. Watanabe and M. Shibayama, Polymer, 2011, 52, 1589–1595 CrossRef CAS.
- K. Kodama, R. Tsuda, K. Nitsuma, T. Tamura, T. Ueki, H. Kokubo and M. Watanabe, Polym. J., 2011, 43, 242–248 CrossRef CAS.
- T. Ueki and M. Watanabe, Langmuir, 2007, 23, 988–990 CrossRef CAS.
- T. Ueki, T. Karino, Y. Kobayashi, M. Shibayama and M. Watanabe, J. Phys. Chem. B, 2007, 111, 4750–4754 CrossRef CAS.
- K. Kodama, H. Nanashima, T. Ueki, H. Kokubo and M. Watanabe, Langmuir, 2009, 25, 3820–3824 CrossRef CAS.
- S. Tamura, T. Ueki, K. Ueno, K. Kodama and M. Watanabe, Macromolecules, 2009, 42, 6239–6244 CrossRef CAS.
- T. Ueki, A. Yamaguchi, N. Ito, K. Kodama, J. Sakamoto, K. Ueno, H. Kokubo and M. Watanabe, Langmuir, 2009, 25, 8845–8848 CrossRef CAS.
- V. Strehmel, A. Laschewsky and H. Wetzel, e-Polymer, 2006, 011, 1–10 Search PubMed.
- M. Khoel, Ionic Liquids in Chemical Analysis, 2009, CRC Press, Taylor & Francis Group, USA Search PubMed.
- A. Kumar, H. R. Krishnamurthy and E. S. R. Gopal, Phys. Rep., 1983, 98, 57–143 CrossRef CAS.
- D. T. Jacobs, J. Chem. Phys., 1989, 91, 560–563 CrossRef CAS.
- A. Toumi and M. Bouanz, Eur. Phys. J. E: Soft Matter Biol. Phys., 2000, 2, 211–221 CrossRef CAS.
- R. Okamoto and A. Onuki, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2010, 82, 051501 CrossRef.
- P. Venkatesu, J. Chem. Phys., 2005, 123, 024902 CrossRef.
- P. Venkatesu, J. Phys. Chem. B, 2006, 110, 17339–17346 CrossRef CAS.
- P. M. Reddy and P. Venkatesu, J. Phys. Chem. B, 2011, 115, 12065–12075 CrossRef CAS.
- D. Broseta and L. Leibler, J. Chem. Phys., 1989, 90, 6652–6655 CrossRef CAS.
- K. To, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2001, 63, 026108 CrossRef CAS.
- M. Bouanz and D. Beysens, Chem. Phys. Lett., 1994, 231, 105–110 CrossRef CAS.
- H. L. Bianchi and M. L. Japas, J. Chem. Phys., 2001, 115, 10472–10478 CrossRef CAS.
- K. I. Gutkowski, H. L. Bianchi and M. L. Japas, J. Chem. Phys., 2003, 118, 2808 CrossRef CAS.
- M. Wangner, O. Stanga and W. Schroer, Phys. Chem. Chem. Phys., 2003, 5, 1225–1234 RSC.
- P. K. Madhavan Unni, J. Chem. Phys., 2006, 124, 054505 CrossRef CAS.
- K. I. Gutkowski, H. L. Bianchi and M. L. Japas, J. Phys. Chem. B, 2007, 111, 2554–2564 CrossRef CAS.
- K. I. Gutkowski, R. Fernández-Prini, P. F. Aramendia and M. L. Japas, J. Phys. Chem. B, 2011, 115, 15303–15312 CrossRef CAS.
- Z. Adamezyk, A. Bratek, B. Jachimska, T. Jasinski and P. Warszynski, J. Phys. Chem. B, 2006, 110, 22426–22435 CrossRef.
- E. A. Litmanovich, S. O. Zakharchenko and G. V. Stoichev, J. Phys. Chem. B, 2007, 111, 8567–8571 CrossRef CAS.
- N. Tsukida, H. Muranaka, M. Ide, Y. Maeda and H. Kitano, J. Phys. Chem. B, 1997, 101, 6676–6679 CrossRef CAS.
- B. Li, L. Xu, Q. Wu, T. Chen, P. Sun, Q. Jin, D. Ding, X. Wang, G. Xue and A. Shi, Macromolecules, 2007, 40, 5776–5786 CrossRef CAS.
- T. Ueki and M. Watanabe, Macromolecules, 2008, 41, 3739–3749 CrossRef CAS.
- N. Winterton, J. Mater. Chem., 2006, 16, 4281–4293 RSC.
- A. A. Aerov, A. R. Khokhlov and I. I. Potemkin, J. Phys. Chem. B, 2006, 110, 16205–16207 CrossRef CAS.
- R. Marcilla, J. A. Blazquez, J. Rodriguez, J. A. Pomposo and D. Mecerreyes, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 208–212 CrossRef CAS.
- E. S. Sashina and N. P. Novoselov, Russ. J. Gen. Chem., 2009, 79, 1057–1062 CrossRef CAS.
- L. T. Costa and M. C. C. Riberio, J. Chem. Phys., 2006, 124, 184902 CrossRef.
- K. Noack, P. S. Schulz, N. Paape, J. Kiefer, P. Wasserscheid and A. Leipertz, Phys. Chem. Chem. Phys., 2010, 12, 14153–14161 RSC.
- J. J. Magda, G. H. Fredrickson, R. G. Larson and E. Helfand, Macromolecules, 1988, 21, 726–732 CrossRef CAS.
- K. To and H. J. Choi, Phys. Rev. Lett., 1998, 80, 536–539 CrossRef CAS.
- C. A. Grabowski and A. Mukhopadhyay, Phys. Rev. Lett., 2007, 98, 207801 CrossRef.
- D. Balasubramanian and P. Mitra, J. Phys. Chem., 1979, 83, 2724–2727 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental setup (Fig. IS) and its representative data (Table IS). See DOI: 10.1039/c2ra20251d |
| This journal is © The Royal Society of Chemistry 2012 |
