Signatures of specificity of interactions of binary protein mixtures with citrate-stabilized gold nanoparticles†
Rumi
Khandelia
a,
Jashmini
Deka
a,
Anumita
Paul
*a and
Arun
Chattopadhyay
*ab
aDepartment of Chemistry, Indian Institute of Technology, Guwahati 781039, India. E-mail: arun@iitg.ernet.in; anumita@iitg.ernet.in; Fax: + 91 361 2582349; Tel: +91 3612582304
bCentre for Nanotechnology, Indian Institute of Technology, Guwahati 781039, India
First published on 27th March 2012
Abstract
In this article we report on the observation of specificity of interactions of binary mixtures of proteins with citrate-stabilized gold nanoparticles (Cit-Au NPs), by following the changes in the optical properties of the NPs. The protein mixtures consisted of α-amylase and bovine serum albumin (BSA) or α-amylase and amyloglucosidase (AMG) or glucose oxidase (GOD) and peroxidase (POD). The results observed herein indicated that interaction between a binary protein mixture and Cit-Au NPs depended on the nature and concentration of the component proteins. For example, addition of increasing concentrations of proteins containing α-amylase and BSA consistently broadened the extinction spectrum of Cit-Au NPs. The area under the curves when plotted against the concentration of either of the proteins increased linearly. FTIR, fluorescence, starch agar plate assay and gel electrophoresis results indicated that both α-amylase and BSA were present in the agglomerated structures of proteins and NPs, indicating that both of the proteins played a role in the association of NPs. On the other hand, when the mixture contained increasing concentration either of α-amylase or AMG, the broadening as well as the change in the area under the curve varied randomly rather than following any linearity. Interestingly, when the mixtures of GOD and POD were used—although broadening was observed—the change in the area was linear only for low concentrations of GOD in the medium and was very sensitive to its concentration. Transmission electron microscopy (TEM) results indicated agglomeration of the NPs in the presence of the protein mixtures as the primary reason behind the optical property change. Our observations indicated that the preferential attachment of one protein to Cit-Au NPs—in presence of the other—primarily depended on the overall charge of the protein in the medium.
1 Introduction
Interaction of nanoparticles (NPs) with biomolecules (especially proteins) has been an important research area in the field of biomedicine with potential applications in diagnostics and therapeutics. When a biomolecule such as protein, DNA, or RNA interacts with NPs, the properties of the NP may undergo changes which can be probed using optical and magnetic techniques. This can form the basis for detection and assay of the biomolecule. For example, the optical properties of Au NPs have been used for the development of assays for important proteins and for studying the conformational changes of proteins consequent upon pH changes or heat.1–8 Recently, based on their investigations of the interaction of Au NPs with common human blood proteins, Lacerda et al. concluded that the degree of co-operativity of particle-protein binding depends on particle size and the native protein structure.9 Tang et al. have reported that in the presence of a mixture of Ag NPs and Au NPs the interaction between bovine serum albumin (BSA) and Ag NPs was reduced in comparison to that in absence of Au NPs.10 In addition, the induced aggregation of a protein by Au NPs and the partial unfolding of a protein on the surface of a NP have recently been demonstrated.4,11–12 Interestingly, while a recent report suggests affinity of a protein as the deciding factor in its attachment to Au NP in the presence of a mixture of proteins,13 there is no other report on the behavior of a mixture of proteins with respect to their attachment to NPs. It would be important to know the consequence of attachment of a protein to a NP in relation to the other proteins present in the medium. Recent reports from our laboratory indicated that a protein at its lower concentration gets attached to Au NP. However, when the concentration of protein is increased these protein-NP composites agglomerate forming larger structures.4 The observations further indicated that the area under the extinction curve varied linearly with the concentration of the protein. On the other hand, the slope of the curve depended on the nature and conformational state of the protein. The method allowed estimation of protein with the ability to distinguish conformation and its fractional content.4,11 A pertinent question may be asked about the consequences of the presence of a binary mixture of proteins in a medium containing citrate-stabilized Au NPs (Cit-Au NPs). A systematic study may give answers to some fundamental questions related to the presence of NPs in the milieu of proteins among other biomolecules. This is essential as nanomaterials are poised to be used on a larger scale and may become increasingly ubiquitous—with the possibility of being uncared for over long periods of time.In many circumstances, a mixture of proteins plays a vital role either in desired product formation or in the development of a suitable sensor. For example, the binary mixture of α-amylase and amyloglucosidase (AMG) is used to convert starch to glucose via maltose,14 while a mixture of glucose oxidase (GOD) and peroxidase (POD) is used for the estimation of glucose levels in the blood and in other serums.15 In fact, the importance of the glucose detection using these enzymes has been underscored by the development of nanotechnology-based electrodes for higher sensitivity and faster detection.16 In this regard, Au NPs have played a significant role in the attachment of an enzyme to the electrode and in enhanced electron transfer.17
Herein we report on the changes in the optical properties of Cit-Au NPs in the presence of binary mixtures of proteins, namely, α-amylase and BSA, α-amylase and AMG, and GOD and POD. The choice of proteins reflects the differential sensitivity of their interactions with the NPs and subsequent changes in the optical properties, which acted as a sensitive probe. Interaction of the binary protein mixture with Cit-Au NPs was observed to be very specific depending on the nature and concentration of the component proteins. For a binary protein mixture, preferential attachment of one protein over the other to Cit-Au NPs primarily depended on the overall charge of the protein in the solution, which is a reflection of the isoelectric point (pI) of the protein. For example, the mixture of α-amylase and BSA interacted with the NPs in such a way that there was a consistent increase in the broadening of the extinction spectrum of Cit-Au NPs with the increase in concentration of one of the proteins, while keeping the other constant. Also, the ratio of area under the extinction curve of NP-protein solution to that of NPs only, varied linearly with the concentration of the protein. On the other hand, upon addition of the mixture of α-amylase and AMG the change in the broadening of the extinction spectrum of Cit-Au NPs was not consistent and as a result the change in the ratio of the area under the extinction curve versus the concentration of a protein (in the presence of the other) did not follow a clear linear behavior across the range of concentrations used. Interestingly, for the GOD and POD mixture the change in the broadening, as well as the area under the extinction curve, was sensitive to the concentration of GOD. Essentially, the results reported herein highlight the specificity of interactions of the binary protein mixtures with Cit-Au NPs. The results also indicate that the study of interactions between Au NPs and a protein mixture may provide new information about the specificity of their interactions, which may not be available from investigation using single proteins.
2 Experimental
2.1 Materials
Proteins α-amylase from porcine pancreas (43.6 U/mg activity), AMG from Aspergillus niger (59.9 U/mg activity), and POD from horse radish (969.65 U/mg activity) were purchased from Fluka. GOD from Aspergillus niger (158![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 U/g activity) and hydrogen tetrachloroaurate trihydrate were purchased from Sigma-Aldrich. BSA, trisodium citrate 2-hydrate and all other reagents required for experiments were obtained from Merck Specialities Private Limited, India. Milli-Q grade water was used in all of the experiments.
900 U/g activity) and hydrogen tetrachloroaurate trihydrate were purchased from Sigma-Aldrich. BSA, trisodium citrate 2-hydrate and all other reagents required for experiments were obtained from Merck Specialities Private Limited, India. Milli-Q grade water was used in all of the experiments.
2.2 Preparation of Cit-Au NP dispersion
1.5 mL of 1.73 × 10−2 M HAuCl4 solution was added to 60.0 mL of Milli-Q grade water and then heated to boiling under reflux conditions. Then 1.0 mL of 0.857 M trisodium citrate 2-hydrate solution was added to the above solution (that was being stirred) all at once. The solution first turned blue and then deep red indicating the formation of a Cit-Au NP dispersion. The stirring was continued for another 30 min to ensure complete reduction of Au(III) ions. The synthesis of Cit-Au NPs was confirmed by UV-vis and TEM analysis. The dispersion of Cit-Au NPs thus prepared was diluted 2× with sodium phosphate buffer (0.01 M, pH 7.0) so that the maximum extinction was ∼1.0 and the final pH of Cit-Au NP dispersion was ∼7.0. It may be mentioned here that treatment of phosphate buffer saline (PBS) with Cit-Au NPs led to a broadening of the extinction spectrum, indicating possible agglomeration. This was not the case for 0.01 M sodium phosphate, which was thus used in all further experiments. This diluted Cit-Au NP dispersion was used for all experiments and Cit-Au NP dispersion refers to this diluted form hereafter in the manuscript. The NP dispersion was diluted just before performing the experiments.2.3 Preparation of protein solution
1.0 mg mL−1 solution of each protein, namely α-amylase, BSA, GOD, and POD was prepared by dissolving the respective protein in 0.01 M sodium phosphate buffer of pH 7.0. For the preparation of 1.0 mg mL−1 AMG solution, sodium acetate buffer of 0.05 M and pH 4.5 was used. The sparingly soluble α-amylase, in phosphate buffer, was stirred for 15 min in a magnetic stirrer at room temperature and then centrifuged at 5000 rpm. The supernatant was collected and used for the experiments. Each solution was diluted 10× to obtain 0.1 mg mL−1 protein solution. POD and GOD solutions were further diluted 10× with phosphate buffer to obtain 0.01 mg mL−1 POD and GOD solutions respectively. The actual protein content in the as-prepared solution for each protein was calculated based on the Bradford test using BSA as the standard protein (see ESI†, Fig. S1). The concentration of each protein expressed hereafter in the manuscript is thus the actual protein content present in the final NP-protein solution.2.4 UV-vis measurements
For set I of the binary mixture of α-amylase and BSA, the following procedure was followed. 3.0 mL of Cit-Au NP dispersion was taken in a disposable plastic cuvette and its UV-vis spectrum was recorded (using a Hitachi U-2900 Double Beam spectrophotometer). To this, 160.0 μL (10.0 μL BSA + 10.0 μL α-amylase + 140.0 μL sodium phosphate buffer, mixed beforehand in an eppendorf) of binary protein mixture was added drop by drop. The concentrations of BSA and α-amylase were 0.316 and 0.024 μg mL−1 respectively in the final solution. The above solution was mixed well and kept for 5 min. The UV-vis spectrum was again recorded. Similar additions of the binary protein mixture were made to Cit-Au NPs in separate cuvettes, keeping the BSA volume constant and changing the volume of α-amylase solution (and addition of buffer so as to maintain the final volume of the protein mixture at 160.0 μL). UV-vis experiments for the other sets are reported in ESI†.2.5 Calculation of the average area under the UV-vis spectrum
The area under the UV-vis extinction curve was calculated using software which is a part of the operating software of the equipment. The average area (i.e. the area under the extinction curve in between the two wavelengths, divided by the range of wavelength) was calculated by selecting two wavelengths in the extinction spectrum. Further, the ratios of area in the presence and absence of proteins were calculated from these average values. For calculating average areas, the extreme wavelengths were always set at 405 and 630 nm. A typical view of the total area under the extinction curve is shown in Fig. S2 (ESI†).2.6 Sample preparation for TEM analysis
For TEM analysis, the sample was prepared by drop casting the Cit-Au NP dispersion on a carbon-coated copper grid and left overnight for drying. Similarly, samples were prepared with Cit-Au NPs containing six different protein mixtures: 0.071 μg mL−1 α-amylase and 0.316 μg mL−1 BSA, 0.071 μg mL−1 α-amylase and 1.899 μg mL−1 BSA, 0.094 μg mL−1 α-amylase and 1.266 μg mL−1 BSA, 0.118 μg mL−1 α-amylase and 0.157 μg mL−1 AMG, 0.903 μg mL−1 GOD and 0.051 μg mL−1 POD and 0.045 μg mL−1 GOD and 0.043 μg mL−1 POD. These samples were analyzed by a JEOL JEM 2100 TEM operating at a maximum accelerating voltage of 200 kV.3 Results and discussion
It is known that the addition of proteins such as α-amylase or BSA to a dispersion of Cit-Au NP leads to agglomeration of the NPs, which is accompanied by broadening of the UV-vis extinction spectrum.4 Also, the area under the extinction curve varies linearly with the concentration of the protein when the concentration is sufficiently small. On the other hand, the slope of the curve varies depending on the nature of the protein and its conformation (native versus denatured). A natural extension of this idea would be to elucidate the effect of a binary mixture of proteins on the NPs. The results reported here point out interesting consequences depending on the nature of the proteins and their concentrations in the mixture. For example, upon addition of α-amylase and BSA mixture to Cit-Au NPs, the deep red color of the dispersion changed to pink at low total protein concentration. The color changed to purple when the total concentration of proteins was increased. There were observable changes in the surface plasmon resonance (SPR) extinction spectrum of Cit-Au NPs in the presence of mixture of α-amylase and BSA. UV-vis spectra of Cit-Au NPs in absence and presence of binary mixture of proteins (having different fractional content of α-amylase and BSA) are shown in Fig. 1. Quantitatively, when a mixture of 0.316 μg mL−1 BSA and 0.024 μg mL−1 α-amylase was added to 3.0 mL of Cit-Au NP dispersion, the spectrum broadened with a slight red shift from 522 to 524 nm in the SPR peak of Cit-Au NPs. The broadening increased further upon increasing the concentration of α-amylase (up until 0.165 μg mL−1 of protein), while keeping the concentration of BSA constant at 0.316 μg mL−1. At a concentration of α-amylase higher than 0.165 μg mL−1, no further broadening of the SPR peak was observed. Also, when keeping the concentration of BSA constant at 0.633 μg mL−1 and changing the concentration of α-amylase, broadening of the SPR peak of Cit-Au NP dispersion was observed to increase and further spectral broadening was no longer observed beyond a concentration of 0.165 μg mL−1 α-amylase. When the concentration of BSA was kept constant at 1.266 and 1.899 μg mL−1, saturation occurred after addition of 0.165 and 0.118 μg mL−1 α-amylase respectively. However, when the concentration of BSA was kept constant at 2.532 μg mL−1 and that of α-amylase was changed from 0.024–0.188 μg mL−1, broadening increased systematically up to a concentration of 0.071 μg mL−1 α-amylase. Beyond that, the change in broadening was not systematic (ESI†, Fig. S3). On the other hand, when the concentration of α-amylase was kept constant at 0.047 μg mL−1 and that of BSA was varied from 0.316 to 4.114 μg mL−1 in the Cit-Au NP dispersion, then also broadening of the SPR peak of Cit-Au NPs increased (up to a BSA concentration of 2.215 μg mL−1) (ESI†, Fig. S4). At a concentration of BSA higher than 2.215 μg mL−1, saturation occurred and no further increase in broadening was observed. | ||
| Fig. 1 SPR extinction spectra of Cit-Au NP dispersion before and after addition of binary protein mixtures having different fractional content of α-amylase and BSA. Concentration of α-amylase was varied in the range of 0.024–0.165 μg mL−1 (for A–C) and from 0.024–0.118 μg mL−1 (for D), keeping the concentration of BSA constant at (A) 0.316, (B) 0.633, (C) 1.266, and (D) 1.899 μg mL−1. The arrows in the figures show the increase in broadening of the SPR spectrum of Cit-Au NPs with increasing concentration of α-amylase. | ||
Further, when the α-amylase-AMG mixtures, having a constant concentration of AMG (0.157 μg mL−1) and varying concentration of α-amylase (0.024 to 0.259 μg mL−1), were added to the Cit-Au NP dispersion, broadening was also observed. However with an increase in concentration of α-amylase, there was no consistent change in broadening (ESI†, Fig. S5). For example, when 0.047 μg mL−1 of α-amylase was present in the mixture, the spectrum was broadened, whereas when the mixture contained 0.071 μg mL−1 of α-amylase there was broadening but less than that in the presence of 0.047 μg mL−1 of α-amylase. On the other hand, when 0.094 μg mL−1 α-amylase containing protein mixture was added to the dispersion, there was broadening again—higher than that at 0.047 μg mL−1. In addition, when the concentration of α-amylase was kept constant at 0.114 μg mL−1 and that of AMG was varied from 0.046–0.457 μg mL−1, the results were similar.
On the other hand, the results with respect to a mixture of GOD and POD were dependent on the concentration of GOD. For example, when a mixture of 0.903 μg mL−1 GOD and 0.017 μg mL−1 POD was added to a Cit-Au NP dispersion, a broadening of the SPR peak was observed. However, with an increase in the concentration of POD, the broadening was observed not to change consistently (ESI†, Fig. S6). For example, when 0.017 μg mL−1 of POD was present in the mixture the spectrum was broadened, whereas when the mixture contained 0.034 μg mL−1 of POD there was broadening but less than that at 0.017 μg mL−1 of POD. Similarly, when 0.051 μg mL−1 POD containing protein mixture was added to the dispersion then there was broadening again, which was higher than that in the presence of 0.017 μg mL−1. Interestingly, when the concentration of GOD was fixed at 0.045 μg mL−1 (lower than the sensitivity range of individual GOD; see the discussion below) and that of POD was varied, broadening was observed to increase consistently with an increase in concentration (up to 0.146 μg mL−1) of POD (ESI†, Fig. S7). However, when the concentration of POD was kept constant at 0.034 μg mL−1 and that of GOD was varied from 0.008 μg mL−1–0.241 μg mL−1, no systematic change in broadening was observed.
TEM investigations of the Cit-Au NPs in the presence of mixtures of proteins indicated that the origin of the spectral broadening may be attributed to the agglomerations of the NPs. It may be mentioned here that TEM of the as-synthesized Cit-Au NPs (i.e. in absence of protein) indicated no such agglomeration and the particle size distribution of Cit-Au NPs was found to be 10.0 ± 1.0 nm (Fig. 2A). On the other hand, Cit-Au NPs in the presence of mixtures of 0.071 μg mL−1 α-amylase and 1.899 μg mL−1 BSA (Fig. 2B and ESI†, Fig. S8 D, E and F), 0.071 μg mL−1 α-amylase and 0.316 μg mL−1 BSA (ESI†, Fig. S8 A, B and C), 0.094 μg mL−1 α-amylase and 1.266 μg mL−1 BSA (ESI†, Fig. S8 G, H and I), 0.118 μg mL−1 α-amylase and 0.157 μg mL−1 AMG (Fig. 2C and ESI†, Fig. S9 A, B and C), and 0.045 μg mL−1 GOD and 0.043 μg mL−1 POD (Fig. 2D and ESI†, Fig. S9 D, E and F) exhibited significant agglomeration of the NPs. Interestingly, when a mixture of 0.903 μg mL−1 GOD and 0.051 μg mL−1 POD was added to a Cit-Au NP dispersion, agglomeration was present but less widespread (ESI†, Fig. S9 G, H and I). This indicates that an increase in the concentration of GOD (in the presence of POD) in the solution led to a lowering of the extent of NP agglomeration. Thus, the presence of the mixture of proteins in the dispersion of Cit-Au NP led to the agglomeration of the NPs which caused the broadening of the extinction spectrum. Further, the extent of the broadening of the spectrum and agglomeration of the NPs were dependent not only on the nature of the protein but also on their content in the mixture.
 | ||
| Fig. 2 TEM images of (A) Cit-Au NPs only, (B) Cit-Au NPs in presence of mixture of 0.071 μg mL−1 α-amylase and 1.899 μg mL−1 BSA, (C) Cit-Au NPs in presence of mixture of 0.118 μg mL−1 α-amylase and 0.157 μg mL−1 AMG and (D) Cit-Au NPs in presence of mixture of 0.045 μg mL−1 GOD and 0.043 μg mL−1 POD. Scale bar is 50 nm in all images. | ||
It has previously been observed that a more quantitative understanding of the interaction between proteins and Cit-Au NPs could be achieved if the area under the extinction curve (related to oscillator strength) was plotted against the protein concentration.4 This turned out to be quite useful for assays of individual proteins with distinction of conformations. It can be expected that similar analyses would throw new light on the interactions of proteins and NPs in the presence of a second protein. The results of such analyses involving a binary mixture of α-amylase and BSA are shown in Fig. 3. The ratio of the area under the curve in the presence of a particular mixture of proteins to that in their absence was plotted against the concentration of a particular protein, while keeping the other protein constant. The concentration range of component proteins was chosen preferably from the linear region of area under the extinction curve versus concentration plot, obtained from the spectra when individual proteins were added to Cit-Au NPs.4 It is interesting to observe that the ratio of the area under the UV-vis spectrum of Cit-Au NPs (in the presence of a binary mixture of α-amylase and BSA) to that of Cit-Au NPs only, increased linearly with the increase in concentration of one of the component proteins (until a certain concentration). For example, when the concentration of BSA was kept constant at 0.316 μg mL−1, linearity was observed for the change in concentration of α-amylase from 0.024 to 0.165 μg mL−1 (Fig. 3A). Additionally, for constant concentrations of BSA of 0.633, 1.266 and 1.899 μg mL−1, linearity was observed from 0.024 to 0.165, 0.165 and 0.118 μg mL−1 of α-amylase concentration respectively (Fig. 3B–3D). The plot of ratio of area versus α-amylase concentration (μg mL−1), when the concentration of BSA was kept constant at 2.532 μg mL−1 is shown in Fig. S10 (ESI†). At this concentration of BSA, the linearity was observed up to an α-amylase concentration of 0.071 μg mL−1. However at higher concentrations—while broadening was significant—clear linearity with an increase in concentration of α-amylase was not apparent (ESI†, Fig. S10). On the other hand, when the α-amylase concentration was fixed at 0.047 μg mL−1 and that of BSA was varied, then linearity was also observed for changes in concentration of BSA from 0.316–2.215 μg mL−1 (ESI†, Fig. S11). At several other concentrations of α-amylase, the linearity was maintained with respect to the increase in the concentration of BSA. A three-dimensional representation of the results of areas of the extinction spectra of Cit-Au NPs in the presence of binary mixtures of α-amylase and BSA is depicted in Fig. 4. Clearly the figure indicates that the area ratio was linear in the concentration range of α-amylase from 0.024 to 0.165 μg mL−1, while that of BSA was from 0.316 to 1.899 μg mL−1. A schematic representation of the process of agglomeration of the NPs in the presence of a binary mixture of α-amylase and BSA is shown in Scheme 1b (Case 1). An important point which needs to be noted here is the concentration of individual protein at which agglomerates are formed. It was observed that even in the mixture the concentration of α-amylase required for agglomeration was less in comparison to that of BSA. Earlier works from our laboratory indicated that when only α-amylase was used then the concentration of the protein required for agglomeration was less in comparison to that when only BSA was used.4 Thus the trend in concentration-dependent agglomeration in the binary mixture was commensurate with the behavior in the presence of individual proteins. Further, the linear increase in area under the extinction curve with concentration of either of the proteins indicated that this could be a facile method for estimation of proteins in a binary mixture, at least when the concentration of one is known.
 | ||
| Fig. 3 Ratio of area under the UV-vis spectrum of Cit-Au NPs in presence of different fractional content of α-amylase and BSA to that of Cit-Au NPs only, plotted against α-amylase concentration (μg mL−1). The areas were calculated based on the results in Fig. 1. Concentration of α-amylase was varied keeping the concentration of BSA constant in the final solution. Concentrations of BSA were (A) 0.316, (B) 0.633, (C) 1.266, and (D) 1.899 μg mL−1. The error bars were calculated from the results of three independent experiments. | ||
 | ||
| Fig. 4 Three-dimensional plot of ratio of area as a function of α-amylase concentration (μg mL−1) in x-axis and BSA concentration (μg mL−1) in y-axis. | ||
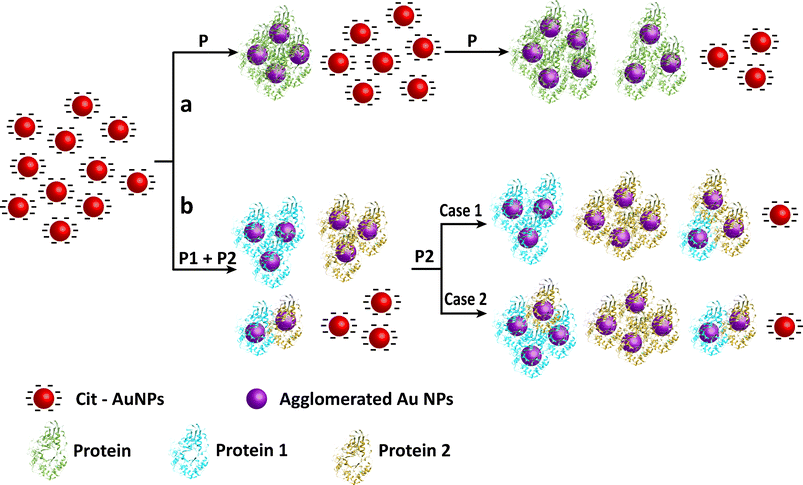 | ||
| Scheme 1 A schematic representation of the process of agglomeration of the NPs in the presence of (a) single protein and (b) binary mixtures of proteins. | ||
Further, the results from the study involving α-amylase and AMG mixtures did not clearly exhibit linearity in the increase in area, under the extinction spectrum, with concentration of either of the proteins. As an example, for a mixture of α-amylase and AMG where the concentration of AMG was fixed at 0.157 μg mL−1 and that of α-amylase was varied from 0.024 to 0.259 μg mL−1, the ratio of area under the UV-vis spectrum of Cit-Au NPs and proteins solution to that of Cit-Au NPs only did not change linearly with an increase in concentration of α-amylase (ESI†, Fig. S12). Additionally, when the concentration of α-amylase was kept constant at 0.114 μg mL−1 and that of AMG varied from 0.046–0.457 μg mL−1, the results were similar.
In case of a GOD-POD mixture, when the concentration of GOD was kept constant at 0.903 μg mL−1 and that of POD varied from 0.017–0.206 μg mL−1, the change in the ratio of area versus POD concentration was not linear (ESI†, Fig. S13). However, when changing the concentration of GOD to 0.045 μg mL−1 (which is lower than the sensitivity range of individual GOD; see the discussion below) and then varying the concentration of POD, a linear increase in the ratio of area was observed in the concentration range 0.017–0.146 μg mL−1 of POD (ESI†, Fig. S14). Interestingly, when the concentration of POD was kept constant at 0.034 μg mL−1 and that of GOD varied from 0.008–0.241 μg mL−1, again no linearity was observed. The results indicated that linearity was maintained at a low concentration of GOD in the solution. The processes of agglomeration in the case of α-amylase—AMG (Case 2) and GOD—POD (Case 1, at low GOD concentration and Case 2, at high GOD concentration) mixtures are depicted in Scheme 1b.
Further, DLS-based particle size analysis indicated that for Cit-Au NPs, the maximum of the particle size distribution curve was at 18 nm. In the presence of a low concentration of GOD (0.045 μg mL−1), when POD was added increasingly from 0.045 to 0.240 μg mL−1, the maximum gradually shifted from 22 to 37 nm (ESI†, Fig. S15 A). A closer analysis of the distribution curves (ESI†, Fig. S16 A) revealed that upon addition of binary protein mixtures (GOD and POD) to Cit-Au NPs, the number of particles in the region 9–24 nm decreased whereas the number of particles in the region 24–72 nm increased, indicating an association of particles to form agglomerated structures. Moreover, significant peaks at higher sizes (166–4936 nm) appeared on the addition of the proteins. On the other hand, at a higher concentration of GOD, when POD amount was increased from 0.017 to 0.206 μg mL−1, the number of smaller particles decreased and that of larger particles increased. However, the maxima of the distribution curves did not vary consistently. For example, in the presence of 0.903 μg mL−1 of GOD when 0.017, 0.086, and 0.120 μg mL−1 of POD were added, the maxima of the distribution curves were at 28, 24 and 28 nm respectively. In general, the maximum of the particle size distribution curves varied between 28 and 24 nm; however, the changes were not consistent with the protein concentrations (ESI†, Fig. S15 B). Peaks corresponding to higher particle size distribution also appeared (ESI†, Fig. S16 B); and their behaviors were similar. The results indicated that in the presence of a higher concentration of GOD, the addition of POD changed the particle sizes, but there was no simple correlation between them. In other words, although there were agglomerations, they did not follow any simple behaviour. Moreover, in the presence of one protein, the assay sensitivity of the second protein in the mixture could be improved in the case where linearity is observed. For example, as shown in the ESI† (Fig. S17 and S18) the sensitivity of detection of POD and GOD (in their individual protein solutions) could be as low as 0.009 and 1.091 μg mL−1 respectively. On the other hand, in the presence of a binary mixture of proteins, the sensitivity of the detection of GOD could be even higher with a value as low as 0.045 μg mL−1. Thus, this indicates an additional advantage of assay of binary protein mixture with improved sensitivity which may otherwise not be feasible using a single protein in the current method.
It has been demonstrated that proteins, upon attachment with the NPs, become unfolded and interact with excess proteins in the solution leading to agglomeration.12 The interactions between colloidal particles in a medium have traditionally been addressed by DLVO theory.18 In this case, the Coulombic forces and van der Waals interactions play major roles in the stability as well as agglomeration behaviors of the colloids. On the other hand, it has been reported lately that in the presence of high salt concentrations, i.e. 0.1 M or higher, DLVO theory fails to explain the stability and agglomeration behaviors of colloidal particles.19 In the present case, a clearer understanding of the phenomena based on DLVO theory may not be easy, as the concentration of citrate and other salts is rather high (6.5 M). The agglomeration of the NPs in the presence of proteins could be due to a combination of electrostatic and van der Waals interactions, hydrogen bonding, steric, entropic and hydrophobic interactions (following unfolding of proteins attached to the NPs). A clear and quantitative understanding of these forces is out of the bounds of the present context as the focus here is on the consequences of aggregation rather than its origin. Thus we shall restrict our conclusions based on a more qualitative analysis of the effect of agglomeration.
Considering the observations described above it can be speculated that when a binary mixture of proteins interacts with Cit-Au NPs, the composites of the NPs and proteins could form structures with three different kinds of component molecules (as depicted in Scheme 1b)—Au NPs + protein 1 (P1), Au NPs + protein 2 (P2) and Au NPs + P1 and P2. Each of these aggregates would contribute to the broadening of the spectrum. The contribution of each of the aggregate to the overall broadening could be accounted for by invoking the broadening (and accompanying shift in the peak position) of the spectral line for each constituent. For a colloidal solution with N particles per unit volume the extinction of light could be written as follows:20
 | (1) |
Here IO and I are the intensities of incident and transmitted light, l is the path length and Qext is the extinction coefficient of a single particle.
 | (2) |
Here R is the radius of the spherical NP, λ is the wavelength of the light, εm is the dielectric function of the medium, ε1 and ε2 are the complex dielectric functions of the particle core and the surface coating and g is the volume fraction occupied by the surface coating. The extinction of light integrated over wavelengths (i.e. the area under the extinction curve) could be expressed as,
 | (3) |
Here Atotal(λ) is the wavelength-dependent extinction of light by Cit-Au NPs in the presence of proteins, ANP(λ) is the wavelength-dependent extinction of light by Cit-Au NPs which remain free and do not form agglomerate, AP1(λ) is the wavelength-dependent extinction of light by P1 agglomerated Au NPs, AP2(λ) is the wavelength-dependent extinction of light by P2 agglomerated Au NPs and AP1p2(λ) is the wavelength-dependent extinction of light by Au NPs agglomerated with both P1 and P2. Further, the integrated extinction of light could be rewritten as,
 | (4) |
Here, Ntotal = NNP + NP1 + NP2 + NP1P2, where Ntotal is the total number of Au NPs present in the medium, NNP is the number of Cit-Au NPs which are free, NP1 is the number of Au NPs agglomerated with P1, NP2 is the number of Au NPs agglomerated with P2 and NP1P2 is the number of Au NPs agglomerated with both P1 and P2, all measured at the same instant of time. It has been observed earlier4 and also reported herein that the interaction of a single protein with Cit-Au NPs led to the formation of agglomerated structures for which the change in the area of extinction was linear with the concentration of protein. Thus it can be approximated that the number of particles associated with the composite formation linearly depends on the protein concentration. In other words, NP = kCP, where CP is the concentration of the protein involved and k is the proportionality constant. So far as the composite with two proteins is concerned, one can write the number of NPs involved as NP1P2 = f(P1,P2). For the integrated extinction of light to be linear with either of the protein concentrations (while the second is kept constant), the following condition needs to be satisfied:
N P1P2 = k3CP1 + k4CP2 and the integrated extinction of light could be written as,
 | (5) |
In different sets of preparations of Cit-Au NPs there are variations in the size and distribution of Au NPs and this will be reflected in the extinction spectra. Thus the total area under the curve would vary from sample to sample. If the variation is sufficiently small then the associated changes could be accommodated by dividing both sides of eqn (5) by ∫ ANP (λ) dλ. The resultant equation could be written as,
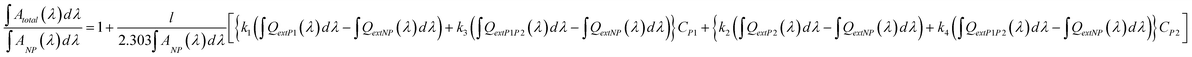 | (6) |
Therefore, the ratio of area under the extinction curve would vary linearly with either of the protein concentrations provided the concentration of the second protein is kept constant. The linear increase in the area under the curve with respect to concentrations of one of the proteins while keeping the other constant may at first indicate that interactions between the proteins and Cit-Au NPs could possibly be independent events. In other words, each protein could interact independently with the NPs and there is no secondary interaction between the two proteins involved. However, it could also be possible that the interactions between these two proteins and Cit-Au NPs were identical even when present as a mixture. In other words, if agglomeration took place with increasing concentration of one protein, the behavior would be similar for the other protein. Thus, the extent of agglomeration in the presence of increasing concentration of either of the proteins, while that of the other being kept constant, was similar. Essentially, a mixture of proteins would behave like that of a single protein, with the protein whose concentration is being increased leading the nature of the agglomerate. This would mean increasing concentration of α-amylase (with keeping BSA constant) would have a different slope than that of increasing concentration of BSA (keeping α-amylase constant). That the slopes were different (Fig. 3, 4 and ESI†, S11) support the above. Also, the concentration ranges where the linearity was maintained were similar to the results when individual proteins were used instead of a mixture.4 On the other hand, when the area is not linearly dependent on any of the protein concentration then the expression could be written as,
 | (7) |
Thus, when one of the protein concentrations is sufficiently low and hence the last term involving the concentrations of both the proteins is small, the dependence is again linear with either of the protein concentration. It is important to mention here that the extinction coefficients of agglomerated structures have been assumed to be invariant with an increase in the extent of agglomeration upon serial addition of proteins. In other words, with increase in protein concentrations, although the number of agglomerated structures and extent of agglomeration increased, the environment surrounding each NP might not have changed further in the higher agglomerated structures. Thus any change in extinction coefficient (i.e. complex dielectric function of the surface coating and the volume fraction occupied by the surface coating) might be assumed to be minimal.
Scheme 1a shows that in case of single protein the agglomeration increases systematically and thus the area under the curve increases linearly with concentration of the protein. In case of binary mixtures if the Au NPs agglomerated systematically with both P1 and P2 (as depicted in Scheme 1b, Case 1), the area under the curve would vary linearly. However, if the interaction of Au NPs with both P1 and P2 changes agglomeration abruptly (as depicted in Scheme 1b, Case 2), the area under the curve would vary non-linearly.
A binary mixture of proteins may interact with the Cit-Au NPs present in the medium leading to partial or complete replacement of the stabilizers (citrate). It is likely that both proteins would be attached simultaneously; however, their population ratio in each particle may be decided by the properties of individual protein especially its affinity toward the NP. Thus, the equilibrium population of the proteins attached to a particle would not only be decided by their concentrations in the medium but also by their three-dimensional structures with constituent amino acids, especially those exposed to the medium.21 This specificity has been observed in the interaction between a protein and Cit-Au NP, as reflected in the difference in changes of optical property of Cit-Au NPs in the presence of different mixtures of proteins. However, the interaction between a binary protein mixture and Cit-Au NP would not only depend on the specificity but also on the relative affinity. For example, one may ask the question whether a change in the area under the extinction curve would depend on the method of protein addition to the NPs. In other words, what would be the possible differences if proteins were added as a mixture versus in sequence? In this regard, when the binary mixture of α-amylase and BSA was added to 3.0 mL of Cit-Au NPs, with the final concentrations being 0.071 and 0.633 μg mL−1 respectively, broadening of the SPR peak of Cit-Au NPs was observed and the ratio of area under the UV-vis spectrum of Cit-Au NP-protein solution to that of Cit-Au NPs only was found to be 1.028 ± 0.010. Peak broadening was also observed upon addition of 0.633 μg mL−1 BSA to 3.0 mL of Cit-Au NPs already containing 0.071 μg mL−1 of α-amylase, and the ratio of area under the spectra was observed to be 1.025 ± 0.003. On the other hand, addition of 0.071 μg mL−1 of α-amylase to 3.0 mL of Cit-Au NPs already containing 0.633 μg mL−1 of BSA not only broadened the spectrum but also led to a comparatively significant increase in the ratio of the area under the spectra (to a value of 1.043 ± 0.008). The above results are from experiments performed in triplicates and the concentrations of the proteins refer to concentrations of the final solution. It could be noted that the ratios of area under the spectra for the first and second mode of addition of proteins to Cit-Au NPs were similar suggesting that the mechanism of interaction of proteins with Cit-Au NPs in these two cases were similar. However, the different value of the ratio of area under the spectra for the third mode of addition indicated the involvement of different kind of mechanism of interaction between the proteins and Cit-Au NPs. Similar results were obtained for a dispersion of Cit-Au NPs containing 0.118 μg mL−1 α-amylase and 1.899 μg mL−1 BSA. The ratios of area corresponding to different sequences of addition of proteins to the NP dispersion to reach the final mixture are shown in Tables S2 and S3 of the ESI†. The UV-vis graphs are shown in Fig. S19 (ESI†).
The above experiments were also carried out for a mixture of 0.157 μg mL−1 AMG and 0.118 μg mL−1 α-amylase as well as for a mixture of 0.045 μg mL−1 GOD and 0.043 μg mL−1 POD. In the case of α-amylase and AMG (Table S4, ESI†), when a mixture of 0.157 μg mL−1 AMG and 0.118 μg mL−1 α-amylase was added to 3.0 mL of Cit-Au NPs, the ratio of the area under the curves was found to be 1.032 ± 0.004. On addition of 0.157 μg mL−1 AMG to 3.0 mL of Cit-Au NPs already containing 0.118 μg mL−1 α-amylase, the ratio of the area was found to be 1.037 ± 0.006. On the other hand when 0.118 μg mL−1 α-amylase was added to 3.0 mL of Cit-Au NPs already containing 0.157 μg mL−1 AMG, the ratio of the area was observed to be 1.087 ± 0.014. For GOD and POD (Table S5, ESI†), when 0.045 μg mL−1 GOD and 0.043 μg mL−1 POD were added as a mixture to Cit-Au NPs, the ratio of the area was noted to be 1.001 ± 0.004. Additionally, when 0.045 μg mL−1 GOD was added to 3.0 mL of Cit-Au NPs already containing 0.043 μg mL−1 POD, the ratio of the area was almost same (1.001 ± 0.001). However, when 0.043 μg mL−1 POD was added to 3.0 mL of Cit-Au NPs already containing 0.045 μg mL−1 GOD, the ratio of the area changed to 1.017 ± 0.004.
The dependence of the ratio of area on the sequence of addition indicated different affinities of proteins for their attachment to the NPs as well as their tendencies for forming agglomerates. For example, between α-amylase and BSA, α-amylase may not only have higher affinity for the NPs but also have higher tendency for agglomeration. Thus when present as a mixture or with α-amylase being added initially, the preferential attachment of the protein in combination with a lower tendency of BSA (for agglomeration) leads to the same final structure. Thus the changes upon addition of BSA following α-amylase would be too feeble to lead to further agglomeration. On the other hand, when BSA is added initially there is a lower level of agglomeration occurring upon attachment of the protein to the NPs. However, following subsequent addition of α-amylase while some of the BSA molecules attached to the NPs may be replaced, further agglomeration would occur without disrupting the initial agglomerates. In other words, in the presence of BSA alone (at its low concentration) the level of agglomeration would be low and there may be considerable presence of NPs without agglomeration even if they contained the protein. Upon subsequent addition, α-amylase would not only replace the BSA (at least partially) present in non-agglomerated structures but would also enhance aggregation thus increasing the overall agglomeration. This would lead to an increased broadening of the spectrum, as observed. An important factor that would decide the attachment of the protein to Cit-Au NP as well as higher tendency for agglomeration could be the overall charge of the protein present in the medium. The net charge of a protein present in a medium is dependent on the pI of the protein. For example, pI values of α-amylase, BSA, AMG, GOD, and POD are 6.5, 4.7, 4.35, 4.2, and 7.2 respectively.22–25 This means BSA, AMG, and GOD would be present in the solution (at pH 7.0) with overall negative charge. On the other hand, α-amylase and POD are expected to be present close to their zwitterionic form in the solution. When the proteins are present close to their zwitterionic form it might be that they would interact with negatively charged Cit-Au NPs, either with an exposed thiol group or a positively charged functional group present in the protein. On the other hand, the proteins with overall negative charge might tend to be repelled from the negatively charged Cit-Au NPs. However, if any of those proteins contain an exposed thiol group, the interaction could be due to the thiol moiety, as thiol groups are known to have a higher affinity for Au NPs in comparison to other functional groups in proteins.26 Furthermore, it could be possible that with time the negatively charged protein molecules might replace the negatively charged citrate ions from the NP. The electrostatic stabilization of NPs by citrate could involve dynamic exchanges of citrate ions present in the solution in the absence of proteins. Thus, likewise proteins with overall negative charge could also be exchanged. Once attached to the NPs, the proteins might partially unfold and lead to agglomeration with additional proteins and NPs present in the medium. In the case of a binary mixture, the overall interaction would involve preferential attachment of one protein over the other with subsequent agglomeration in the presence of both. This would depend on the overall charge and amino acid residues of both the proteins. It may also be dependent on the partially unfolded structure of the protein attached to the NP. Thus, in the case of a α-amylase-BSA mixture, α-amylase being close to its zwitterionic form and having two exposed thiol groups,27 would bind preferentially to Cit-Au NPs over BSA, with the latter being present as a negatively charged moiety. As a result, when α-amylase and BSA were added as a mixture to Cit-Au NPs, the ratio of area was similar to that when α-amylase was added followed by BSA to Cit-Au NPs. Similar was the case for α-amylase and AMG mixture. Moreover, in the case of the GOD-POD mixture, POD (having no thiol group;27 pI 7.2) interacted with Cit-Au NPs preferentially over GOD (although having exposed thiol groups;28 pI 4.2). This might be due to the presence of its zwitterionic form which leads to the preferential attachment of the protein over GOD to the NPs. This indicated that overall charge of the protein in the solution may play a pivotal role in interaction with Cit-Au NPs. However, the role of exposed thiol groups cannot be ruled out completely.
Since among the combinations of binary mixture of proteins mentioned above the linearity of increase of area under the extinction curve of Cit-Au NPs versus the protein concentration was clearly observed for α-amylase and BSA, the interactions between the two proteins and Cit-Au NPs were investigated in more detail. This was further pursued by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), enzymatic activity assay of α-amylase, photoluminescence, and FTIR studies. SDS-PAGE analysis (Fig. 5) revealed the presence of a major band around 66 kDa, corresponding to BSA (lane 2). In the case of pure α-amylase, a band around 50 kDa was evident corresponding to the expected size of porcine pancreatic α-amylase (lane 3). It could also be seen that other minor bands were visible in both lanes 2 and 3 and presumably were due to degradation products of pure proteins in the presence of the denaturing gel. In case of composite of Cit-Au NPs (lane 4), an upper band was observed whose mobility coincided with that of pure BSA (indicated by line ‘a’ in lane 4). Likewise, a band of lesser intensity was observed corresponding to the size of α-amylase (indicated by line ‘b’ in lane 4). This confirmed the presence of both α-amylase and BSA in the NP-protein composite. The fact that the bands migrated separately in the gel indicates that the proteins dissociated from the composite and migrated as individual proteins in denaturing gel conditions. The bands were less intense due to a smaller amount of proteins present in the NP-protein composite. The other minor bands in the composite (lane 4) were likely the degradation products of the original proteins as observed in lanes 2 and 3. No band was observed in lane 5, which corresponded to Cit-Au NPs only.
 | ||
| Fig. 5 SDS-PAGE analysis of Au NP-protein composite. Wells correspond to (1) Protein Marker, (2) BSA, (3) α-amylase, (4) α-amylase-BSA-Au NP composite and (5) Cit-Au NPs only. | ||
For enzymatic activity of α-amylase, a starch agar plate assay was performed (Fig. 6). Around wells a and c (corresponding to pure α-amylase and composite respectively), clear zones were observed which indicated the presence of α-amylase with observable retention of activity. As seen from the figure, the zone of clearance for the composite was smaller than for pure α-amylase. This could be because of limited diffusion of Au NP-protein composite in the solid medium compared to that of the free enzyme. Moreover, the amount of enzyme associated with the composite was less compared to that of the added free enzyme. On the other hand, no such clearing zone was observed around wells b, d, and e (corresponding to phosphate buffer, BSA, and Cit-Au NPs respectively). Further experiments were performed with three different concentrations of the protein mixtures (1.266 μg mL−1 BSA and 0.094 μg mL−1 α-amylase, 1.266 μg mL−1 BSA and 0.165 μg mL−1 α-amylase and 1.266 μg mL−1 BSA and 0.235 μg mL−1 α-amylase). All of them exhibited enzyme activity. Thus not only was the α-amylase present in the mixture, but its activity was also considerably retained (ESI†, Fig. S20).
 | ||
| Fig. 6 Starch agar plate assay for enzymatic activity of (a) pure α-amylase, (b) phosphate buffer, (c) α-amylase-BSA-Au NP composite, (d) BSA and (e) Cit-Au NPs only. | ||
It is known that when protein is mixed with Cit-Au NPs the fluorescence due to tryptophan residue occurring at 360 nm gets quenched, indicating the attachment of the protein to the NP.29 In the present set of experiments, the emission peak at 360 nm, for α-amylase, BSA, and the mixture of the two, disappeared in the presence of Cit-Au NPs. The fluorescence spectroscopic results are shown in Fig. 7. The loss of fluorescence of the mixture of proteins in the presence of Cit-Au NPs indicated that both of them possibly were attached to the NPs at the total protein concentration. Interestingly, when BSA (1.582 μg mL−1) was added to α-amylase (0.118 μg mL−1) treated Cit-Au NPs, its fluorescence was also quenched. This indicates that even in the presence of α-amylase, at its sufficiently low concentration, BSA interacted with Cit-Au NPs. A similar observation was made when α-amylase (0.118 μg mL−1) was added to Cit-Au NPs containing BSA (1.582 μg mL−1). The time-dependent study of Cit-Au NPs in the presence of 0.118 μg mL−1 α-amylase and 1.582 μg mL−1 BSA (ESI†, Fig. S21) indicated that within 1 min of mixing the proteins with the NPs, the fluorescence was quenched. This indicated that the proteins were attached to the NPs immediately upon addition.
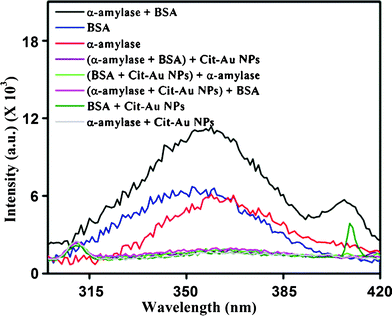 | ||
| Fig. 7 Fluorescence spectra of α-amylase and BSA in the presence and absence of Cit-Au NPs. | ||
FTIR spectroscopy is also an important tool to study the interaction between a biomolecule and NP.27 For example, it has been used to study the conformational changes in the secondary structure of proteins upon interaction with Au NPs.6 There are three types of amide linkages in proteins—amide I, amide II, and amide III—of which amide I absorbs between 1600–1700 cm−1.30 For BSA (Fig. 8A) as well as α-amylase (Fig. 8B), the FTIR band observed in the region 1625–1700 cm−1 is due to amide I linkage whereas for Cit-Au NPs (Fig. 8C), the band around 1589 cm−1 is due to the presence of the acidic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of citrate on its surface (ESI†, Fig. S22). In the NP-protein composite containing 0.118 μg mL−1 α-amylase and 1.899 μg mL−1 BSA (Fig. 8D), a broad band in the region 1500–1750 cm−1 was observed and it appears to be a combination of three bands. The band around 1589 cm−1 matched with that of Cit-Au NPs and the appearance of a shoulder at around 1663 cm−1 indicated the presence of proteins on the surface of Au NPs, in addition to citrate. This also supports the partial replacement of trisodium citrate by proteins.4 Furthermore, the occurrence of an additional peak at 1615 cm−1 indicated an interaction between the protein and Au NP. The proteins, on interacting with Au NPs, possibly adopt a more incompact conformational state as such changes at both the secondary and tertiary structure levels are known to occur.6 The appearance of this band can also be due to H–bonding of the amide C
O group of citrate on its surface (ESI†, Fig. S22). In the NP-protein composite containing 0.118 μg mL−1 α-amylase and 1.899 μg mL−1 BSA (Fig. 8D), a broad band in the region 1500–1750 cm−1 was observed and it appears to be a combination of three bands. The band around 1589 cm−1 matched with that of Cit-Au NPs and the appearance of a shoulder at around 1663 cm−1 indicated the presence of proteins on the surface of Au NPs, in addition to citrate. This also supports the partial replacement of trisodium citrate by proteins.4 Furthermore, the occurrence of an additional peak at 1615 cm−1 indicated an interaction between the protein and Au NP. The proteins, on interacting with Au NPs, possibly adopt a more incompact conformational state as such changes at both the secondary and tertiary structure levels are known to occur.6 The appearance of this band can also be due to H–bonding of the amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O with water molecules.31 As the protein structure becomes more flexible on interacting with Cit-Au NPs, the amide linkages become more prone to H–bonding with water molecules, and possibly, as a result, the band downshifts. The above experiment along with SDS-PAGE, enzymatic activity assay of α-amylase, and fluorescence results indicated that both α-amylase and BSA interacted with Cit-Au NPs. Thus when binary mixtures of α-amylase and BSA were added to Cit-Au NPs not only were both proteins attached to the NPs but they were also a part of the composite of the proteins and NPs, which led to systematic spectral broadening.
O with water molecules.31 As the protein structure becomes more flexible on interacting with Cit-Au NPs, the amide linkages become more prone to H–bonding with water molecules, and possibly, as a result, the band downshifts. The above experiment along with SDS-PAGE, enzymatic activity assay of α-amylase, and fluorescence results indicated that both α-amylase and BSA interacted with Cit-Au NPs. Thus when binary mixtures of α-amylase and BSA were added to Cit-Au NPs not only were both proteins attached to the NPs but they were also a part of the composite of the proteins and NPs, which led to systematic spectral broadening.
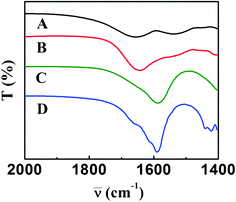 | ||
| Fig. 8 FTIR spectra of (A) BSA, (B) α-amylase, (C) Cit-Au NPs only and (D) Cit-Au NPs in presence of a mixture of 0.118 μg mL−1 α-amylase and 1.899 μg mL−1 BSA. | ||
4 Conclusions
In summary, we have been able to demonstrate that there is specificity in the interaction of a binary mixture of proteins with Cit-Au NPs and the interactions could be different from those with individual proteins or simple summation of interactions of the component proteins. While a mixture of α-amylase and BSA consistently changed the level of agglomeration with an increase of one in the presence of other; the mixture of α-amylase and AMG behaved completely differently. However for a GOD-POD mixture, the consistent increase in agglomeration of NPs depended on the concentration of GOD in the solution. High concentrations of GOD led to lowering of agglomeration of Cit-Au NP-protein mixture, while that at low concentrations gave rise to systematic agglomeration. Thus, it is not necessary that the presence of proteins will lead to significant agglomeration of Cit-Au NP; however, that would be dependent on the nature of proteins and their concentrations. In the case of individual proteins, the area under the SPR spectrum of Cit-Au NPs changed consistently with an increase in the concentration of the protein.4 In the case of α-amylase-BSA and GOD-POD (at low concentrations of GOD) mixtures, the trend was maintained but not in case of the α-amylase-AMG mixture, nor in the case of high concentrations of GOD in the GOD-POD mixture. Interestingly, the results also suggest that a particular protein may preferentially bind with Cit-Au NPs over another one, depending on the overall charges of the proteins in a solution. These behaviors were also reflected in the optical properties of the Cit-Au NPs in the presence of the mixtures of the proteins.Finally, the present investigations indicated the possibility of a rich and diverse nature of interactions between Cit-Au NPs and mixtures of proteins. Thus, when Au NPs are present inside a living cell, their interactions with the biomolecules in the milieu may possibly be more than the sum of their interactions with individual molecules of the cell. Further, the study also points out that in order to understand and address the issue of the persistent presence of nanomaterials in living beings and in the environment, it is imperative that efforts may be made to probe the complexity of the interactions rather than assuming them to be a simple summation of individual interactions.
Acknowledgements
We thank the Department of Science and Technology (SR/S1/PC-30/2008), and the Department of Biotechnology (BT/49/NE/TBP/2010), India for funds. R. K. thanks the Council of Scientific and Industrial Research (CSIR) for a fellowship (09/731(0105)/2010-EMR-I). We also thank Dr S. S. Ghosh and Dr A. Ramesh for valuable suggestions, Dr Pallab Sanpui and Mr. Manab Deb Adhikari for help, and Prof. K. Ismail for allowing us to carry out DLS measurements.References
- A. T. Gates, S. O. Fakayode, M. Lowry, G. M. Ganea, A. Murugeshu, J. W. Robinson, R. M. Strongin and I. M. Warner, Langmuir, 2008, 24, 4107–4113 CrossRef CAS.
- Y. M. Chen, C. J. Yu, T. L. Cheng and W. L. Tseng, Langmuir, 2008, 24, 3654–3660 CrossRef CAS.
- C. M. Stoscheck, Anal. Biochem., 1987, 160, 301–305 CrossRef CAS.
- J. Deka, A. Paul and A. Chattopadhyay, J. Phys. Chem. C, 2009, 113, 6936–6947 CAS.
- S. Chah, M. R. Hammond and R. N. Zare, Chem. Biol., 2005, 12, 323–328 CrossRef CAS.
- L. Shang, Y. Wang, J. Jiang and S. Dong, Langmuir, 2007, 23, 2714–2721 CrossRef CAS.
- G. Harrison, P. Haffey and E. E. Golub, Anal. Biochem., 2008, 380, 1–4 CrossRef CAS.
- M. Moeremans, G. Daneels and J. D. Mey, Anal. Biochem., 1985, 145, 315–321 CrossRef CAS.
- S. H. D. P. Lacerda, J. J. Park, C. Meuse, D. Pristinski, M. L. Becker, A. Karim and J. F. Douglas, ACS Nano, 2010, 4, 365–379 CrossRef.
- B. Tang, S. Xu, J. Tao, Y. Wu and W. Xu, J. Phys. Chem. C, 2010, 114, 20990–20996 CAS.
- J. Deka, A. Paul and A. Chattopadhyay, Nanoscale, 2010, 2, 1405–1412 RSC.
- D. Zhang, O. Neumann, H. Wang, V. M. Yuwono, A. Barhoumi, M. Perham, J. D. Hartgerink, P. W. Stafshede and N. J. Halas, Nano Lett., 2009, 9, 666–671 CrossRef CAS.
- M. P. Monopoli, D. Walczyk, A. Campbell, G. Elia, I. Lynch, F. B. Bombelli and K. A. Dawson, J. Am. Chem. Soc., 2011, 133, 2525–2534 CrossRef CAS.
- C. Lopez, A. Torrado, P. Fucinos, N. P. Guerra and L. Pastrana, J. Agric. Food Chem., 2004, 52, 2907–2914 CrossRef CAS.
- L. L. Salomon and J. E. Johnson, Anal. Chem., 1959, 31, 453–456 CrossRef CAS.
- D. Aussawasathien, P. He and L. Dai, ACS Symp. Ser., 2006, 918, 246–268 CrossRef CAS.
- S. Zhang, N. Wang, H. Yu, Y. Niu and C. Sun, Bioelectrochemistry, 2005, 67, 15–22 CrossRef CAS.
- J. N. Israelachvili, Intermolecular & Surface Forces, 2nd ed.; New York: Academic Press, 1992 Search PubMed.
- M. Bostrom, D. R. M. Williams and B. W. Ninham, Phys. Rev. Lett., 2001, 87, 168103 (1)–168103(4) CrossRef.
- P. Mulvaney, Langmuir, 1996, 12, 788–800 CrossRef CAS.
- C. Rocker, M. Potzl, F. Zhang, W. J. Parak and G. U. Nienhaus, Nat. Nanotechnol., 2009, 4, 577–580 CrossRef.
- A. Lamanda, Z. Cheaib, M. D. Turgut and A. Lussi, PLoS One, 2007, 2, e263 Search PubMed.
- K. Y. Chun and P. Stroeve, Langmuir, 2002, 18, 4653–4658 CrossRef CAS.
- J. H. Pazur and K. Kleppe, Biochemistry, 1964, 3, 578–583 CrossRef CAS.
- C. Xialing and M. Lin, J. Biochem. Tech., 2009, 1, 92–95 Search PubMed.
- A. Faccenda, C. A. Bonham, P. O. Vacratsis, X. Zhang and B. Mutus, J. Am. Chem. Soc., 2010, 132, 11392–11394 CrossRef CAS.
- A. Rangnekar, T. K. Sarma, A. K. Singh, J. Deka, A. Ramesh and A. Chattopadhyay, Langmuir, 2007, 23, 5700–5706 CrossRef CAS.
- V. R. S. Babu, M. A. Kumar, N. G. Karanth and M. S. Thakur, Biosens. Bioelectron., 2004, 19, 1337–1341 CrossRef CAS.
- M. Iosin, F. Toderas, P. L. Baldeck and S. Astilean, J. Mol. Struct., 2009, 924-926, 196–200 CrossRef CAS.
- W. K. Surewicz, H. H. Mantsch and D. Chapman, Biochemistry, 1993, 32, 389–394 CrossRef CAS.
- N. S. Myshakina, Z. Ahmed and S. A. Asher, J. Phys. Chem. B, 2008, 112, 11873–11877 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Additional UV-vis, SDS-PAGE, enzymatic assay of α-amylase, fluorescence, FTIR and DLS experimental details, Bradford assay UV-vis and standard curve, additional UV-vis spectra and their analyses, TEM images, DLS-based particle size analysis results, time dependent fluorescence spectra, FTIR spectra, sensitivity graphs of individual GOD and POD, and various tables are available. See DOI: 10.1039/c2ra20096a/ |
| This journal is © The Royal Society of Chemistry 2012 |
