Triacylglycerol composition of British bluebell (Hyacinthoides non-scripta) seed oil
Vera
Thoss
*a,
Patrick J.
Murphy
a,
Ray
Marriott
b and
Thomas
Wilson
ac
aSchool of Chemistry, Bangor University, Bangor, LL57 2UW, UK
bBioComposites Centre, Bangor University, Bangor, LL57 2UW, UK
cInstitute of Biological, Environmental and Rural Science, Aberystwyth University, Gogerddan, Aberystwyth, SY23 3EB, UK
First published on 13th April 2012
Abstract
Bluebell seeds were collected from the same location for five different growth periods (2006–2010). The composition of fatty acids in the triacylglycerols present in bluebell seeds was determined using 1H- and 13C NMR and GC-MS of fatty acid methyl esters with good agreement between the different methods of analysis for the proportion of individual fatty acids. The seed oil comprised 80% ω-9 monounsaturated (C18:1, C20:1, C22:1), 10% ω-6,9 biunsaturated (C18:2) and 10% saturated fatty acids (C16:0, C18:0, C20:0, C22:0). The oil contained 25% of fatty acids with 20 or 22 carbon chain length. Gondoic acid (C20:1) was present at 20% and there was a consistency in the composition of the seed oil for the different harvest years. Based on the composition of bluebell seed oil, possible future uses are suggested and the combination of bio refining bluebell seeds in tandem with conservation efforts is proposed.
Introduction
The current drive to shift from mineral feedstock to renewable resources requires assessing the potential of different plant species to provide chemical compounds for different uses. The non-food use of rapeseed oil, for example, has increased sevenfold in the last decade.1 Seed crops are assessed for their oil content and suitability for biodiesel production,2–4 nutritional supplements or industrial chemicals.1,5,6 However, seed crops are mainly food sources and an emphasis is now placed on finding species that do not compete with food crops for the land required for their production while being ideally high yielding in oil and containing oil with unusual fatty acids.1 Here we report on the fatty acid composition of the seeds of the wildflower British Bluebell (Hyacinthoides non-scripta (L.) Chouard ex Rothm.) which has not been reported on previously.British bluebells (from here on referred to as “bluebells”) are woodland plants that carpet the woodland floor in broadleaved forests. Populations often contain millions of individuals which result in characteristic blue carpets in spring. This annual spectacle is worthy of reporting in the national news and the British bluebell has been voted the Nation's favourite wildflower (Plantlife International survey 2002). Bluebells also often grow on grasslands and in conjunction with bracken (Pteridium aquilinum) which is deemed to be indicative of sites of ancient woodlands.7 Bluebell populations are sensitive to drought and are found more often on the Western side of the British Isles including exposed cliffs. The geographical extent of British bluebells also includes the North-Western part of the European mainland.8,9
Bluebells propagate predominantly via seeds with vegetative reproduction being reported for between 3 to 8% of individual plants occurring in the populations.10,11 Germination is triggered by the reduction in the mean temperature to below 10 °C.12 In the first year of growth one leaf and a bulblet is formed. Bluebells are perennial plants and each year of growth results in an increase in biomass above and below ground. They have contractile roots which result in the bulb being drawn deeper into the ground each year up to a depth of 25 cm.13 After five years of growth flowers may be formed which, after successful cross pollination,14 yield around 100 seeds per plant. The consumption of the leaves and flowers has been observed for muntjac deer15 and roe deer16 and seed predation has been documented for wood mice and bank voles.17 (For a general ecological description of British bluebells consult Blackman and Rutter, 19548).
British bluebells are now protected under the Wildlife and Countryside Act 1981 (as amended in 1998) due to habitat loss,18 atmospheric deposition of nitrogen19 and hybridisation with congenerics.9 The sale of wild British bluebells, whole plants, bulbs, seeds or derivatives, is only permitted through a licence.
Bluebells are considered poisonous plants with little reference of medicinal usage transmitted in British folklore. One mention in the “Physicians of Meddvai”, a collection of herbal treatments used in 13th century Wales, included “wild hyacinth” as part of a leper treatment. Ethnobotanical use of boiled bluebell bulbs for treating coughs and whitlows was reported from Ireland while in Scotland fried bluebell bulbs were used as a plaster to promote suppuration.20
Bluebell bulbs, leaves and flowers have been chemically analysed previously, however, the main focus has been on the carbohydrate content which consists predominantly of fructans as reserve carbohydrate.21 Considerable interest was shown in the iminosugar content of bluebells with several groups22–25 investigating this area. Iminosugars are monosaccharide analogues in which the endocyclic oxygen is replaced by a nitrogen. The main iminosugar found in bluebells is DMDP ((2R,3R,4R,5R)-2,5-dihydroxymethyl-3,4-dihydroxypyrrolidine). Several other pyrrolidine containing iminosugars were also isolated in lower amounts as well as an arabinose analogue. Iminosugars in bluebells are the most likely chemical constituents that could be attributed to the toxicity of bluebells implicated in livestock poisoning.26,27
Our work describes the first stage in bio refining bluebell seeds through extraction and chemical characterisation of the lipid fraction. The fatty acid composition is given based on high temperature GC-MS, 1H NMR and 13C NMR data of crude oil, and GC-MS of fatty acid methyl esters (FAMEs) and fatty acid trimethylsilyl (TMS) esters.
Experimental
Bluebell Seed Collection
Bluebell seeds were collected under licence from the location N 53° 07′ and W 04° 08′ at a height of 250 m above sea level. The site encompasses about one hectare with a bluebell coverage of half the area. The main vegetation present is bracken with a height of up to 1.5 m. Also present are Gelium saxatila, Horcus linatus, Tucrium scordunium, Agrostis vineale and Rumex acitocellar. Seeds were manually collected in the months of July and August in the years 2006 to 2010.Every year the seeds were manually removed from the husks soon after harvesting and dried spread out on netting at ambient temperature. Once dried (the water content was not determined) the seeds were placed in sealed plastic containers and stored at room temperature. Processing and storage conditions were varied.
Seed weights were recorded for each harvest year by weighing 100 seeds (repeated five times). For the water content measurement seeds were dried at 100 °C overnight and their loss in mass recorded.
Extraction of Bluebell Seed Oil
Seeds (100 g) were flaked three times using the Eschenfelder Seed and Grain Flaker 1200. Flaked seeds were stirred in n-hexane (200 cm3) at room temperature (RT) for 2 h. This was repeated until no further oil was extracted (up to four times). The extract was dried over MgSO4 to remove co-extracted water, and then filtered under gravity. n-Hexane was removed by rotary evaporation at 45 °C to yield a clear golden oil. The oil was stored at room temperature until further use.1H NMR and 13C NMR
1H NMR characterisation was performed on a Bruker BioSpin 500 MHz at 500 MHz and 13C NMR recorded at 125 MHz. Samples (approx. 10 mg) were run in CDCl3 at 25 °C, with a relaxation time of 2.0 s.In order to calculate the relative percentage of saturated (SFA), monounsaturated (MUFA) and polyunsaturated fatty acids (PUFA) in the bluebell seed oil using 1H NMR (Fig. 1), the integrals of the different proton environments were used.
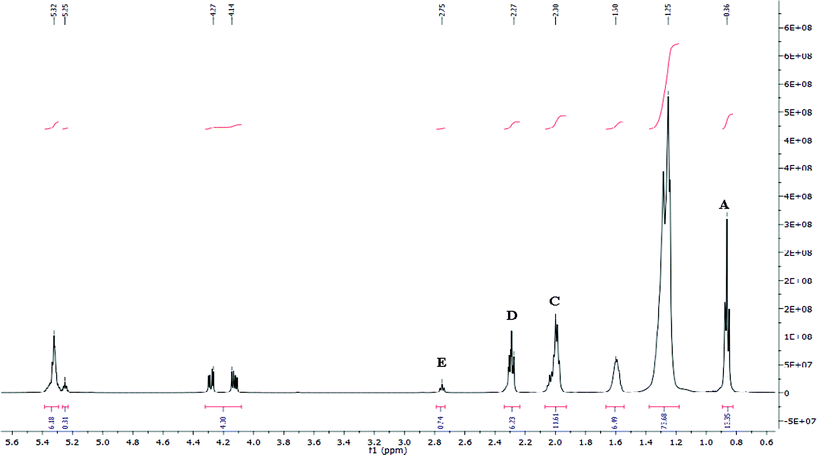 | ||
| Fig. 1 1H NMR spectrum of crude bluebell seed oil, with labelled proton environments which are used for the determination of the relative percentages of MUFA, PUFA and SFA. | ||
The terminal methyl group (A) at δ0.86 ppm is equivalent to 3 protons, and is present in both saturated and unsaturated fatty acids. The relative amount of the PUFA can be calculated using the integrations of the acyl group at δ2.27 ppm (D) and the protons attached to the bis-allylic carbon at δ2.75 ppm (E). The acyl group at δ2.27 ppm is equivalent to two protons and contributes one methylene group to all types of fatty acids. The protons attached to the bis-allylic carbon are seen at δ2.75 ppm, and contribute two protons but are only present in the biunsaturated fatty acids present within the bluebell seed oil. The relative amount of PUFA can be calculated by;
| PUFA = (E/D) |
The shift at δ2.00 ppm (C) is characteristic of the allylic region which is present in all unsaturated fatty acids, irrelevant of the degree of unsaturation. The relative amount of MUFA is calculated using the integrations of the α-allylic proton environment (C) and the acyl group (D). The α-allylic environment contributes two methylene groups in total, for both MUFA and PUFA. The fraction (C/2D) gives the relative amount of the total unsaturation present, so therefore the relative MUFA content can be calculated using;
| MUFA = [(C/2D)] − PUFA |
Finally the amount of SFA can be determined by subtracting the relative total amount of unsaturation from the total FA amount.
| SFA = 1 − (C/2D) |
Ozonolysis of Bluebell Seed Oil
Bluebell seed oil (0.5 g) was dissolved in dichloromethane (200 cm3) in a three necked 500 cm3 flask. After cooling to −70 °C, using a liquid N2 acetone bath, ozone was passed through the flask at a rate of 4 mmol min−1 for 1 min 6 s, when a pale blue colour was visible in the flask. Excess O3 was removed using a stream of N2. At this point NaBH4 (0.3 g) and MeOH (100 cm3) was added, the solution slowly warmed to RT and stirred for 3 h under N2. Deionised H2O (200 cm3) and H2SO4 (2 cm3, 10% v/v) were added and the resulting mixture separated. The aqueous phase was extracted with dichloromethane (3 × 100 cm3). The combined organic extracts were dried (MgSO4) and filtered.GC-MS analysis of the extract was performed on a Perkin Elmer Clarus 680 GC equipped with a VF-5 MS column (L 30 m × ID 0.25 mm × DF 0.25 μm). Injection was split at a ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with an injection volume of 1 μl at 300 °C. The initial oven temperature was 60 °C which was held for 1 min then increased at a rate of 60 °C min−1 up to a temperature of 300 °C, then held for 10 min. The phase transfer line temperature was kept at 270 °C. Mass spectra were collected on a Perkin Elmer 600 C (Qp) MS. Electron impact (70 eV) ionisation was used for fragmentation with a rate of 1 scan per second. All processing and analysis was carried out on a Turbo Mass V.5.4.7.
1 with an injection volume of 1 μl at 300 °C. The initial oven temperature was 60 °C which was held for 1 min then increased at a rate of 60 °C min−1 up to a temperature of 300 °C, then held for 10 min. The phase transfer line temperature was kept at 270 °C. Mass spectra were collected on a Perkin Elmer 600 C (Qp) MS. Electron impact (70 eV) ionisation was used for fragmentation with a rate of 1 scan per second. All processing and analysis was carried out on a Turbo Mass V.5.4.7.
High Temperature GC-MS Analysis of Bluebell Seed Oil
Direct GC-MS analysis of the bluebell seed oil was performed on a Perkin Elmer Clarus 680 GC equipped with a ZB-5HT column (L 15 m × ID 0.25 mm × DF 0.25 μm) using helium as carrier gas at 2 cm3 min−1. Injection was split at a ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with an injection volume of 1 μl at 300 °C. The injector was fitted with a packed (silanized glass wool) liner and the column was calibrated with a C10–C60 alkane standard which showed no mass discrimination across this wide mass range. The initial oven temperature was 100 °C increasing at a rate of 10 °C min−1 up to a final temperature of 425 °C and then held for 10 min. The transfer line temperature was kept at 300 °C and mass spectra were acquired using a Perkin Elmer Clarus 600 MS. All spectra were obtained using electron impact (70 eV) ionisation with a scan rate of 1 scan per second. All processing and analysis was carried out using Perkin Elmer Turbo Mass V.5.4.7 software. GC analysis was carried out using the same conditions except a Perkin Elmer Autosystem XL was used with a flame ionisation detector. Samples were prepared by dissolving 10 mg bluebell seed oil in 1 cm3n-heptane.
1 with an injection volume of 1 μl at 300 °C. The injector was fitted with a packed (silanized glass wool) liner and the column was calibrated with a C10–C60 alkane standard which showed no mass discrimination across this wide mass range. The initial oven temperature was 100 °C increasing at a rate of 10 °C min−1 up to a final temperature of 425 °C and then held for 10 min. The transfer line temperature was kept at 300 °C and mass spectra were acquired using a Perkin Elmer Clarus 600 MS. All spectra were obtained using electron impact (70 eV) ionisation with a scan rate of 1 scan per second. All processing and analysis was carried out using Perkin Elmer Turbo Mass V.5.4.7 software. GC analysis was carried out using the same conditions except a Perkin Elmer Autosystem XL was used with a flame ionisation detector. Samples were prepared by dissolving 10 mg bluebell seed oil in 1 cm3n-heptane.
Silylation of the Free Fatty Acids Present in Bluebell Seed Oil
Bluebell seed oil (5 mg) was dissolved in 1 cm3 of a 20% solution of 1-(trimethylsilyl)-imidazole in dry pyridine. The mixture was incubated at 70 °C for 60 min with continuous stirring. When cool 2 cm3n-heptane was added, mixed and the upper layer analysed using the method described for the ozonolysis reaction mixture above.Transesterification of Bluebell Seed Oil
Bluebell seed oil (2.0 g) was dissolved in sodium methoxide (8 mmol, 20 cm3) and heated under reflux for 20 min to produce FAMEs. Deionised H2O (50 cm3) was then added, and the solution extracted with n-hexane (3 × 100 cm3). The combined organic extracts were dried (MgSO4), filtered and evaporated at 45 °C.The transesterified samples, diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 with hexane containing 100 mg dm−3 ethylpalmitate as internal standard, were analysed using two different methods:
1000 with hexane containing 100 mg dm−3 ethylpalmitate as internal standard, were analysed using two different methods:
Method 1 Gas chromatography-quadrupole-mass spectrometry (GC-Qp-MS) used a Thermoquest Finnigan Trace GC 2000, with a Chrompack silica fused DB-5 column (L 25 m × ID 0.32 mm × DF 0.25 μm). A 1 μl sample was injected at 270 °C in splitless mode, after 0.5 min the split valve was opened with a split flow of 20 cm3 min−1. The initial oven temperature was 100 °C (held for 2 min), ramped at 5 °C min−1 up to 270 °C (held for 10 min). The phase transfer line temperature was held at 270 °C. Mass spectra were collected on a Thermoquest Voyager Qp MS. Electron impact (70 eV) ionisation was used for fragmentation with a rate of 1 scan per second. Processing was carried out on Xcalibur v1.2.
Method 2 Gas chromatography-time of flight-mass spectrometry (GC-ToF-MS) used an Agilent 6890N GC fitted with an OmegaWax DB-5 column (L 30 m × ID 0.25 mm × DF 0.25 μm). Split injection of 1 μl at 250 °C was used with a split flow of 20 cm3 min−1. The initial oven temperature was 150 °C (held for 2 min), ramped at 5 °C min−1 up to 260 °C (held for 5 min). Mass spectra were collected on a Micromass GCT ToF-MS. Electron impact (70 eV) ionisation was used for fragmentation with a rate of 2 scans per second. Processing was carried out on MassLynx v4.0.
FAMEs were identified using a 37 FAME standard mixture (Supelco) based on retention time, molecular ion peak and characteristic fragmentation pattern in the mass spectrum. Percentage peak areas, as reported, were calculated by using Xcalibur and MassLynx. Manual integration was performed when necessary.
The robustness of the transesterification of the crude bluebell seed oil was assessed by triplicate determination of a total of six times (n = 17). The relative standard error for the proportion of individual FAMEs using method 1 ranged from 0.01 to 0.29%.
Hydrolysis of Bluebell Seed Oil
Bluebell seed oil (5.0 g) was dissolved in ethanolic KOH (50 cm3, 2.6 M) and heated under reflux for 4 h to produce free fatty acids. Deionised H2O (100 cm3) and HCl (25 cm3, 18 M) was added. The solution was then extracted with n-hexane (3 × 100 cm3). The organic phase was dried (MgSO4), filtered and residual solvent evaporated at 45 °C. The presence of free fatty acids was confirmed by 1H NMR and then analysed by 13C NMR as described above, for the determination of the position of unsaturation.Results
Bluebell Seeds
After flowering, bluebell flowers turn into green and fleshy “fruits” while the stem slowly erects. The seeds gradually ripen and once ripe the plant draws down its resources leaving behind a translucent stem and fruit husks. The fruit husks open gradually from the bottom to the top of the stem. All the above ground biomass was collected as seeds spill out easily from the husks. Bluebell seeds are round, black and shiny and their maximum length is 2 mm. They have a pointed end which allows the seed to attach onto the desiccated husk. An individual seed weighed 6.06 ± 0.06 mg for all harvest years whilst the water content ranged from 10 to 15%. It was observed that there was no physical injury on the seeds of any harvest year. However, some batches (not used for oil extraction) displayed fungal growth on prolonged storage at room temperature.Chemical Characteristics of Bluebell Seed Oil
As the seeds are very hard the best method for extracting the oil was via flaking them first which physically breaks and flattens the seeds. The extracted oil is golden in colour and the yields for the different harvest years ranged from 22.2 to 24.9% by mass for dry seeds.The refractive index of the bluebell seed oil ranged from 1.461 to 1.468 depending on the harvest year and batch. The unsaponifiable fraction of the oil was 1.5% (harvest 2009). Different techniques were employed to further characterise the triglycerides: 1H NMR, 13C NMR and GC-MS of the FAMEs.
1H- and 13C NMR Spectroscopy
The 1H NMR of the crude oil showed the characteristic resonances reported for other seed oils29 (Table 1, Fig. 1). Based on the integration of the chemical shifts the relative % of SFA, MUFA and PUFA present was calculated (Table 2). The absence of a proton shift at δ0.93 ppm (Fig. 1) indicated no trivinyl methylene environments and thus the absence of α-triunsaturated fatty acids, such as α-linolenic acid, or any ω3-monounsaturated fatty acids.| δ (ppm) | Proton | Compound |
|---|---|---|
| 0.86 | –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3 3 |
Terminal methyl |
| 1.25 | C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2 2 |
Methylene |
| 1.60 | C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–CH2–COO 2–CH2–COO |
All acyl chains |
| 2.00 | C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–CH 2–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH CH |
All unsaturated fatty acids |
| 2.27 | C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–COO 2–COO |
All acyl chains |
| 2.75 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C C–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2C 2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
Protons attached to bis allylic carbon |
| 4.14–4.27 | C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2O(α) 2O(α) |
Glycerol (triglycerides) |
| 5.25 | C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) O (β) O (β) |
Glycerol (triglycerides) |
| 5.32 | C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) |
Olefinic (all unsaturated fatty acids) |
| 2006 | 2007 | 2008 | 2009 | 2010 | |
|---|---|---|---|---|---|
| MUFA | 79 | 80 | 78 | 79 | 82 |
| PUFA | 11 | 11 | 12 | 11 | 11 |
| SFA | 10 | 9 | 10 | 10 | 7 |
13C NMR of the crude bluebell oil showed resonances for both the carbon chains of the fatty acid tails and the glycerol (Fig. 2, Table 3). Resonances at 172.68 ppm and 173.10 ppm were assigned to the carboxy carbons which can be used to assign α and β substitution of the glycerol backbone. 13C NMR also showed the unsaturated region of the fatty acid carbon chains. The presence of the glycerol backbone resulted in an α- and β-carbon signal for carbons in the alkene region.
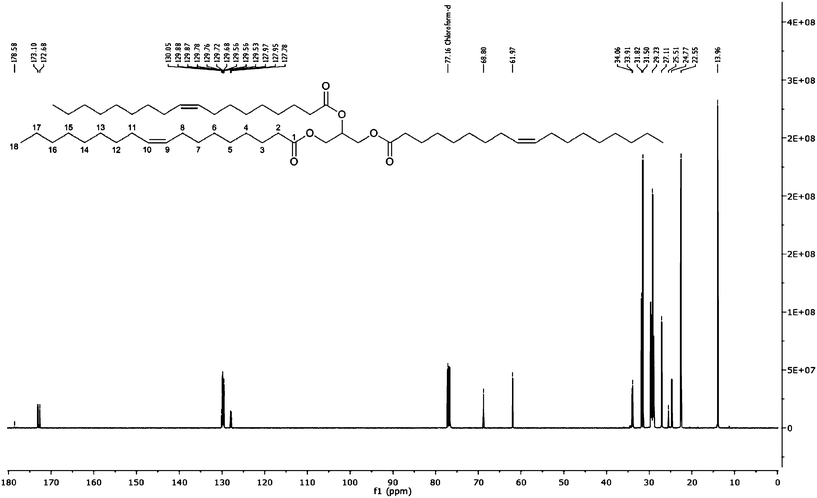 | ||
| Fig. 2 13C NMR spectrum of crude bluebell seed oil with labelled carbons of triolein, showing the 13C regions used for the assignment in Table 3. | ||
| δ (ppm) | Carbon | Assignment |
|---|---|---|
| 13.96 | α-CH3 | All acyl chains |
| 22.55 | β-CH3 | All acyl chains |
| 24.77 | C3 | All acyl chains |
| 25.51 | C11 | Diallylic |
| 27.11 | C8–11 (oleyl), C8–14 (linoleyl) | Allylic |
| 28.95–29.67 | CH2n | All acyl chains |
| 31.50 | C16 | Linoleyl |
| 33.91 | α- C2 | All acyl chains |
| 34.06 | β-C2 | All acyl chains |
| 61.97 | α-CH2O | Glycerol (triacylglycerols) |
| 68.80 | β-CH2O | Glycerol (triacylglycerols) |
| 127.78 | C12 | Linoleyl |
| 127.97 | C13 | Linoleyl |
| 129.53 | β-C9 | Oleyl |
| 129.56 | α-C9 | Oleyl |
| 129.68 | C11 | Gondoyl |
| 129.72 | C13 | Erucyl |
| 129.76 | C14 | Erucyl |
| 129.78 | α-C12 | Gondoyl |
| 129.81 | β-C12 | Gondoyl |
| 129.84 | C9 | Linoleyl |
| 129.87 | α-C10 | Oleyl |
| 129.88 | β-C10 | Oleyl |
| 130.05 | C10 | Linoleyl |
| 172.68 | α-C1 | Glycerol (triacylglycerols) |
| 173.10 | β-C1 | Glycerol (triacylglycerols) |
| 178.58 | C1 | Free fatty acids |
The resonances between 127.78–130.05 ppm were used to assign the position of unsaturation within the fatty acids, as the alkene carbons resonated within this frequency range (Fig. 3). The α and β signals arose from the difference in the chemical shift of the fatty acid carbonyl when bound to the 1,3-glycerol (α) or the 2-glycerol carbon (β). For the more abundant fatty acids, such as C18:1 an α and β signal was apparent for both alkene carbons; C9 at α- = 129.56 ppm and β- = 129.53 ppm, plus C10 at α- = 129.87 ppm and β- = 129.88 ppm. For the less abundant C22:1 due to the low resolution of the 13C spectrum, only one signal was seen for each carbon, which suggested either overlaying of the α and β signals, resulting in a single observable shift, or substitution in only one position, due to the decreased abundance of C22:1 compared with C18:1 and C20:1.
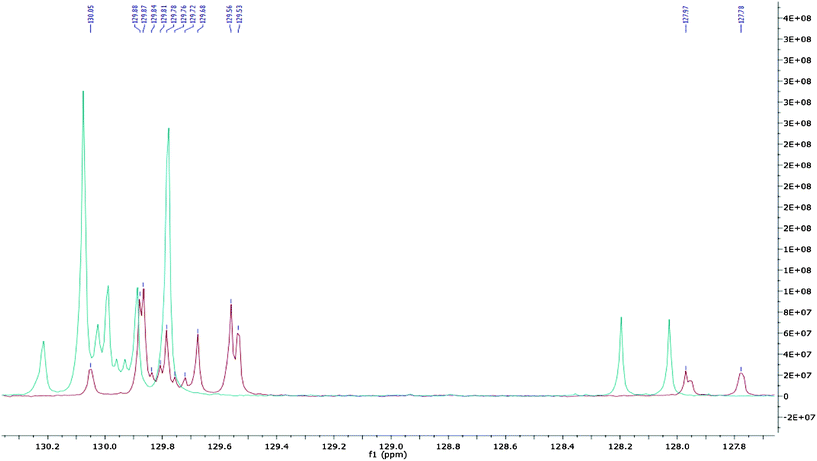 | ||
| Fig. 3 The 13C NMR olefinic region (127–130 ppm) of crude bluebell seed oil (red) and the hydrolysed oil (blue). | ||
After hydrolysis of the crude bluebell seed oil, free fatty acids were produced which were used for the assignment of the position of unsaturation (Fig. 3). Each alkene group gives a pair of signals, one for each carbon of the alkene group. The removal of the glycerol backbone during alkaline hydrolysis resulted in the loss of α- and β-substitution so only a single signal was seen for each carbon resonance. The difference (ppm) between the alkene carbons indicated the position of unsaturation. For C18:1 ω9, the distance between the C9 and C10 signal, was 0.32 ppm (129.88–129.65). This distance was comparable with studies of C18:1 ω9 used as a standard for 13C NMR analysis. The unsaturated carbon signals for C20:1 were observed at 129.68 and 129.78 ppm, a difference of 0.10 ppm indicating that the unsaturation lies between the C11 and C12 carbon, making it ω9 unsaturated.28–31 The C22:1 unsaturated region was identified on the 13C NMR spectrum as the signals at 129.72 and 129.76 ppm, a difference of 0.04 ppm indicating that the unsaturation lies between the C13 and C14 carbons. The C22:1 unsaturated carbon signals had the lowest observable intensity, which was in agreement with a relative percentage of C22:1 at 4.2–4.6% (as determined by the GC-MS of the FAMEs).
GC-Qp-MS of the products of ozonolysis confirmed that the unsaturation within the monounsaturated fatty acids found within the bluebell seed oil was of the ω-9 position. The relative percentage of C9 fragments (nonanol and nonanal) was determined as 85.3% (GC-Qp-MS) which is in agreement with the total relative percentage of MUFA at 81.6% (determined by GC-Qp-MS). The remaining 14.7% of the ozonolysis reaction mixture was found to be C6 fragments (hexanol and hexanal) which were produced by the Criegee rearrangement of the C18:2 ω6, 9 fatty acids. The 1,3-propandiol produced from the presence of a skip double bond is absent as it remained in the water phase during the reaction work-up. The total relative percentage of C18:2 was approximately 8.4% with the relative percentage of C6 fragments being slightly higher at 14.7%. The C6 fragments also supported that the C18:2 is unsaturated in the 6 and 9 position as 1H NMR already confirmed the absence of ω-3 monounsaturated fatty acids because of the absence of a trivinyl methylene environment: no proton shift at δ 0.93 ppm.
GC-MS Analysis of Triglycerides
The use of a column that can be operated in excess of 400 °C allows the direct chromatography of triacylglycerols. Fig. 4 shows the GC trace obtained from the bluebell seed oil with five triacylglycerols eluting between 28.5 and 31.0 min. The free fatty acids are also visible but exhibit poor resolution without derivatisation (relative % in Table 4 as TMS derivatives). Calculation of the Kovats retention index for the five triacylglycerols indicated that they were separated by 200 KI units and therefore by two carbon chain units. The peaks appeared to be rather broader than would be expected and this is most likely due to co-elution of triacylglycerols with equivalent total chain lengths but having a combination of SFA, MUFA and PUFA.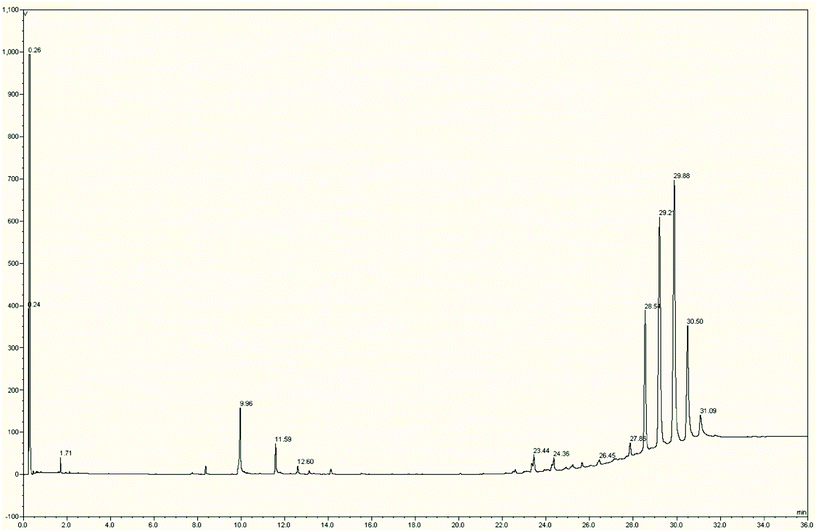 | ||
| Fig. 4 The high temperature GC-MS chromatogram of crude bluebell seed oil showing free fatty acids (Rt 10.0–15.0 mins) and triacylglycerols (Rt 29.5–32.0 mins). | ||
| Fatty Acid | m/z | % a | m/z | %b | %c |
|---|---|---|---|---|---|
| a determined as TMS esters of free fatty acids. b determined as FAMEs (method 1). c determined as FAMEs (method 2). | |||||
| C16:0 | 328 | 6.1 | 270 | 7.1 | 5.5 |
| C18:2(ω9,6) | 352 | 8.0 | 294 | 8.4 | 8.7 |
| C18:1(ω9) | 354 | 62.4 | 296 | 57.6 | 54.9 |
| C18:1(ω6) | 354 | 1.1 | – | – | – |
| C18:0 | 356 | 0.6 | 298 | 0.8 | 0.4 |
| C20:1(ω9) | 382 | 18.2 | 324 | 19.8 | 24.8 |
| C20:0 | 384 | 0.7 | 326 | 1.2 | 0.5 |
| C22:1(ω9) | 412 | 0.5 | 352 | 4.2 | 4.6 |
| C22:0 | 410 | 2.5 | 354 | 1.1 | 0.6 |
GC-MS Analysis of Fatty Acid Derivatives
Prior to GC-MS analysis, free fatty acids require derivatisation in order to improve volatility and subsequent resolution. Derivatisation can be achieved by transesterification with TMS and alkyl esters. Table 4 shows the relative percentages obtained for both derivatisation methods and utilising non-polar and mid-polar capillary columns. Fatty acids with chain lengths of 16, 18, 20 and 22 carbon atoms were detected. The monounsaturated fatty acids found to be present were the C18:1, C20:1 and C22:1. Only C18:2 was observed as a polyunsaturated fatty acid, confirmed by the characteristic proton shift at δ 2.75 ppm in the 1H NMR. Oleic acid methyl ester (C18:1) was the most abundant FAME with a relative percentage of 54 to 58% and 62% for the TMS ester. The C20:1 FAME was the second most abundant MUFA and was quantified with relative proportions between 18 to 25%. The retention times observed for the monounsaturated FAMEs (C18:1, C20:1 and C22:1) matched those of the oleic acid methyl ester (C18:1 Z9), cis-11-eicosenoic acid methyl ester (C20:1 Z11) and erucic acid methyl ester (C22:1 Z13). GC-MS analysis of the TMS esters showed the presence of two C18:1 fatty acids. Because of the products generated from ozonolysis oleic acid is the most abundant fatty acid in bluebell seed oil triacylglycerols.In summary the combination of NMR and GC-MS of the FAMEs and TMS esters showed agreement for the abundance of the different fatty acids, either by the degree of unsaturation or the relative proportion of transesterified fatty acids. Both GC-MS methods 1 & 2 detected C16:0, C18:1, C18:2, C18:0, C20:1, C20:0, C22:1 and C22:0. C18:1 was detected as the most abundant by all GC-MS methods.
Method 1 using a non-polar column and quadrupole MS detection was chosen for the assessment of the variability for different harvest years. The concentration of SFA and the relative percentage of SFA determined by 1H NMR range from 7.0 to 10.1%. On a year by year comparison between the methods the largest discrepancy was for the 2010 harvest with 1H NMR giving 7% and GC-MS for the FAMEs giving 9.2%. C18:2 is the only PUFA present and its relative percentage ranged from 8.4 to 12%. On a year by year comparison between the methods the largest discrepancy was for the harvests in 2007 and 2008 with a variation of 2.6%. The relative proportion of MUFA ranged from 78.0 to 82.0%. On a year by year comparison between the methods the largest discrepancy was for the harvest in 2008 with a variation of 3.2%.
Discussion
Overall the composition of the bluebell seed oil obtained from the seeds harvested in the different years showed little variability (Table 2 and 5). This stability in composition suggests that climatic factors do not influence the triacylglycerol profile significantly.| 2006 | 2007 | 2008 | 2009 | 2010 | |
|---|---|---|---|---|---|
| C16:0 | 5.4 | 7.1 | 6.2 | 5.9 | 6.3 |
| C18:0 | 0.7 | 0.8 | 0.8 | 1.0 | 0.8 |
| C18:1 | 54.7 | 57.6 | 56.6 | 54.4 | 54.5 |
| C18:2 | 11.9 | 8.4 | 9.4 | 9.9 | 11.9 |
| C20:0 | 1.0 | 1.2 | 1.2 | 1.3 | 1.0 |
| C20:1 | 19.9 | 19.8 | 20.0 | 20.5 | 19.7 |
| C22:0 | 1.1 | 1.1 | 1.2 | 1.7 | 1.1 |
| C22:1 | 5.2 | 4.2 | 4.6 | 5.5 | 4.7 |
The most variable component of the bluebell seed oil was its free fatty acid content that reached up to 40% based on integration values determined from 13C NMR analysis of the carbonyl resonances at 178.58 ppm versus the glycerol resonances at 172.68 and 173.10 ppm. As the seeds were kept in different batches under varying storage conditions, the free fatty acid content is attributed to the suboptimal seed conditions. The appearance of mould on some batches was noted previously. While we only visually inspected the seed prior to extraction, the increasing free fatty acid content is hypothesised to be an early indicator for seed quality.
Yield and Composition of Bluebell Oil
The seed weight (6 mg) and oil yield (22 to 25%) determined in this study for bluebells agrees with the averages reported for flowering herbs growing in shaded habitats in the temperate region with 5.07 ± 0.12 mg (n = 65) and 27.9 ± 0.3% (n = 84).32 The bluebell seed oil obtained in this study has an unusual profile for a seed oil as it contains about 20% gondoic acid (C20:1 ω9). In addition it has a high degree of unsaturation with about 90%. Most seed oils have predominantly C16 and C18 fatty acids in their make-up.3,4 The exception for commonly used seed oil is rape with a high concentration in erucic acid C22:1, which has been reduced or enhanced in relative abundance through plant breeding depending on the end use as either biodiesel or in erucimide production. Genetic engineering of oilseed crops is advancing to enable or increase the yield of rare or unusual fatty acids, such as developing a plant source for ω3-polyunsaturated fatty acids.1 False flax (Camelina sativa) also contains about 35% fatty acids in the triacylglycerols with carbon chain lengths of 20 or more.33 Meadowfoam (Limanthes alba L.) is another plant whose seed oil is rich in high chain length unsaturated fatty acids with 95% of the fatty acids being C20 or longer and comprising of 64% of C20:1 (Z5).34Sources
The potential for utilising bluebell seeds as a source of base oil and fine chemicals through bio refining depends on their abundance and an estimation of the yield of seeds that could be gained. As a rough estimate one square metre densely covered in bluebells (approximately 500 individuals) yielding 100 seeds each would result in 350 g of seeds. Working on an area of half a hectare yielded about 10 kg of seeds (Thoss unpublished) or 20 g per metre squared, which reflects that coverage is unequal. In addition, bluebell seeds ripen gradually and once ripe expel easily from the plant.10 For conservation purposes this is desirable as it leaves ripe seeds behind for the next generation.Bluebells are ubiquitous in Great Britain on the scale of 10 km squares.9,35 More detailed figures on bluebell coverage were not available (Fred Ramsey, Natural History Museum, personal communication). This may be a reflection on bluebells growing predominantly on marginal land: woodlands and bracken covered rough grazing land, particularly on hillsides and coastal cliffs. In view of the utilisation of bluebells as a sustainable source of fine chemicals, this trait is advantageous as it does not compete with agricultural land used for food production.
Potential Applications
Often the use of seed oils is historic with a human use of a wild or cultivated plant that has been growing in abundance in the vicinity of human settlements, referred to as ethnobotany.20 Despite its abundance, the bluebell in Britain has had few uses and to date there is no product containing an extract of bluebells. Anecdotal evidence suggests that a glue used for bookbinding could be obtained from bluebell bulbs and similarly a bluebell derived starch had been used to stiffen clothes.Currently seed oils are most often investigated as potential sources for biodiesel.2–4,34 The specifications for biodiesel are described in fuel standards ASTM D6751 and EN 124143 and relate to their performance when used in a compression engine. Biodiesel is a modified seed oil in which the fatty acids present in the triacylglycerols have undergone transesterification. Predictions for biodiesel performance can be made based on the fatty acid profile: an increase in unsaturation decreases the energy content. An increase in chain length increases the CETANE number, a measurement of the combustion quality of diesel fuel, and other general rules.4 However, due to the limited quantity of bluebell seeds available this is unlikely to be a significant application.
Historically seed oils were used for cosmetic applications. The triacylglycerol profile in seed oils does not correlate with its application in cosmetic products.5,37 Examples of seed oils found as cosmetic ingredient are borage (Borago officinales), sea buckthorn (Hippophae rhamnoides), evening primrose (Oenothera biennis), safflower (Carthamus tinctorius), almond (Prunus dulcis), and apricot kernel (Prunus armeniaca).36 The minor constituents with attributed biological activities are sterols, fat-soluble vitamins and anti-oxidants that comprise less than 3% of the seed oil. The chemical identities of the minor compounds in bluebell seed oil are currently under investigation.
Triglycerides do not give colour or scent to base oils. Culinary uses of base oils depend on their physicochemical properties, such as smoke point and sensory properties. Again the use for culinary purposes is thought to mostly derive from the shared history of man and plant.
It is hypothesised that bluebells were neither used as food nor cosmetics due to their iminosugar content, which is present in all parts of the plant.24,25 The advances in bio refining allow various plant extracts and specific compounds to be obtained, due to their polarity iminosugars do not partition into the seed oil when the oil is extracted with a non-polar solvent such as n-hexane. The seed residue remaining after oil extraction could be further refined to, for example, obtain specific iminosugars and leave the carbohydrate fraction for other uses.
Conservation
Bluebells are a protected species and the collection of seeds for chemical exploitation is only permitted under license. A prerequisite for the license for the commercial use of bluebells was the confirmation that the population was genetically pure Hyacinthoides non-scripta. The oil extracted from bluebell seeds as described in this study is sufficiently unusual to generate interest in the chemical exploitation of other bluebell populations. This could be beneficial for two reasons: the maintenance of existing populations and the assessment of bluebell coverage. A benefit to the conservation of the species could derive from this chemical exploitation if a percentage of gathered seeds are used for re-seeding suitable habitats devoid of bluebells. This is particularly relevant for bluebells as these regenerate predominantly by seeds,12 however, the seeds are too heavy to be windborne. Suitable habitats may be existing woodlands, newly established woodlands, parklands or roadside verges.Conclusions
Bluebell seed oil has been extracted and the fatty acid profile characterised using different analytical techniques: NMR on crude oil, high temperature GC-MS analysis of crude oil, as well as GC-MS analysis of FAMEs and TMS esters. Seed material from five different growth periods was used which showed little variability. The oil yield ranged from 22 to 25% dry weight. The agreement between the different techniques was good and showed the bluebell seed oil to be highly unsaturated (> 85%). It contained about 20% of C20:1 (Z9) and had an unusually high proportion of fatty acids with 20 or more carbon atoms in a quarter of the fatty acids presentAcknowledgements
Thomas Wilson would like to acknowledge the funding by the European Social Fund (ESF) in conjunction with Vera Bluebell Ltd. Patrick Murphy, Vera Thoss and Thomas Wilson acknowledge funding through the BEACON project (European Regional Development Fund). We would like to thank Denis Williams and Louise Simpson from the School of Chemistry, Bangor for GC-MS and NMR analysis, Viacheslav Tverezovskiy for his help with the ozonolysis, Andy Jones (CCW) for providing species identification on the sampling site, and Mark Baird and Dave Preskett for advice on free fatty acid quantification using 13C NMR. Two anonymous referees are acknowledged for their constructive comments.References
- C. Lu, J. A. Napier, T. E. Clemente and E. B. Cahoon, Curr. Opin. Biotechnol., 2011, 22, 252–9 CrossRef CAS.
- Y. C. Sharma, B. Singh and J. Korstad, J. Agric. Food Chem., 2010, 58, 242–7 CrossRef CAS.
- B. R. Moser, In Vitro Cellular Developmental Biology-Plant, 2009, 45, 229–266 CrossRef CAS.
- A. Karmakar, S. Karmakar and S. Mukherjee, Bioresour. Technol., 2010, 101, 7201–10 CrossRef CAS.
- F. Saloua, N. I. Eddine and Z. Hedi, Ind. Crops Prod., 2009, 29, 1–8 CrossRef CAS.
- J. M. Dyer, S. Stymne, A. G. Green and A. S. Carlsson, Plant J., 2008, 54, 640–55 CrossRef CAS.
- F. Rose, British Wildlife, 1999, 11, 241–251 Search PubMed.
- G. E. Blackman and A. J. Rutter, J. Ecol., 1954, 629–638 CrossRef.
- D. D. Kohn, P. E. Hulme, P. M. Hollingsworth and A. Butler, Biol. Conserv., 2009, 142, 61–74 CrossRef.
- G. H. Knight, J. Ecol., 1964, 52, 405–421 CrossRef.
- J. Y. Wilson, New Phytol., 1959, 58, 155–163 CrossRef.
- P. A. Thompson and S. A. Cox, Ann. Bot-London, 1978, 42, 51–62 Search PubMed.
- P. W. Grabham and J. R. Packham, Biol. Conserv., 1983, 26, 105–126 CrossRef.
- S. A. Corbet, Oecologia, 1998, 114, 349–360 CrossRef.
- A. S. Cooke, Journal of Zoology, 1997, 242, 365–369 CrossRef.
- N. Pettorelli, S. Dray, J.-M. Gaillard, D. Chessel, P. Duncan, A. Illius, N. Guillon, F. Klein and G. Van Laere, Oecologia, 2003, 137, 363–9 CrossRef.
- C. H. S. Watts, J. Anim. Ecol., 1968, 37, 25 CrossRef.
- J. M. Hoekstra, T. M. Boucher, T. H. Ricketts and C. Roberts, Ecol. Lett., 2004, 8, 23–29 CrossRef.
- F. S. Gilliam, J. Ecol., 2006, 94, 1176–1191 CrossRef CAS.
- D. E. Allen and G. Hatfield, Medicinal Plants in Folk Tradition: An Ethnobotany of Britain & Ireland, Timber Press, Portland, Cambridge, 2004 Search PubMed.
- K. J. Brocklebank and G. A. F. Hendry, New Phytol., 1989, 112, 255–260 CrossRef CAS.
- A. A. Watson, G. W. J. Fleet, N. Asano, R. J. Molyneux and R. J. Nash, Phytochemistry, 2001, 56, 265–295 CrossRef CAS.
- R. J. Nash and A. A. Watson, Chemoecology, 1994, 5–6(3–4), 167–171 CrossRef.
- A. Kato, I. Adachi, M. Miyauchi, K. Ikeda, T. Komae, H. Kizu, Y. Kameda, A. Watson, R. J. Nash, M. R. Wormald, G. W. Fleet and N. Asano, Carbohydr. Res., 1999, 316, 95–103 CrossRef CAS.
- A. A. Watson, R. J. Nash, D. J. Wormald, S. D. Harvey, E. Lees, N. Asano, H. Kizu, A. Kato and R. C. Griffiths, Phytochemistry, 1997, 46, 255–259 CrossRef CAS.
- R. H. Thursby-Pelham, Vet. Rec., 1967, 80, 709–10 CAS.
- K. Cutler, Livest. Sci., 2007, 12, 44–47 Search PubMed.
- R. Sacchi, L. Mannina, P. Fiordiponti, P. Barone, L. Paolillo, M. Patumi and A. Segre, J. Agric. Food Chem., 1998, 46, 3947–3951 CrossRef CAS.
- L. Mannina, C. Luchinat, M. C. Emanuele and A. Segre, Chem. Phys. Lipids, 1999, 103, 47–55 CrossRef CAS.
- G. Vlahov, A. A. Giuliani and P. D. Re, Anal. Methods, 2010, 2, 916–923 RSC.
- G. Vlahov, P. K. Chepkwony and P. K. Ndalut, J. Agric. Food Chem., 2002, 50, 970–975 CrossRef CAS.
- A. Levin, Am. Nat., 1974, 108, 193–206 Search PubMed.
- S. Bail, G. Stuebiger, H. Unterweger, G. Buchbauer and S. Krist, Eur. J. Lipid Sci. Technol., 2009, 111, 170–182 CrossRef CAS.
- B. R. Moser, G. Knothe and S. C. Cermak, Energy Environ. Sci., 2010, 3, 318–327 CAS.
- C. D. Preston, D. A. Pearman and T. D. Dines, New Atlas of the British and Irish Flora, Oxford University Press, 2002 Search PubMed.
- M. Nogala-Kalucka, M. Rudzinska, R. Zadernowski, A. Siger and I. Krzyzostaniak, J. Am. Oil Chem. Soc., 2010, 87, 1481–1487 CrossRef CAS.
- M. Lísa and M. Holcapek, J. Chromatogr., A, 2008, 1198–1199, 115–30 Search PubMed.
| This journal is © The Royal Society of Chemistry 2012 |
