DOI:
10.1039/C2RA01362B
(Paper)
RSC Adv., 2012,
2, 2842-2847
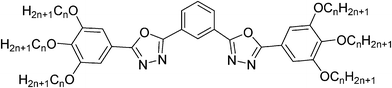
Columnar mesophases of luminescent polycatenar liquid crystals incorporating a 1,3-substituted benzene ring interconnecting two 1,3,4-oxadiazoles†
Received
27th December 2011
, Accepted 4th January 2012
First published on 6th January 2012
Abstract
New polycatenar liquid crystals containing two 1,3,4-oxadiazole rings interconnected by a bent 1,3-substituted benzene ring as the central linking unit and three alkoxy chains at each terminal have been synthesized and investigated by polarizing microscopy, DSC, XRD scattering, UV-vis spectroscopy and photoluminescence measurements. All compounds form enantiotropic hexagonal columnar phases and have broader mesophase ranges than the corresponding para substituted analogues and show strong blue fluorescence emission with a large Stokes shift in solution.
1. Introduction
Liquid crystals (LCs) incorporating 1,3,4-oxadiazole rings have received significant attention since the time when they were first reported as a new class of heterocyclic LCs.1,2 In recent years not only smectic phase forming rod-like molecules,3 their dimers,4 and polymers,5,6 but also 1,3,4-oxadiazole based polycatenar7–10 and star-shaped11 LC molecules have been reported. Nowadays, major interest is focused on two subjects. At first, the so-called bent-core LCs incorporating a bent central oxadiazole unit12 are of significant interest as they could possibly lead to the elusive biaxial nematic phase.13 Secondly, oxadiazole derivatives have been extensively investigated with respect to their high quantum yield of luminescence,14 as well as their potential application in development of organic light-emitting diodes (OLEDs), lasers and optical sensors.10,14–17 For applications as organic electronic materials the 1,3,4-oxadiazoles are especially interesting as they represent chemically stable electron transport materials.15,16 Liquid crystalline functional materials provide the advantages of self assembly and self healing.18–23 Therefore, attention has been given in recent years to LC 1,3,4-oxadiazoles, especially those with more than one 1,3,4-oxadiazole ring in the π-conjugated system, among them nearly linear21,24–28 and significantly bent molecules incorporating two oxadiazoles,5,15,21 and also star-shaped molecules incorporating three12,29–31 or four such units.32 The molecular shape as well as the number of attached alkyl chains decides if lamellar (smectic) or columnar phases are formed. In linear molecules the two oxadiazoles were either directly connected with each other28 or via a para-substituted benzene ring (e.g. compounds p/n in Scheme 1),25,26 whereas for star-shaped mesogens incorporating three or four oxadiazole units 1,3,5-trisubstituted benzenes were used, in which all oxadiazoles are meta-connected with respect to each other (e.g. compound 1 in Scheme 1).21,30
 |
| | Scheme 1 Compounds m/n under investigation and relationships to the star-shaped tris-oxadiazoles 130 and the para-connected polycatenar bis-oxadiazoles p/n.25 | |
Herein we report the “missing link” between these two groups of oxadiazole based materials, the star-shaped meta-connected tris-oxadiazoles 130 and the polycatenar para-connected bis-oxadiazoles p/n25 (see Scheme 1). The investigated compounds represent 5-ring mesogens incorporating two 1,3,4-oxadiazoles interconnected by a meta-substituted benzene ring and having in total 6 alkyl chains attached equally to the two terminal benzene rings. These compounds are assigned as m/n, where m stands for the meta-connection and n is the alkyl chain length. These molecules can be considered as polycatenar mesogens33–36 with the difference to classical compounds of this type that the aromatic core is more bent than usual.37 Their mesophase behavior was studied by POM, DSC and X-ray scattering, indicating enantiotropic hexagonal columnar phases (Colhex) for all investigated compounds. A model of self assembly in these columnar phases is proposed and the effects of meta-connection (compounds m/n) compared to the para-connection in the previously reported isomeric compounds p/n25 on mesophase type, mesophase stability, mesophase range, UV-vis absorption and photoluminescence (PL) are compared. All meta-connected compounds m/n have much lower melting points and show broader enantiotropic mesophase range than the corresponding para substituted analogues and the reported compounds show strong blue fluorescence emission with large Stokes shift.
2. Results and discussion
2.1 Synthesis
The synthetic pathway to compounds m/n is described in Scheme 2. Both oxadiazole rings were formed simultaneously by reaction of isophthaloyl dichloride with appropriate 3,4,5-trialkoxybenzhydrazides, and subsequent cyclisation with phosphorus oxychloride (POCl3). Isophthaloyl dichloride was freshly prepared from isophthalic acid with thionyl chloride and used immediately. Purification was done by column chromatography. Experimental procedures and analytical data are collated in the ESI.†
 |
| | Scheme 2 Synthesis of compounds m/n; Reagents and conditions: (i) hydrazine hydrate, n-BuOH, 115 °C, 40 h; (ii) SOCl2, 70 °C, 6 h; (iii) Et3N, THF, 25 °C, 8 h; (iv) POCl3, reflux, 12 h. | |
2.2 Investigations
The synthesized compounds m/n were investigated by POM, DSC and for selected representatives the liquid crystalline phases were also investigated by XRD. A Mettler heating stage (FP 82 HT) was used for polarizing microscopy (Optiphot 2, Nikon) and DSCs were recorded with a DSC-7 calorimeter (Perkin-Elmer) at 10 K min−1. X-Ray diffraction (XRD) of surface aligned samples was performed using a 2D-detector (HI-Star, Siemens). Orientation of the samples was achieved by slowly cooling a drop of the compound on a glass surface; the X-ray beam was applied parallel to the substrate surface (exposure time 30 min).
2.3 Liquid crystalline phases
DSC investigation indicates two phase transitions for all compounds, one nearly reversible transition with an enthalpy value between 2.4 and 5 kJ mol−1 at temperatures between 77 and 84 °C, corresponding to the transition from the LC phase to the isotropic liquid state and a second one around 50–69 °C with much larger enthalpy values (19–89 kJ mol−1) assigned to the melting of the crystalline material with formation of the LC phase (see Table 1). This transition can be overcooled by about 10 K (cooling rate 10 K min−1).
Table 1 Transition temperatures and associated enthalpy values (in brackets) of compounds m/na
| Compound |
n
|
T/°C [ΔH/kJ mol−1] |
Colhex–Cr/°C |
|
Transition temperatures were determined by DSC (peak temperatures, first heating scans, 5 °C min−1); Cr = crystal; Colhex = hexagonal columnar phase; Iso = isotropic liquid; Colhex–Cr = crystallization temperature observed on cooling.
|
|
m/6
|
6 |
Cr 50 [18.9] Colhex 77 [2.4] Iso |
40 |
|
m/8
|
8 |
Cr 67 [28.0] Colhex 80 [2.6] Iso |
55 |
|
m/10
|
10 |
Cr 67 [29.6] Colhex 79 [2.5] Iso |
57 |
|
m/14
|
14 |
Cr 66 [88.6] Colhex 84 [3.9] Iso |
56 |
|
m/16
|
16 |
Cr 69 [39.6] Colhex 82 [4.9] Iso |
61 |
The textures of all mesogens, as observed between crossed polarizers under a polarizing microscope, are characterized by typical birefringent spherulitic domains or filaments occurring together with homeotropically aligned areas which appear completely dark, indicating that this mesophase is optically uniaxial (see Fig. 1, S1†) as typical for hexagonal columnar mesophases.
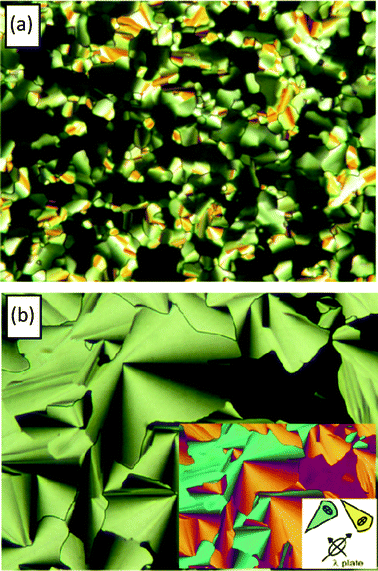 |
| | Fig. 1 Optical textures of the Colhex phases as seen between crossed polarizers (a) for m/8 at T = 79 °C and (b) for m/14 at T = 75 °C, inset: texture of m/14 with λ-plate in the same area and indicatrix orientation in the compensator. | |
The LC phases of the homologues with the shortest (m/6) and the second longest alkyl chains (m/14) were investigated by XRD of surface aligned samples (see Fig. 2, S2† and Table 2). The 2D X-ray diffraction pattern of both compounds are characterized by a diffuse halo in the wide-angle region with a maximum corresponding to d = 0.45 nm, which is attributed to the disordered alkyl chains and aromatic cores. There is no separate scattering which could be assigned to a defined π-stacking distance, indicating a disordered phase. The small-angle reflections of the mesophase of compound m/6 have a ratio of their positions corresponding to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31/2
31/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Fig. 2a), indicating a hexagonal lattice (Colhex/p6mm). Though in the small-angle region of compound m/14, only one scattering (d = 3.28 nm) is found (Fig. S2†), in the diffraction pattern of an oriented sample it is split into 6 spots on a regular hexagon, confirming a hexagonal columnar mesophase also for this compound (Fig. 2b and Table 2). The lattice parameters ahex were calculated to be ahex = 3.0 nm (at T = 70 °C) for m/6 and ahex = 3.8 nm (at T = 78 °C) for m/14 (Table 2). Though XRD has not been carried out for all columnar phases of compounds m/n, based on textural similarities (e.g. homeotropic alignment, indicating uniaxiality of all LC phases, spherulitic textures indicating columnar organization in all cases), it is highly reasonable to assume that also the LC phases of the intermediate homologues represent hexagonal columnar phases. The number of molecules organized in a slice of the columns with a height of h = 0.45 nm (maximum of the diffuse wide angle scattering), estimated using eqn (1) and assuming a density of ρ = 1 g cm−3, is about n = 2 for the investigated compounds (NA = Avogadro constant, M = molecular mass, see Table 2).
2 (Fig. 2a), indicating a hexagonal lattice (Colhex/p6mm). Though in the small-angle region of compound m/14, only one scattering (d = 3.28 nm) is found (Fig. S2†), in the diffraction pattern of an oriented sample it is split into 6 spots on a regular hexagon, confirming a hexagonal columnar mesophase also for this compound (Fig. 2b and Table 2). The lattice parameters ahex were calculated to be ahex = 3.0 nm (at T = 70 °C) for m/6 and ahex = 3.8 nm (at T = 78 °C) for m/14 (Table 2). Though XRD has not been carried out for all columnar phases of compounds m/n, based on textural similarities (e.g. homeotropic alignment, indicating uniaxiality of all LC phases, spherulitic textures indicating columnar organization in all cases), it is highly reasonable to assume that also the LC phases of the intermediate homologues represent hexagonal columnar phases. The number of molecules organized in a slice of the columns with a height of h = 0.45 nm (maximum of the diffuse wide angle scattering), estimated using eqn (1) and assuming a density of ρ = 1 g cm−3, is about n = 2 for the investigated compounds (NA = Avogadro constant, M = molecular mass, see Table 2).
| |  | (1) |
 |
| | Fig. 2 XRD patterns (a) of m/6 at T = 70 °C and (b) of m/14 at T = 78 °C. | |
Table 2 Comparison of X-ray data and molecular dimensions of the columnar phases of compounds m/6 and m/14a
| Compound |
n
|
a
hex/nm (T/°C) |
L/nm |
n
|
|
Abbreviations: ahex = lattice parameter determined by XRD; L = length of a molecule measured between the ends of the terminal chains and assuming a most stretched shape with all-trans conformation of the alkyl chains;38n = number of molecules in the cross section of a column in the Colhex phases (assumed height of 0.45 nm).
|
|
m/6
|
6 |
3.0 (70) |
3.4 |
2.1 |
|
m/14
|
14 |
3.8 (78) |
5.4 |
2.1 |
Based on the above investigation, we can propose the following model for the molecular organization in the hexagonal columnar phase. In principle, the molecules can adopt different conformations, among them the nearly linear one shown in Fig. 3a and the bent-shape shown in Fig. 3b. The calculated number of two molecules in each virtual column slice with a height of 0.45 nm suggests that on average two molecules should be organized side-by-side. Hence, during self assembly the individual polycatenar molecules should adopt a half disc-like average shape39 which is best realized with the bent conformation shown in Fig. 3b. The side-by-side packing of the aromatic cores allows a nearly complete surrounding of these cores by the flexible alkyl chains (Fig. 3c). Remaining space at the periphery between the alkyl chains can be filled by intercalation of the chains of adjacent columns and by chain folding. Considering this interdigitation, the effective diameters of the columns corresponds to the experimentally observed hexagonal lattice parameters ahex. An on average circular cross sectional shape of the columns results from time and space averaging of the slightly elliptical columns. Investigation of the Colhex phases by polarizing microscopy between crossed polarizers with an additional λ-retarder plate (Fig. 1b, S1d†) indicates that the columnar phases of all compounds are optically negative.40 This means that the major intramolecular π-conjugation pathway, which is along the long axis of the aromatic cores, is perpendicular to the column long axis. This is in line with the proposed organization of the molecules in the columns.
 |
| | Fig. 3 (a,b) CPK-models of two possible molecular conformations of compound m/14; (c) the cross section through a single column; the regions inside the dotted circles show the dimensions provided by the experimentally observed hexagonal lattice parameters (ahex = 3.8 nm). | |
In contrast to the meta-connected bis-oxadiazoles m/n reported herein, for related para-connected bis-oxadiazoles p/n rectangular columnar phases were reported for the homologues with n = 10–14, and it was proposed that the molecules are tilted in these columns.25 Remarkably, all meta-connected bis-oxadiazoles m/n have much lower melting points (40–70 °C) compared with the para-connected analogues p/n (74–140 °C)25,42 whereas the stability of the columnar LC phase is about the same for both series of compounds. Therefore, much broader enantiotropic LC regions can be found for the meta-connected bis-oxadiazoles m/n; the enantiotropic mesomorphic range being between 13 and 28 K for compounds m/n whereas it is only 5–9 K for the related compounds p/m.25 Moreover, in the series of compounds m/n, liquid crystalline properties can be retained even for molecules with relatively short alkyl chains, whereas in the series of compounds p/n already compound p/8 is a crystalline solid.25 The reason is that the melting points of compounds m/n tend to decrease with decreasing alkyl chain length, whereas there is a strong increase of the melting point for the short chain compounds p/n. This becomes especially clear if the isomeric compounds p/641 and m/6 are compared. Whereas the para-connected compound p/6 is a crystalline solid with a melting point of 140 °C the meta-connected compound m/6 has a melting point of only 50 °C and a Colhex phase which is stable up to 77 °C. Hence, with respect to the LC properties the meta-connected bis-oxadiazoles m/n are advantageous compared to the para-connected analogues. In order to understand these differences it must be considered that the meta connection reduces the molecular symmetry and allows the formation of an increased number of different conformations. This leads to the coexistence of a larger number of different molecular shapes, which is unfavorable for crystallization. This effect becomes especially strong for compounds with short alkyl chains as the contribution of chain disorder to distortion of crystallization is reduced for these compounds. That increased conformational flexibility of the aromatic core does not significantly influence the mesophase stability indicates that micro-segregation42 of the aromatic cores from the aliphatic chains should be the main driving force for mesophase formation, instead of the specific shape, favoring an alignment of the molecules in the column cores. This flexibility also removes any tilt and allows the formation of hexagonal columnar phases for all homologues instead of lower symmetry phases as reported for most compounds p/n.25
2.4 Photophysical properties
It could be expected that the change of the substitution pattern should have an influence on the photophysical properties of the bis-oxadiazoles as the conjugation path along the aromatic core is interrupted by the meta-substitution pattern. The UV-vis absorption and fluorescence spectroscopic data in THF solution (c = 10−6 mol l−1) are shown in Fig. 4 for compounds m/n (for spectra recorded in CH2Cl2, see Fig. S3†). All compounds m/n show a maximum absorption peak at 309 nm which may be attributed to a π–π* transition. All compounds described in this work exhibited blue emission with maxima of the emission peaks at 425 nm, hence, the Stokes shift has a remarkably large value of 116 nm. Comparable values of the para-connected compound p/6 (in THF) are 339 nm for excitation and 465 nm for emission25 (Fig. 5). Hence, all maxima of compounds m/n are blue shifted compared with the related para-connected compounds by 30 nm (absorption), respectively 40 nm (emission).
 |
| | Fig. 5 Comparison of the normalized absorption spectra (a) and photoluminescence spectra (b) of compounds m/6 and p/6 recorded in THF solution (c = 10−6 mol l−1) at room temperature. | |
Fluorescence quantum yields of compounds m/n were determined relative to quinine sulfate in sulfuric acid aqueous solution (Φ = 0.546) and calculated according to the literature.43 All the compounds exhibited high quantum yield (Φ > 73%) in dichloromethane (10−6 mol L−1) at λexc =341 nm.
3. Conclusion
A series of bis-1,3,4-oxadiazole based polycatenar liquid crystals incorporating a bent 1,3-disubstituted benzene ring as the central linking unit was synthesized. Exclusively hexagonal columnar phases were observed and a packing model is proposed where the molecules adopt a half disc-like average conformation and these half-disc-like units pack back-to-back in the center of the columns. All compounds reported here have lower melting points than isomeric compounds having a 1,4-disubstituted benzene ring as the central unit. As the mesophase stabilities of related isomers are comparable, the meta-connected compounds reported herein show broader mesophase ranges than the corresponding para-connected analogues. The difference is especially large for compounds with relatively short alkyl chains. All compounds exhibit intensive blue fluorescence emission with large Stokes shifts. Potential applications for these compounds, such as charge carrier or luminescent LC materials will be further explored.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21074105 and No. 20973133), the Yunnan Science Foundation (2010CD018); M.P. acknowledges the support by the Cluster of Excellence “Nanostructured Materials”.
References
- No LC phases were observed for the first reported symmetrically substituted 1,3,4-oxadiazoles: K. Dimitrowa, J. Hauschild, H. Zaschke and H. Schubert, J. Prakt. Chem., 1980, 322, 933–944 Search PubMed
 .
.
- First examples of LC 1,3,4-oxadiazoles: D. Girdziunaite, C. Tschierske, E. Novotna, H. Kresse and A. Hetzheim, Liq. Cryst., 1991, 10, 397–407 Search PubMed
 .
.
-
(a) L. A. Karamysheva, S. I. Torgova, I. F. Agafonova and V. F. Petrov, Liq. Cryst., 2000, 27, 393–405 CrossRef CAS
 ;
(b) M. L. Parra, E. Y. Elgueta, V. Jimenez and P. I. Hidalgo, Liq. Cryst., 2009, 36, 301–317 CrossRef CAS
;
(b) M. L. Parra, E. Y. Elgueta, V. Jimenez and P. I. Hidalgo, Liq. Cryst., 2009, 36, 301–317 CrossRef CAS  ;
(c) A. K. Prajapati and V. Modi, Phase Transitions, 2010, 83, 634 Search PubMed
;
(c) A. K. Prajapati and V. Modi, Phase Transitions, 2010, 83, 634 Search PubMed  ;
(d) M. L. Parra, P. I. Hidalgo and E. Y. Elgueta, Liq. Cryst., 2008, 35, 823 CrossRef CAS
;
(d) M. L. Parra, P. I. Hidalgo and E. Y. Elgueta, Liq. Cryst., 2008, 35, 823 CrossRef CAS  ;
(e) M. Parra, P. Hidalgo, E. Carrasco, J. Barbera and L. Silvino, Liq. Cryst., 2006, 33, 875 CrossRef CAS
;
(e) M. Parra, P. Hidalgo, E. Carrasco, J. Barbera and L. Silvino, Liq. Cryst., 2006, 33, 875 CrossRef CAS  .
.
-
(a) R. M. Srivastava, R. A. W. Neves Filho, R. Schneider, A. A. Vieira and H. Gallardo, Liq. Cryst., 2008, 35, 737 CrossRef
 ;
(b) J. Han, F.-L. Wang, F.-Y. Zhang and L.-R. Zhu, Liq. Cryst., 2010, 37, 1521 Search PubMed
;
(b) J. Han, F.-L. Wang, F.-Y. Zhang and L.-R. Zhu, Liq. Cryst., 2010, 37, 1521 Search PubMed  ;
(c) J. Han, M. Zhang, F. Wang and Q. Geng, Liq. Cryst., 2010, 37, 1471 CrossRef CAS
;
(c) J. Han, M. Zhang, F. Wang and Q. Geng, Liq. Cryst., 2010, 37, 1471 CrossRef CAS  ;
(d) K. C. Majumdar, P. K. Shyam, D. S. S. Rao and S. K. Prasad, J. Mater. Chem., 2011, 21, 556 RSC
;
(d) K. C. Majumdar, P. K. Shyam, D. S. S. Rao and S. K. Prasad, J. Mater. Chem., 2011, 21, 556 RSC  .
.
- Y. Xu, Q. Yang, Z. Shen, X. Chen, X. Fan and Q. Zhou, Macromolecules, 2009, 42, 2542 CrossRef CAS
 .
.
- A.P. Kulkarni, C. J. Tonzola, A. Babel and S. A. Jeneke, Chem. Mater., 2004, 16, 4556 CrossRef CAS
 .
.
- C. K. Lai, Y.-C. Ke, J.-C. Su, C. Shen and W.-R. Li, Liq. Cryst., 2002, 29, 915 CrossRef CAS
 .
.
- C. F. He, G. R. Richards, S. M. Kelly, A. E. A. Contoret and M. O'Neill, Liq. Cryst., 2007, 34, 1249 CrossRef CAS
 .
.
- C. R. Wen, Y.-J. Wang, H.-C. Wang, H.-S. Sheu, G.-H. Lee and C. K. Lai, Chem. Mater., 2005, 17, 1646 CrossRef CAS
 .
.
- A. E. A. Contoret, A. J. Eastwood, S. R. Farrar, S. M. Kelly, J. E. Nicholls, M. O'Neill, G. J. Richards and C. Wu, Mol. Cryst. Liq. Cryst., 2001, 368, 271 Search PubMed
 .
.
- J. B. M. A. Godoy, P. I. Hidalgo, M. L. Parra, J. A. Ulloa and J. M. Vergara, Liq. Cryst., 2011, 38, 679 Search PubMed
 .
.
-
(a) K. J. K. Semmler, T. J. Dingemans and E. T. Samulski, Liq. Cryst., 1998, 24, 799 CrossRef CAS
 ;
(b) T. J. Dingemans and E. T. Samulski, Liq. Cryst., 2000, 27, 131 CrossRef CAS
;
(b) T. J. Dingemans and E. T. Samulski, Liq. Cryst., 2000, 27, 131 CrossRef CAS  ;
(c) O. Francescangeli and E. T. Samulski, Soft Matter, 2010, 6, 2413 RSC
;
(c) O. Francescangeli and E. T. Samulski, Soft Matter, 2010, 6, 2413 RSC  ;
(d) N. A. Zafiropoulos, W. Lin, E. T. Samulski, T. J. Dingemans and S. J. Picken, Liq. Cryst., 2009, 36, 649 CrossRef CAS
;
(d) N. A. Zafiropoulos, W. Lin, E. T. Samulski, T. J. Dingemans and S. J. Picken, Liq. Cryst., 2009, 36, 649 CrossRef CAS  ;
(e) V. Görtz, C. Southern, N.
W. Roberts, H. F. Gleeson and J. W. Goodby, Soft Matter, 2009, 5, 463 RSC
;
(e) V. Görtz, C. Southern, N.
W. Roberts, H. F. Gleeson and J. W. Goodby, Soft Matter, 2009, 5, 463 RSC  ;
(f) M. Lehmann, C. Köhn, H. Kresse and Z. Vakhovskaya, Chem. Commun., 2008, 1768 RSC
;
(f) M. Lehmann, C. Köhn, H. Kresse and Z. Vakhovskaya, Chem. Commun., 2008, 1768 RSC  ;
(g) P. J. Martin and D. W. Bruce, Liq. Cryst., 2007, 34, 767 CrossRef CAS
;
(g) P. J. Martin and D. W. Bruce, Liq. Cryst., 2007, 34, 767 CrossRef CAS  ;
(h) S.-W. Choi, S. Kang, Y. Takanishi, K. Ishikawa, J. Watanabe and H. Takezoe, Chirality, 2007, 19, 250 CrossRef CAS
;
(h) S.-W. Choi, S. Kang, Y. Takanishi, K. Ishikawa, J. Watanabe and H. Takezoe, Chirality, 2007, 19, 250 CrossRef CAS  ;
(i) D. Apreutesei and G. H. Mehl, J. Mater. Chem., 2007, 17, 4711 RSC
;
(i) D. Apreutesei and G. H. Mehl, J. Mater. Chem., 2007, 17, 4711 RSC  .
.
- C. Tschierske and D. Photinos, J. Mater. Chem., 2010, 20, 4263 RSC
 .
.
- G. Hughes and M. R. Bryce, J. Mater. Chem., 2005, 15, 94 RSC
 .
.
-
(a) C. Adachi, T. Tsutsui and S. Saito, Appl. Phys. Lett., 1989, 55, 1489 CrossRef CAS
 ;
(b) H. Tokuhisa, M. Era, T. Tsutsui and S. Saito, Appl. Phys. Lett., 1995, 66, 3433 Search PubMed
;
(b) H. Tokuhisa, M. Era, T. Tsutsui and S. Saito, Appl. Phys. Lett., 1995, 66, 3433 Search PubMed  .
.
-
(a) J. Bettenhausen, P. Strohriegl, W. Brütting, H. Tokuhisa and T. Tsutsui, J. Appl. Phys., 1997, 82, 4957 CrossRef CAS
 ;
(b) J. Bettenhausen and P. Strohriegl, Adv. Mater., 1996, 8, 507 CAS
;
(b) J. Bettenhausen and P. Strohriegl, Adv. Mater., 1996, 8, 507 CAS  .
.
- D. Acierno, S. Concilio, A. Diodati, P. Iannelli, S. P. Piotto and P. Scarfato, Liq. Cryst., 2002, 29, 1383 CrossRef CAS
 .
.
-
(a) M. O'Neill and S. M. Kelly, Adv. Mater., 2003, 15, 1135 CrossRef
 ;
(b) W. Pisula, M. Zorn, J. Y. Chang, K. Müllen and R. Zentel, Macromol. Rapid Commun., 2009, 30, 1179 CrossRef
;
(b) W. Pisula, M. Zorn, J. Y. Chang, K. Müllen and R. Zentel, Macromol. Rapid Commun., 2009, 30, 1179 CrossRef  ;
(c) S. Sergeyev, W. Pisula and Y. H. Geerts, Chem. Soc. Rev., 2007, 36, 1902 RSC
;
(c) S. Sergeyev, W. Pisula and Y. H. Geerts, Chem. Soc. Rev., 2007, 36, 1902 RSC  ;
(d) Y. Sagara and T. Kato, Nat. Chem., 2009, 1, 605 CrossRef CAS
;
(d) Y. Sagara and T. Kato, Nat. Chem., 2009, 1, 605 CrossRef CAS  .
.
- R. Cristiano, F. Ely and H. Gallardo, Liq. Cryst., 2005, 32, 15 CrossRef CAS
 .
.
-
(a) R. Cristiano, A. A. Vieira, F. Ely and H. Gallardo, Liq. Cryst., 2006, 33, 381 CrossRef CAS
 ;
(b) M. Parra, J. Alderete, C. Zuniga, H. Gallardo, P. Hidalgo, J. Vergara and S. Hernandez, Liq. Cryst., 2001, 28, 1659 CrossRef CAS
;
(b) M. Parra, J. Alderete, C. Zuniga, H. Gallardo, P. Hidalgo, J. Vergara and S. Hernandez, Liq. Cryst., 2001, 28, 1659 CrossRef CAS  .
.
- C. Chai, Q. Yang, X. Fan, X. Chen, Z. Shen and Q. Zhou, Liq. Cryst., 2008, 35, 133 Search PubMed
 .
.
- R. Cristiano, D. M. P. O. Santos and H. Gallardo, Liq. Cryst., 2005, 32, 7 CrossRef CAS
 .
.
- H. Tokuhisa, M. Era and T. Tsutsui, Appl. Phys. Lett., 1998, 72, 2639 CrossRef CAS
 .
.
- H. T. Wang, F. L. Zhang, B. L. Bai, P. Zhang, J. H. Shi, D.Y. Yu, Y. F. Zhao, Y. Wang and M. Li, Liq. Cryst., 2008, 35, 905 CrossRef CAS
 .
.
- E. J. Choi and F. Xu, Mol. Cryst. Liq. Cryst., 2010, 529, 147 Search PubMed
 .
.
- J. Seo, S. Kim, S. H. Gihm, C. R. Park and S. Y. Park, J. Mater. Chem., 2007, 17, 5052 RSC
 .
.
-
(a) S. Qu, L. Wang, X. Liu and M. Li, Chem.–Eur. J., 2011, 17, 3512 CrossRef CAS
 ;
(b) S. Qu and M. Li, Tetrahedron, 2007, 63, 12429 CrossRef CAS
;
(b) S. Qu and M. Li, Tetrahedron, 2007, 63, 12429 CrossRef CAS  ;
(c) S. Qu, L. Zhao, Z. Yu, Z. Xiu, C. Zhao, P. Zhang, B. Long and M. Li, Langmuir, 2009, 25, 1713 CrossRef CAS
;
(c) S. Qu, L. Zhao, Z. Yu, Z. Xiu, C. Zhao, P. Zhang, B. Long and M. Li, Langmuir, 2009, 25, 1713 CrossRef CAS  ;
(d) S. Qu, X. Chen, X. Shao, F. Li, H. Zhang, H. Wang, P. Zhang, Z. Yu, K. Wu, Y. Wang and M. Li, J. Mater. Chem., 2008, 18, 3954 RSC
;
(d) S. Qu, X. Chen, X. Shao, F. Li, H. Zhang, H. Wang, P. Zhang, Z. Yu, K. Wu, Y. Wang and M. Li, J. Mater. Chem., 2008, 18, 3954 RSC  ;
(e) S. Qu, Y. Li, L. Wang, Q. Lu and X. Liu, Chem. Commun., 2011, 47, 4207 RSC
;
(e) S. Qu, Y. Li, L. Wang, Q. Lu and X. Liu, Chem. Commun., 2011, 47, 4207 RSC  .
.
- C.-P. Chai, X.-Q. Zhu, P. Wang, M.-Q. Ren, X.-F. Chen, Y.-D. Xu, X.-H. Fan, C. Ye, E.-Q. Chen and Q.-F. Zhou, Macromolecules, 2007, 40, 9361 CrossRef CAS
 .
.
-
(a) Y.-D. Zhang, K. G. Jespersen, M. Kempe, J. A. Kornfield, S. Barlow, B. Kippelen and S. R. Marder, Langmuir, 2003, 19, 6534 CrossRef CAS
 ;
(b) S. H. Gihm, B. G. Kim, S. Kim, J. Seo, S. Y. Park and C. R. Park, J. Mol. Struct., 2010, 984, 371 Search PubMed
;
(b) S. H. Gihm, B. G. Kim, S. Kim, J. Seo, S. Y. Park and C. R. Park, J. Mol. Struct., 2010, 984, 371 Search PubMed  .
.
-
(a) B. G. Kim, S. Kim and S. Y. Park, Tetrahedron Lett., 2001, 42, 2697 CrossRef CAS
 ;
(b) B. G. Kim, S. Kim and Y. Y. Park, Mol. Cryst. Liq. Cryst., 2001, 370, 391 Search PubMed
;
(b) B. G. Kim, S. Kim and Y. Y. Park, Mol. Cryst. Liq. Cryst., 2001, 370, 391 Search PubMed  .
.
- S. Varghese, N. S. Saleesh Kumar, A. Krishna, D. S. Shanker Rao, S. Krishna Prasad and S. Das, Adv. Funct. Mater., 2009, 19, 2064 CrossRef
 .
.
-
(a) E. Westphal, I. H. Bechtold and H. Gallardo, Macromolecules, 2010, 43, 1319 CrossRef CAS
 ;
(b) J. Eccher, A. R. Sampaio, R. C. Viscovini, G. Conte, E. Westphal, H. Gallardo and I. H. Bechtold, J. Mol. Liq., 2010, 153, 162 Search PubMed
;
(b) J. Eccher, A. R. Sampaio, R. C. Viscovini, G. Conte, E. Westphal, H. Gallardo and I. H. Bechtold, J. Mol. Liq., 2010, 153, 162 Search PubMed  .
.
-
(a) H.-T. Nguyen, C. Destrade and J. Malthete, Adv. Mater., 1997, 9, 375 CrossRef CAS
 ;
(b)
Handbook of Liquid Crystals, ed. D. Demus, J. W. Goodby, G. W. Gray, H.-W. Spiess and V. Vill, VCH, Weinheim, 1998, vol. 2B, ch. XII Search PubMed
;
(b)
Handbook of Liquid Crystals, ed. D. Demus, J. W. Goodby, G. W. Gray, H.-W. Spiess and V. Vill, VCH, Weinheim, 1998, vol. 2B, ch. XII Search PubMed  .
.
- J. Malthete, H.-T. Nguyen and C. Destrade, Liq. Cryst., 1993, 13, 171 CrossRef CAS
 .
.
-
(a) K. E. Rowe and D. W. Bruce, J. Mater. Chem., 1998, 8, 331 RSC
 ;
(b) D. Fazio, B. Donnio, Y. Galerne, D. Guillon and D. W. Bruce, J. Mater. Chem., 2001, 11, 2852 RSC
;
(b) D. Fazio, B. Donnio, Y. Galerne, D. Guillon and D. W. Bruce, J. Mater. Chem., 2001, 11, 2852 RSC  .
.
- N. H. Sultana, S. M. Kelly, B. Mansoor and M. O'Neill, Liq. Cryst., 2007, 34, 1307 CrossRef CAS
 .
.
-
(a) Examples of rigid bent-core polycatenars: E. Gorecka, D. Pociecha, J. Mieczkowski, J. Matraszek, B. Donnio and D. Guillon, J. Am. Chem. Soc., 2004, 126, 15946 Search PubMed
 ;
(b) E. Gorecka, D. Pociecha, J. Matraszek, J. Mieczkowski, Y. Shimbo, Y. Takanishi and H. Takezoe, Phys. Rev., 2006, 73, 031704 Search PubMed
;
(b) E. Gorecka, D. Pociecha, J. Matraszek, J. Mieczkowski, Y. Shimbo, Y. Takanishi and H. Takezoe, Phys. Rev., 2006, 73, 031704 Search PubMed  ;
(c) J. Matraszek, J. Mieczkowski, D. Pociecha, E. Gorecka, B. Donnio and D. Guillon, Chem.–Eur. J., 2007, 13, 3377 CrossRef CAS
;
(c) J. Matraszek, J. Mieczkowski, D. Pociecha, E. Gorecka, B. Donnio and D. Guillon, Chem.–Eur. J., 2007, 13, 3377 CrossRef CAS  .
.
- The molecular length L was determined with space-filling models (also known as CPK models by Corey, Pauling and Koltun), see R. B. Corey and L. Pauling, Rev. Sci. Instrum., 1953, 24, 621 Search PubMed
 .
.
-
(a) Other examples for columnar phases formed by half-discs: K. Kishikawa, S. Furusawa, T. Yamaki, S. Kohmoto, M. Yamamoto and K. Yamaguchi, J. Am. Chem. Soc., 2002, 124, 1597 Search PubMed
 ;
(b) A. Paraskos, Y. Nishiyama and T. Swager, Mol. Cryst. Liq. Cryst., 2004, 411, 363 Search PubMed
;
(b) A. Paraskos, Y. Nishiyama and T. Swager, Mol. Cryst. Liq. Cryst., 2004, 411, 363 Search PubMed  ;
(c) T. Hegmann, P. Peidis, S. Diele and C. Tschierske, Liq. Cryst., 2000, 27, 1261 CrossRef CAS
;
(c) T. Hegmann, P. Peidis, S. Diele and C. Tschierske, Liq. Cryst., 2000, 27, 1261 CrossRef CAS  .
.
- That the aromatic cores are oriented perpendicular to the column axis is shown by the color of the fans in the optical micrographs (inset in Fig. 1b) taken with a λ-plate. The yellow and blue colors define the orientation of the high-index axis as radial rather than tangential. Since the columns are tangential in the fans, and the high-index axis is known to be parallel to the intramolecular π-conjugation pathway along the aromatic cores, it follows that the rigid cores have a preferred direction perpendicular to the columns; (n‖ < n⊥ = optically negative).
- Compound p/6 was not reported previously and was synthesized as a reference compound to compare it with m/6, for details, see ESI†.
-
(a) C. Tschierske, J. Mater. Chem., 1998, 8, 1485–1580 RSC
 ;
(b) C. Tschierske, J. Mater. Chem., 2001, 11, 2647–2671 RSC
;
(b) C. Tschierske, J. Mater. Chem., 2001, 11, 2647–2671 RSC  .
.
- K. Q. Ye, J. Wang, H. Sun, Y. Liu, Z. C. Mu, F. Li, S. M. Jiang, J. Y. Zhang, H. X. Zhang, Y. Wang and C. M. Che, J. Phys. Chem. B, 2005, 109, 8008–8016 CrossRef CAS
 .
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra01362b |
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. 


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31/2
31/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Fig. 2a), indicating a hexagonal lattice (Colhex/p6mm). Though in the small-angle region of compound m/14, only one scattering (d = 3.28 nm) is found (Fig. S2†), in the diffraction pattern of an oriented sample it is split into 6 spots on a regular hexagon, confirming a hexagonal columnar mesophase also for this compound (Fig. 2b and Table 2). The lattice parameters ahex were calculated to be ahex = 3.0 nm (at T = 70 °C) for m/6 and ahex = 3.8 nm (at T = 78 °C) for m/14 (Table 2). Though XRD has not been carried out for all columnar phases of compounds m/n, based on textural similarities (e.g. homeotropic alignment, indicating uniaxiality of all LC phases, spherulitic textures indicating columnar organization in all cases), it is highly reasonable to assume that also the LC phases of the intermediate homologues represent hexagonal columnar phases. The number of molecules organized in a slice of the columns with a height of h = 0.45 nm (maximum of the diffuse wide angle scattering), estimated using eqn (1) and assuming a density of ρ = 1 g cm−3, is about n = 2 for the investigated compounds (NA = Avogadro constant, M = molecular mass, see Table 2).
2 (Fig. 2a), indicating a hexagonal lattice (Colhex/p6mm). Though in the small-angle region of compound m/14, only one scattering (d = 3.28 nm) is found (Fig. S2†), in the diffraction pattern of an oriented sample it is split into 6 spots on a regular hexagon, confirming a hexagonal columnar mesophase also for this compound (Fig. 2b and Table 2). The lattice parameters ahex were calculated to be ahex = 3.0 nm (at T = 70 °C) for m/6 and ahex = 3.8 nm (at T = 78 °C) for m/14 (Table 2). Though XRD has not been carried out for all columnar phases of compounds m/n, based on textural similarities (e.g. homeotropic alignment, indicating uniaxiality of all LC phases, spherulitic textures indicating columnar organization in all cases), it is highly reasonable to assume that also the LC phases of the intermediate homologues represent hexagonal columnar phases. The number of molecules organized in a slice of the columns with a height of h = 0.45 nm (maximum of the diffuse wide angle scattering), estimated using eqn (1) and assuming a density of ρ = 1 g cm−3, is about n = 2 for the investigated compounds (NA = Avogadro constant, M = molecular mass, see Table 2).




.
.
; (b) M. L. Parra, E. Y. Elgueta, V. Jimenez and P. I. Hidalgo, Liq. Cryst., 2009, 36, 301–317 CrossRef CAS
; (c) A. K. Prajapati and V. Modi, Phase Transitions, 2010, 83, 634 Search PubMed
; (d) M. L. Parra, P. I. Hidalgo and E. Y. Elgueta, Liq. Cryst., 2008, 35, 823 CrossRef CAS
; (e) M. Parra, P. Hidalgo, E. Carrasco, J. Barbera and L. Silvino, Liq. Cryst., 2006, 33, 875 CrossRef CAS
.
; (b) J. Han, F.-L. Wang, F.-Y. Zhang and L.-R. Zhu, Liq. Cryst., 2010, 37, 1521 Search PubMed
; (c) J. Han, M. Zhang, F. Wang and Q. Geng, Liq. Cryst., 2010, 37, 1471 CrossRef CAS
; (d) K. C. Majumdar, P. K. Shyam, D. S. S. Rao and S. K. Prasad, J. Mater. Chem., 2011, 21, 556 RSC
.
.
.
.
.
.
.
.
; (b) T. J. Dingemans and E. T. Samulski, Liq. Cryst., 2000, 27, 131 CrossRef CAS
; (c) O. Francescangeli and E. T. Samulski, Soft Matter, 2010, 6, 2413 RSC
; (d) N. A. Zafiropoulos, W. Lin, E. T. Samulski, T. J. Dingemans and S. J. Picken, Liq. Cryst., 2009, 36, 649 CrossRef CAS
; (e) V. Görtz, C. Southern, N. W. Roberts, H. F. Gleeson and J. W. Goodby, Soft Matter, 2009, 5, 463 RSC
; (f) M. Lehmann, C. Köhn, H. Kresse and Z. Vakhovskaya, Chem. Commun., 2008, 1768 RSC
; (g) P. J. Martin and D. W. Bruce, Liq. Cryst., 2007, 34, 767 CrossRef CAS
; (h) S.-W. Choi, S. Kang, Y. Takanishi, K. Ishikawa, J. Watanabe and H. Takezoe, Chirality, 2007, 19, 250 CrossRef CAS
; (i) D. Apreutesei and G. H. Mehl, J. Mater. Chem., 2007, 17, 4711 RSC
.
.
.
; (b) H. Tokuhisa, M. Era, T. Tsutsui and S. Saito, Appl. Phys. Lett., 1995, 66, 3433 Search PubMed
.
; (b) J. Bettenhausen and P. Strohriegl, Adv. Mater., 1996, 8, 507 CAS
.
.
; (b) W. Pisula, M. Zorn, J. Y. Chang, K. Müllen and R. Zentel, Macromol. Rapid Commun., 2009, 30, 1179 CrossRef
; (c) S. Sergeyev, W. Pisula and Y. H. Geerts, Chem. Soc. Rev., 2007, 36, 1902 RSC
; (d) Y. Sagara and T. Kato, Nat. Chem., 2009, 1, 605 CrossRef CAS
.
.
; (b) M. Parra, J. Alderete, C. Zuniga, H. Gallardo, P. Hidalgo, J. Vergara and S. Hernandez, Liq. Cryst., 2001, 28, 1659 CrossRef CAS
.
.
.
.
.
.
.
; (b) S. Qu and M. Li, Tetrahedron, 2007, 63, 12429 CrossRef CAS
; (c) S. Qu, L. Zhao, Z. Yu, Z. Xiu, C. Zhao, P. Zhang, B. Long and M. Li, Langmuir, 2009, 25, 1713 CrossRef CAS
; (d) S. Qu, X. Chen, X. Shao, F. Li, H. Zhang, H. Wang, P. Zhang, Z. Yu, K. Wu, Y. Wang and M. Li, J. Mater. Chem., 2008, 18, 3954 RSC
; (e) S. Qu, Y. Li, L. Wang, Q. Lu and X. Liu, Chem. Commun., 2011, 47, 4207 RSC
.
.
; (b) S. H. Gihm, B. G. Kim, S. Kim, J. Seo, S. Y. Park and C. R. Park, J. Mol. Struct., 2010, 984, 371 Search PubMed
.
; (b) B. G. Kim, S. Kim and Y. Y. Park, Mol. Cryst. Liq. Cryst., 2001, 370, 391 Search PubMed
.
.
; (b) J. Eccher, A. R. Sampaio, R. C. Viscovini, G. Conte, E. Westphal, H. Gallardo and I. H. Bechtold, J. Mol. Liq., 2010, 153, 162 Search PubMed
.
; (b) Handbook of Liquid Crystals, ed. D. Demus, J. W. Goodby, G. W. Gray, H.-W. Spiess and V. Vill, VCH, Weinheim, 1998, vol. 2B, ch. XII Search PubMed
.
.
; (b) D. Fazio, B. Donnio, Y. Galerne, D. Guillon and D. W. Bruce, J. Mater. Chem., 2001, 11, 2852 RSC
.
.
; (b) E. Gorecka, D. Pociecha, J. Matraszek, J. Mieczkowski, Y. Shimbo, Y. Takanishi and H. Takezoe, Phys. Rev., 2006, 73, 031704 Search PubMed
; (c) J. Matraszek, J. Mieczkowski, D. Pociecha, E. Gorecka, B. Donnio and D. Guillon, Chem.–Eur. J., 2007, 13, 3377 CrossRef CAS
.
.
; (b) A. Paraskos, Y. Nishiyama and T. Swager, Mol. Cryst. Liq. Cryst., 2004, 411, 363 Search PubMed
; (c) T. Hegmann, P. Peidis, S. Diele and C. Tschierske, Liq. Cryst., 2000, 27, 1261 CrossRef CAS
.
; (b) C. Tschierske, J. Mater. Chem., 2001, 11, 2647–2671 RSC
.
.