Organic dyes containing fluorene-substituted indoline core for zinc oxide dye-sensitized solar cell†
Shinji
Higashijima
ab,
Yukiko
Inoue
a,
Hidetoshi
Miura
a,
Yasuhiro
Kubota
b,
Kazumasa
Funabiki
b,
Tsukasa
Yoshida
c and
Masaki
Matsui
*b
aTsukuba Research Center, Chemicrea Inc., 2-1-6, Sengen, Tsukuba, Ibaraki, 305-0047, Japan. E-mail: higashis@chemicrea.co.jp; Fax: +81-29-863-6041; Tel: +81-29-863-6040
bDepartment of Material Science and Technology, Faculty of Engineering, Gifu University, 1-1 Yanagido, Gifu, 501-1193, Japan. E-mail: matsuim@gifu-u.ac.jp; Fax: +81-58-293-2794; Tel: +81-58-293-2601
cEnvironmental and Renewable Energy System Division, Graduate School of Engineering, Gifu University, 1-1 Yanagido, Gifu, 501-1193, Japan
First published on 24th February 2012
Abstract
A novel idoline dye, having dimethylfluorene-substituted indoline donor and dicyanovinylidene-introduced rhodanine acceptor (DN350), gave much higher efficiency than D205 due to bathochromic absorption band and prevention of aggregates formation.
Dye-sensitized solar cells (DSSCs) are currently attracting widespread interest as potential candidates for renewable-energy systems.1 The highest efficiency of 11.2% has been reported for ruthenium complex N719 on nanocrystalline-titanium oxide.2 However, ruthenium metal is quite expensive and the derived complexes are hard to purify compared with metal-free dyes. Therefore, many organic dyes such as oligothiophenes,3 coumarins,4 oligo(phenylenevinylene) derivatives5 and indoline dyes6 have been proposed for DSSC applications. In particular, an indoline dye D205 is widely known as one of the most efficient organic sensitizers on titanium oxide, the conversion efficiency (η) being 9.5%.7 Zinc oxide is a versatile semiconductor having a similar band gap energy to titanium oxide and has been studied with various organic dyes.8 D205 also shows excellent efficiency on zinc oxide.9
We have recently reported that a new indoline dye DN319, having the strongly electron-withdrawing dicyanovinylidene moiety in the terminal rhodanine ring, shows a higher efficiency than D205.10 We consider that this result can come from both the bathochromic shift in the UV-vis absorption band and the positive shift in the Eox level of DN319. On the other hand, organic dyes having a fluorenyl moiety have been reported to prevent aggregation formation due to the sp3-carbon atom at the 9-position and to show high stability for UV-irradiation and heating.11,12 We report herein the photovoltaic properties of new indoline dyes having dicyanovinylidene and fluorenyl moieties.
Fig. 1 shows the synthesis of new indoline dyes. Intermediates 2b–d were prepared by the alkylation of 2-iodofluorene 1. Compounds 1 and 2b–d were allowed to react with indoline 3 to give fluorene-substituted indoline derivatives 4a–d, followed by formylation to afford 5a–d, which were further allowed to react with a double rhodanine having dicyanovinylidene moiety 6 to give DN362, DN350, DN351 and DN363. The details and analytical data are given in the Electronic Supplementary Information (ESI).† Authentic D205 and DN319 were also synthesized as reference compounds.
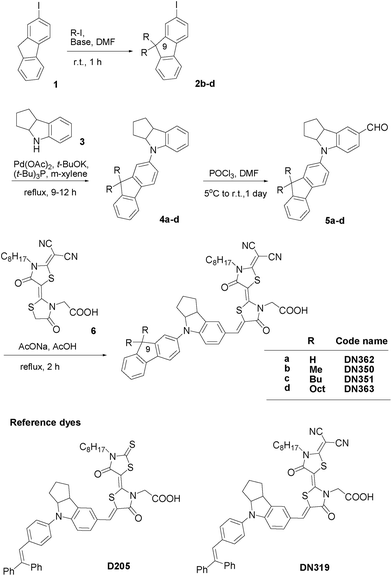 | ||
| Fig. 1 Synthesis route of fluorene-substituted indoline dyes and structures of reference indoline dyes. | ||
Fig. 2 shows the UV-vis absorption and fluorescence spectra of DN362, DN350, DN351, DN363, D205 and DN319 in chloroform. All dyes except for D205 showed the first absorption maxima (λmax) at around 567 nm. Thus, the absorption band showed a bathochromic shift by introducing an electron-withdrawing group into the terminal rhodanine ring. No marked differences in molar absorption coefficient (ε) were observed among these dyes. The results are also listed in Table 1.
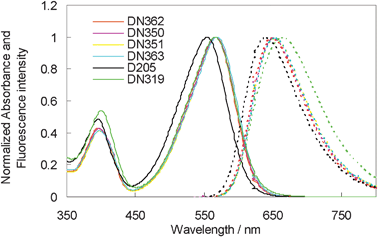 | ||
| Fig. 2 Normalized UV-vis absorption (solid line) and fluorescence (dotted line) spectra of indoline dyes in chloroform. | ||
| Dye | λ max (ε)a/nm | λ max on ZnO/nm | F max a/nm | E ox b/V | E ox − E0–0c/V | HOMOd/eV | LUMOd/eV |
|---|---|---|---|---|---|---|---|
| a Measured on 1.0 × 10−5 mol dm−3 of substrate in chloroform at 25 °C. b vs. Fc/Fc+ in DMF. c Calculated on the basis of Eox and λint. d Calculated by the B3LYP/6-31G(d,p)//B3LYP/3-21G level. | |||||||
| DN362 | 396 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500), 566 (70 500), 566 (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900) 900) |
541 | 651 | + 0.35 | −1.66 | −5.23 | −2.52 |
| DN350 | 397 (31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900), 567 (74 900), 567 (74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800) 800) |
547 | 649 | + 0.36 | −1.66 | −5.22 | −2.51 |
| DN351 | 398 (31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900), 568 (77 900), 568 (77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400) 400) |
547 | 654 | + 0.36 | −1.65 | −5.22 | −2.52 |
| DN363 | 397 (31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500), 568 (76 500), 568 (76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) 100) |
547 | 654 | + 0.36 | −1.65 | −5.22 | −2.51 |
| D205 | 395 (38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100), 554 (74 100), 554 (74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700) 700) |
553 | 641 | + 0.35 | −1.73 | −5.06 | −2.36 |
| DN319 | 400 (36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700), 566 (68 700), 566 (68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
549 | 662 | + 0.37 | −1.66 | −5.18 | −2.53 |
The oxidation potentials of these dyes were measured in DMF as reported in our previous paper.13 The Eox values of DN350, DN351, DN363 and DN319 were observed at around +0.36 V vs. Fc/Fc+, being slightly more positive than that of D205. As the I−/I3− redox level was observed at −0.05 V vs. Fc/Fc+, this Eox level is sufficiently positive for electron-transfer to proceed from the electrolyte to the oxidized indoline dyes. As the reduction peak of these dyes was not observed by the electrochemical measurement, the Eox − E0–0 value, where E0–0 represents the intersection of normalized absorption and the fluorescence spectra in chloroform, was calculated.14 The Eox − E0–0 values of DN362, DN350, DN351, DN363 and DN319 were calculated to be ca. −1.66 V vs. Fc/Fc+. These values were significantly more positive than that of D205. However, as the conducton band level of zinc oxide is located at around −0.95 V vs. Fc/Fc+, these Eox − E0–0 values are negative enough to inject electrons from the excited dyes to zinc oxide.
Fig. 3 depicts the isodensity surface plots of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of DN350 by density functional theory (DFT) calculations. Electrons are located on the fluorene-substituted indoline part in the HOMO level and on the rhodanine rings in the LUMO level, suggesting efficient charge separation by excitation.15 The DFT calculations also indicated that both the HOMO and LUMO levels of DN362, DN350, DN351, DN363 and DN319 were more stable than those of D205, suggesting a high incident photon-to-electron conversion efficiency (IPCE).13 These results are consistent with the results that the Eox and Eox − E0–0 levels of DN362, DN350, DN351, DN363 and DN319 are more positive than those of D205.
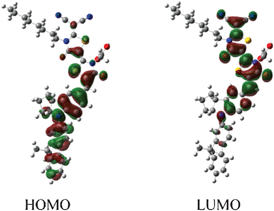 | ||
| Fig. 3 Isodensity surface plots of HOMO and LUMO of DN350. | ||
The procedure of fabrication of zinc oxide DSSCs is described in ESI.† For restraint of aggregation, cholic acid was added.
Fig. 4 indicates the UV-vis absorption band of indoline dyes on zinc oxide. The absorption bands of DN362, DN350, DN351, DN363 and DN319 were more bathochromic than that of D205, showing similarity to those in chloroform. Interestingly, the absorbance at around 460 nm was larger in the order of dyes: D205 > DN363 (Oct) > DN319 > DN351 (Bu) > DN350 (Me) > DN362 (H). The result suggests that ratio of H-aggregates formation on zinc oxide is significantly affected by the shape of the molecule.9
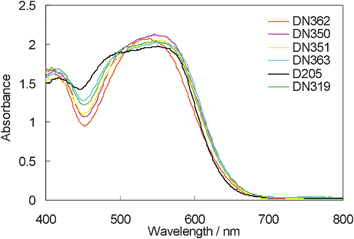 | ||
| Fig. 4 UV-vis absorption spectra of indoline dyes with cholic acid on zinc oxide. | ||
The IPCE action spectra of these dyes are shown in Fig. 5. They showed similar maximum IPCE values. However, interestingly, DN350 showed the additional sensitization of zinc oxide at around 460 and 650 nm, compared with the other indoline dyes.
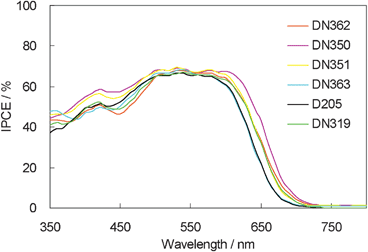 | ||
| Fig. 5 IPCE action spectra of indoline dyes with cholic acid on zinc oxide. | ||
Fig. 6 shows the photovoltaic characteristics of DSSCs under AM1.5 simulated sunlight (100 mW cm−2) illumination. DN350 showed the highest short-circuit photo-current density (Jsc). Table 2 summarizes the results of the photovoltaic properties of indoline dyes on zinc oxide. No significant differences in the open-circuit voltage (Voc) and fill factor (FF) were observed among the indoline dyes. DN350 showed the highest η of 5.55%, corresponding to a 13% improvement of D205, due to the highest Jsc. This result comes from the larger IPCE at around 460 and 650 nm of DN350. As similar molar amounts of dyes were adsorbed, the orientation of the dye and cholic acid on zinc oxide could affect the IPCE. When cholic acid can properly disperse the indoline dyes on zinc oxide, a higher IPCE can be obtained. Fig. 4 and 5 clearly indicate that the H-aggregates formation of DN350 on zinc oxide is least among the indoline dyes to show the higest IPCE at around 460 nm. The IPCE of DN350 at around 650 nm was the highest among DN362, DN351, DN363, and DN319, and DN350 as shown in Fig. 5. This result could be also attributed to the least aggregates formation of DN350 on zinc oxide. The melting points, which represent the intermolecular interactions between the molecules of DN362, DN350, D351 and DN363 were observed to be 216, 254, >300 and >300 °C, respectively. The interactions increased as the alkyl group was made longer. H-aggregates formation of bisazomethine dyes attached with the dialkylamino moiety has been reported to increase as the alkyl group is made longer.16 Meanwhile, the solubilities of DN362, DN350, D351 and DN363 in acetonitrile–tert-butyl alcohol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixed solvent at 25 °C were observed to be 0.4, 3.0, >5.0 and >5.0 × 10−3 g dm−3, respectively. Consequently, DN350 which has proper intermolecular interactions between the molecules and good affinity for the solvent, might form excellent dye–cholic acid orientation on zinc oxide to exhibit the highest conversion effficiency.
1) mixed solvent at 25 °C were observed to be 0.4, 3.0, >5.0 and >5.0 × 10−3 g dm−3, respectively. Consequently, DN350 which has proper intermolecular interactions between the molecules and good affinity for the solvent, might form excellent dye–cholic acid orientation on zinc oxide to exhibit the highest conversion effficiency.
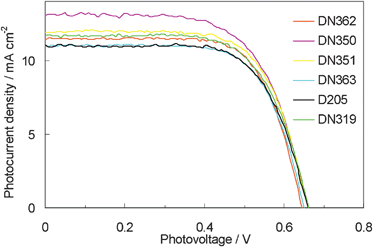 | ||
| Fig. 6 Current density vs. voltage characteristics for DSSCs with indoline dyes as sensitizers under AM 1.5 simulated sunlight (100 mW cm−2) illumination. | ||
| Dye | Dye amounta/10−8 mol cm−2 | J sc/mA cm−2 | V oc/V | FF | η (%) |
|---|---|---|---|---|---|
| a Amount of dye adsorption on zinc oxide measured by UV-vis absorption spectra. | |||||
| DN362 | 5.53 | 11.48 | 0.65 | 0.70 | 5.15 |
| DN350 | 5.41 | 13.07 | 0.66 | 0.65 | 5.55 |
| DN351 | 5.24 | 11.90 | 0.66 | 0.68 | 5.34 |
| DN363 | 5.50 | 10.99 | 0.65 | 0.69 | 4.92 |
| D205 | 6.10 | 11.02 | 0.66 | 0.68 | 4.92 |
| DN319 | 6.25 | 11.69 | 0.66 | 0.67 | 5.15 |
In summary, a new fluorene-substituted indoline dye DN350 exhibited a higher η value than D205, one of the most efficient organic sensitizers for DSSC. This result is attributed to the bathochromic shift based on the introduction of the electron-withdrawing dicyanovinylidene moiety into the terminal rhodanine ring and reduced aggregates formation comes from the dimethylfluorenyl group.
References
- (a) B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef CAS; (b) M. Grätzel, Nature, 2001, 414, 338 CrossRef; (c) P. Wang, C. Klein, R. Humphry-Baker, S. M. Zakeeruddin and M. Grätzel, J. Am. Chem. Soc., 2005, 127, 808 CrossRef CAS; (d) F. Gao, Y. Wang, J. Zhang, D. Shi, M. Wang, R. Humphry-Baker, P. Wang, S. M. Zakeeruddin and M. Grätzel, Chem. Commun., 2008, 2635 RSC.
- Md. K. Nazeeruddin, F. De Angelis, S. Fantacci, A. Selloni, G. Viscadi, P. Liska, S. Ito, B. Takeru and M. Grätzel, J. Am. Chem. Soc., 2005, 127, 16835 CrossRef CAS.
- N. Koumura, Z.-S. Wang, S. Mori, M. Miyashita, E. Suzuki and K. Hara, J. Am. Chem. Soc., 2006, 128, 14256 CrossRef CAS.
- Z.-S. Wang, Y. Cui, Y. Dan-oh, C. Kasada, A. Shinpo and K. Hara, J. Phys. Chem. C, 2007, 111, 7224 CAS.
- S. Hwang, J. H. Lee, C. Park, H. Lee, C. Kim, C. Park, M.-H. Lee, W. Lee, J. Park, K. Kim, N.-G. Park and C. Kim, Chem. Commun., 2007, 4887 RSC.
- (a) T. Horiuchi, H. Miura and S. Uchida, Chem. Commun., 2003, 3036 RSC; (b) T. Horiuchi, H. Miura, K. Sumioka and S. Uchida, J. Am. Chem. Soc., 2004, 126, 12218 CrossRef CAS.
- S. Ito, H. Miura, S. Uchida, M. Takata, K. Sumioka, P. Liska, P. Comte, P. Péchy and M. Grätzel, Chem. Commun., 2008, 5194 RSC.
- (a) E. Hosono, S. Fujihara, I. Honda and H. Zhou, Adv. Mater., 2005, 17, 2091 CrossRef CAS; (b) T. P. Chou, Q. Zhang and G. E. Cao, Adv. Mater., 2007, 19, 2588 CrossRef CAS; (c) T. Yoshida, J. Zhang, D. Komatsu, S. Sawatani, H. Minoura, T. Pouporté, D. Lincot, T. Oekermann, D. Schlettwein, H. Tada, D. Wöhrle, K. Funabiki, M. Matsui, H. Miura and H. Yanagi, Adv. Funct. Mater., 2008, 19, 17 CrossRef; (d) M. Matsui, Y. Asamura, Y. Kubota, K. Funabiki, J. Jin, T. Yoshida and H. Miura, Tetrahedron, 2010, 66, 7405 CrossRef CAS; (e) X.-F. Wang, O. Kitao, E. Hosono, H. Zhou, S. Sasaki and H. Tamaki, J. Photochem. Photobiol., A, 2010, 210, 145 CrossRef CAS; (f) Y. Sakuragi, X.-F. Wang, H. Miura, M. Matsui and T. Yoshida, J. Photochem. Photobiol., A, 2010, 216, 1 CrossRef CAS.
- H.-M. Cheng and W.-F. Hsieh, Energy Environ. Sci., 2010, 3, 442 CAS.
- S. Higashijima, H. Miura, T. Fujita, Y. Kubota, K. Funabiki, T. Yoshida and M. Matsui, Tetrahedron, 2011, 67, 6289 CrossRef CAS.
- (a) I. Jung, J. K. Lee, K. H. Song, K. Song, S. O. Kang and J. Ko, J. Org. Chem., 2007, 72, 3652 CrossRef CAS; (b) H. Qin, S. Wenger, M. Xu, F. Gao, X. Jin, P. Wang, S. M. Zakeeruddin and M. Grätzel, J. Am. Chem. Soc., 2008, 130, 9202 CrossRef CAS; (c) G. Zhou, N. Pschirer, J. C. Schöneboom, F. Eickemeyer, M. Baumgarten and K. Müllen, Chem. Mater., 2008, 20, 1808 CrossRef CAS.
- (a) Y. Shirota, M. Kinoshita, T. Noda, K. Okumoto and T. Ohara, J. Am. Chem. Soc., 2000, 122, 11021 CrossRef CAS; (b) S. Kim, K. H. Song, S. Kang and J. Ko, Chem. Commun., 2004, 68 RSC.
- M. Matsui, T. Fujita, Y. Kubota, K. Funabiki, J. Jin, T. Yoshida and H. Miura, Dyes Pigm., 2010, 86, 143 CrossRef CAS.
- K. Hara, T. Sato, R. Katoh, A. Furube, Y. Ohga, A. Shinpo, S. Suga, K. Sayama, H. Sugihara and H. Arakawa, J. Phys. Chem. B, 2003, 107, 597 CrossRef CAS.
- Y. Oseki, M. Fujitsuka, D. W. Cho, A. Sugimoto, S. Tojo and T. Majima, J. Phys. Chem. B, 2005, 109, 19257 CrossRef CAS.
- K. Kinashi, K-P. Lee, S. Matsumoto, K. Ishida and Y. Ueda, Dyes Pigm., 2011, 92, 783 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis procedure, characterization of all compounds and fabrication of DSSCs. See DOI: 10.1039/c2ra01358d |
| This journal is © The Royal Society of Chemistry 2012 |
