Graphene quantum dots with controllable surface oxidation, tunable fluorescence and up-conversion emission†
Shoujun
Zhu
,
Junhu
Zhang
,
Xue
Liu
,
Bo
Li
,
Xingfeng
Wang
,
Shijia
Tang
,
Qingnan
Meng
,
Yunfeng
Li
,
Ce
Shi
,
Rui
Hu
and
Bai
Yang
*
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun, 130012, P. R. China. E-mail: byangchem@jlu.edu.cn; Fax: +86-431-85193423; Tel: +86-431-85168478
First published on 2nd February 2012
Abstract
In this article, graphene quantum dots (GQDs) with tunable surface chemistry (increasing oxidation degree) were prepared by an efficient two-step method. The GQDs have tunable fluorescence induced by the degree of surface oxidation, fine solubility, high stability and applicable up-conversion photoluminescence (PL). The PL mechanism was investigated based on the surface structure and PL behaviors. More importantly, the GQDs have acid–base response property and can be applied as pH sensors.
Introduction
In recent years, fluorescent carbon-based materials,1 such as fullerene,2 carbon nanotubes,3 nanodiamond,4 carbon nanoparticles5–9 and the rising graphene quantum dots (GQDs),10 have received increasing attention due to their superiority in large optical absorptivity, chemical stability, fine biocompatibility and low toxicity.1 These emerging carbon-based nanomaterials have been exploited in a series of exciting areas, such as bioimaging, medical diagnosis, catalysis, photovoltaic devices and so on. However, these materials demand critical solutions in obtaining effective synthetic and separation routes, controllable fluorescent color with high quantum efficiency, high solubility for practical applications and a clear PL mechanism.1To facilitate the application of graphene as a fluorescent material, a promising strategy is to convert 2D graphene to 0D GQDs.10–20 Till now, all existing approaches to prepare GQDs can be classified as two aspects: “top-down” cutting carbon resources or a “bottom-up” condensation reaction. Several groups have reported the synthesis of fluorescent GQDs. For example, Pan et al. have prepared GQDs with blue photoluminescence by cutting reduced graphene oxide through a hydrothermal route.10 Yan et al. have used solution-based synthetic routes to prepare GQDs with precise structures.12,13 Dai's group has also reported the fabrication of fluorescent nano-graphene oxide by rate separation method.14 GQDs have been fabricated by other methods as well.15–20 To present, it is still highly significant to develop an efficient method to obtain tunable luminescent GQDs in the large scale by an efficient method. Moreover, the PL mechanism is extremely desired to be clarified.
In our previous work, we have reported a one-step solvothermal route to synthesize strongly green-photoluminescent GQDs which showed good fluorescent properties and have been demonstrated to be excellent probes for bioimaging.21 However, the wide size distribution of GQDs and the single PL color are fatal to some practical applications and the investigation of the PL mechanism. In this work, combining “top-down” cutting and separation routes, we demonstrate an efficient two-step method to obtain GQDs with tunable surface chemistry compared to previously reported GQDs. The separated GQDs have strong blue to green fluorescence induced by controllable degree of surface oxidation, fine solubility, high stability and pH-sensitive property. The PL mechanism was investigated based on the surface structure and PL behaviors. Moreover, the GQDs show up-conversion PL which may find exciting applications in bioimaging or energy collection.
Experimental
Preparation
GO was prepared by reported methods.21 For synthesizing GQDs, GO was dispersed in DMF with the concentrations of 270 mg 10 mL−1 (Note: other ratios of GO/DMF can also be used to prepare the GQDs, such as 50–500 mg 10 mL−1 and the optimization ratio is not the same for GO with a different degree of oxidation). The GO/DMF solutions were under ultrasonication for 30 min (120 W, 100 kHz), and then transferred to a poly (tetrafluoroethylene) (Teflon)-lined autoclave (30 mL) and heated at 200 °C for 8 h. After the above reaction, the reactors were cooled to room temperature naturally or by water. The product consisted of a brown transparent suspension and black precipitates, and the black precipitates were wasted. The solid samples can be obtained by evaporating the solvents, and we found out that the GQD yield was about 1.6%. After several tests, we concluded that GQDs have excellent solubility in many polar organic solvents or water with different pH levels.Then, the GQDs were purified by column chromatography on silica (the commercial sephadex can also work) utilizing gradient elution (mobile phase: A was methylene chloride-MeOH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), B was H2O). Under the A phase elution, Batch 1 and 2 were obtained in a sequence. Subsequently, under the B phase elution, Batch 3 was obtained, the Batch 3 is the primary production with a yield of ca. 50–70% among the three batches. There were still residues after the two steps separations, which were supposed to be large GO or reduced GO (over 10 nm) left from solvothermal process. There are intangible components in Batch 1, which may be carbon nanoparticles formed by the carbonization of DMF.
1, v/v), B was H2O). Under the A phase elution, Batch 1 and 2 were obtained in a sequence. Subsequently, under the B phase elution, Batch 3 was obtained, the Batch 3 is the primary production with a yield of ca. 50–70% among the three batches. There were still residues after the two steps separations, which were supposed to be large GO or reduced GO (over 10 nm) left from solvothermal process. There are intangible components in Batch 1, which may be carbon nanoparticles formed by the carbonization of DMF.
Characterization
TEM was conducted using a Hitachi H-800 electron microscope at an acceleration voltage of 200 kV with a CCD cinema. AFM images were recorded in the tapping mode with a Nanoscope IIIa scanning probe microscope from Digital Instruments under ambient conditions. Fluorescence spectroscopy was performed with a Shimadzu RF-5301 PC spectrophotometer. UV-vis absorption spectra were obtained using a Shimadzu 3100 UV-vis spectrophotometer. X-ray Photoelectron Spectroscopy (XPS) was investigated by using ESCALAB 250 spectrometer with a mono X-Ray source Al Kα excitation (1486.6 eV). Binding energy calibration was based on C 1s at 284.6 eV.Result and discussion
GQDs were prepared by our previous method, they have wide size distribution which ranges from several nanometres to over ten nanometres. (Fig. 1).21 After a two-step column chromatography separation, we obtained GQDs with a narrow size distribution compared with our previous results.21 There are three separated batches (Batch 1–3) utilizing gradient elution. The three batches have similar sizes and heights from TEM and AFM characterization (Fig. 2, only the AFM image of Batch 3 was given as Batch 1 and 2 have the same AFM morphologies). Fig. 2a–e show the Transmission Electron Microscopy (TEM) images of GQDs and their size distribution. The average diameters of the GQDs are 3–5 nm, and the three batches have a similar size scale. Atomic Force Microscope (AFM) images of the GQDs (Fig. 2f and g.) show that their average height is 0.95 nm. Consequently, it can be concluded that most of the GQDs are single-layered or double-layered (less than 5 layers).10,21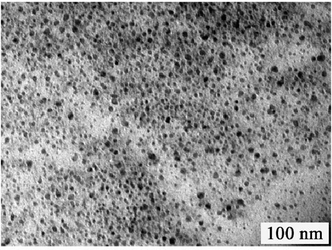 | ||
| Fig. 1 TEM images of un-separated GQDs. | ||
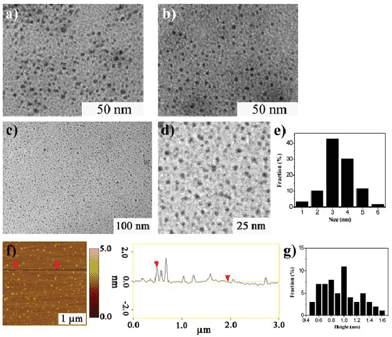 | ||
| Fig. 2 Morphology of GQDs (Batch 1–3). a) TEM image of Batch 1. b) TEM image of Batch 2. c) TEM image of Batch 3 and d) is the enlarge image. e) The size distribution of Batch 3. f–g) AFM image of Batch 3 and its height distribution (the three batches have similar TEM and AFM characterization so only the AFM of Batch 3 was shown). | ||
Though GQDs in three batches possess similar sizes and heights, they have tuned optical properties, which are speculated to be controlled by increasing the degree of surface oxidation10,21 (see mechanism section for detailed analysis). From the UV-vis absorption of the GQDs (Fig. 3a), a typical absorption peak at ca. 320 nm was observed, which is similar to the reported GQDs.11,21 Inset of Fig. 3a is the photograph of the GQD aqueous solution taken under visible lights, and shows brown color. The three batches have tuned fluorescence from blue to green at the same excitation (Fig. 3b), and they each exhibit excitation-dependent PL behaviors (Fig. 3c–e). Interestingly, the PL spectrum shows a strong peak at 427 nm as well as a shoulder peak at 516 nm when excited under the 320 nm wavelength, especially in Batch 3 (Fig. 3 e), where, with red shift of the excitation wavelength, the peak at 427 nm vanishes while the shoulder at 516 nm increases significantly. The full width at half maximum (FWHM) is 90 nm, which is narrower than most reported GQDs.10–21 Using 9,10-bis (phenylethynyl) anthracene as a reference, the PL quantum yields were measured to be 4.1, 9.9 and 12.2% respectively, which are a little higher than that of un-separated GQDs and higher than most reported luminescent carbon nanomaterials.2–21
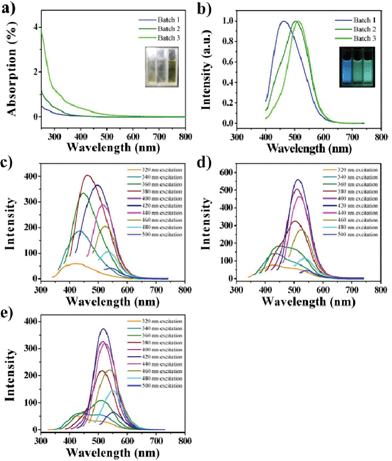 | ||
| Fig. 3 The optical properties of the GQDs aqueous solution. a) UV-vis absorption (ABS) spectra of the GQDs (inset: photograph taken under visible light). b) PL spectra of Batch 1–3 at 380 nm excitation (inset: photograph of three components taken under UV light (365 nm)). c–e) The excitation-dependent PL behaviors of GQDs (Batch 1–3). | ||
The GQDs keep some important properties existing in unseparated GQDs. They possess excellent solubility in water and most polar organic solvents because of the chemical groups, such as –OH, epoxy/ether, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –CO–NR2 (Fig. 4);21 they have solvent-dependent PL behaviors; and they have outstanding stability under UV light and in a wide range of ionic strengths.21 Moreover, the GQDs are much more stable than un-separated GQDs. The fluorescence of the separated GQDs stays constant after many drying/re-dissolving processes, while the un-separated GQDs show poor stability in drying/re-dissolving processes. This might be because the large pieces in un-separated GQDs induce aggregation. From XPS analysis of the GQDs, we can also observe the changes on the degree of oxidation in un-separated and separated GQDs (Table 1). The un-separated GQDs possess a high degree of oxidation, while the separated GQDs possess a decreasing degree of oxidation with the PL peak blue shifting (Fig. 3b) and the degree of oxidation decreasing from Batch 3–1. In addition, the FWHM became narrower compared with un-separated GQDs.
O and –CO–NR2 (Fig. 4);21 they have solvent-dependent PL behaviors; and they have outstanding stability under UV light and in a wide range of ionic strengths.21 Moreover, the GQDs are much more stable than un-separated GQDs. The fluorescence of the separated GQDs stays constant after many drying/re-dissolving processes, while the un-separated GQDs show poor stability in drying/re-dissolving processes. This might be because the large pieces in un-separated GQDs induce aggregation. From XPS analysis of the GQDs, we can also observe the changes on the degree of oxidation in un-separated and separated GQDs (Table 1). The un-separated GQDs possess a high degree of oxidation, while the separated GQDs possess a decreasing degree of oxidation with the PL peak blue shifting (Fig. 3b) and the degree of oxidation decreasing from Batch 3–1. In addition, the FWHM became narrower compared with un-separated GQDs.
| Peak Binding Energy | Un-separated GQDsb | Batch 1 | Batch 2 | Batch 3 | |
|---|---|---|---|---|---|
a Including carbon-hydroxy groups (C–OH), epoxy/ether groups (C–O), carbonyl groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), carboxylate carbon group (O–C O), carboxylate carbon group (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and Nitrous C.
b The data comes from Ref. 21. O) and Nitrous C.
b The data comes from Ref. 21.
|
|||||
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
284.6(±0.2) | 51.32b | 67.15 | 63.53 | 59.19 |
| Other Ca | 285.5–288.5 | 48.68b | 32.85 | 36.46 | 40.81 |
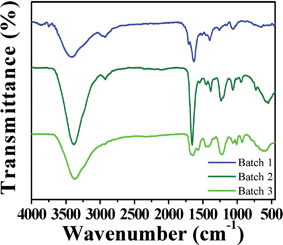 | ||
| Fig. 4 IR spectra of GQDs (Batch 1–3). | ||
The fluorescence mechanism of GQDs is not fully understood, but we can give a possible explanation. Both the surface energy traps and the zig-zag sites contribute to the fluorescence.10,21,22–24 All the GQDs have a similar size and chemical groups, but the oxidation degree increases from Batch 1–3 (Fig. 5 and Table 1). The higher degree of surface oxidation would result in more surface defects, which make the emission red-shift.24 As a result, three components have tunable fluorescent emission from blue to green. In detail, take Batch 3 as an example, they have two emission peaks, one is focused on 427 nm (blue emission) and the other is 516 nm (green emission). The blue emission is attributed to the zig-zag effect,10,22 while the green emission is attributed to surface defects (also called energy traps, because both sp2 and sp3 domains exist in the plane of GQDs).17,21,23 Batch 3 has the highest degree of oxidation (lowest degree of reduction) in the three batches, so the surface defect emission plays the leading roles (green emission).
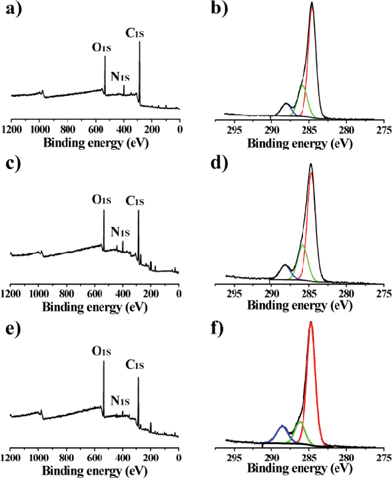 | ||
| Fig. 5 XPS of GQDs. a, b) are Batch 1. c, d) are Batch 2. e, f) are Batch 3. | ||
The GQDs have some important properties for practical applications. For example, the prepared GQDs possess up-conversion PL property. As shown in Fig. 6a, when excited from 600 nm–900 nm, the emissions exhibit similar behaviors compared to that shown for short wavelength excitation (down-conversion).25 The up-conversion PL property was indicated to be possibly attributed to the multiphoton active process and will provide exciting applications in bioimaging or energy collection.9,18 Moreover, they have interesting pH-dependent PL behaviors (Fig. 6b): PL intensities decrease in a solution of high or low pH. Moreover, in an alkaline solution, the PL peak of the GQDs blue-shifts (shown blue color by naked eyes) and the FWHM becomes narrow, so the acid–base may be identified by naked eyes, and the pH can be measured by fluorescent intensity using such GQDs as probes. From the UV-vis absorption of the GQDs in different pH aqueous, the typical absorption peak at ca. 320 nm changed little (Fig. S1, ESI†), which corresponds to the minor shift in the PL peaks and proves the observed PL behaviors.
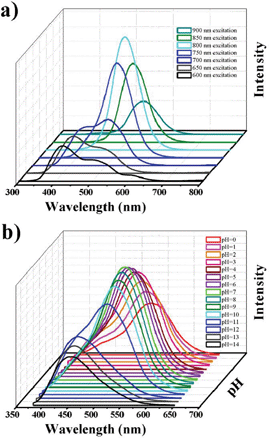 | ||
| Fig. 6 The applicable properties of the GQDs (Batch 3). a) The up-conversion PL properties of GQDs. b) The pH-dependent PL behavior of GQDs. | ||
Conclusions
In conclusion, we have demonstrated a new efficient two-step separated method to obtain GQDs with controllable surface oxidation which induced tunable fluorescence. The reported method promotes the synthesis and application of GQDs as well as comprehension of the PL mechanism. The prepared GQDs have strongly blue and green fluorescence, fine solubility and high stability. In addition, the GQDs have an acid–base response property which can be applied in pH probes. Moreover, by combining free dispersion in water and up-conversion PL properties, GQDs may be applied as an energy collector or bioimaging probe under a near IR range.Acknowledgements
This work was supported by the National Science Foundation of China (Grand No. 50973039, 21074048). The authors thank Prof. Li Niu (Changchun Institute of Applied Chemistry, CAS, Changchun) for the GO preparation and helpful discussion.References
- S. N. Baker and G. A. Baker, Angew. Chem. Int. Ed., 2010, 49, 6726 CrossRef CAS.
- J. Jeong, M. Cho, Y. T. Lim, N. W. Song and B. H. Chung, Angew. Chem. Int. Ed., 2009, 48, 5296 CrossRef CAS.
- K. Welsher, Z. Liu, S. P. Sherlock, J. T. Robinson, Z. Chen, D. Daranciang and H. Dai, Nature Nanotech., 2009, 4, 773 CrossRef CAS.
- (a) S. J. Yu, M. W. Kang, H. C. Chang, K. M. Chen and Y. C. Yu, J. Am. Chem. Soc., 2005, 127, 17604 CrossRef CAS; (b) A. Krueger, Adv. Mater., 2008, 20, 2445 CrossRef CAS.
- (a) Y. P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. Wang, P. G. Luo, H. Yang, M. E. Kose, B. Chen, L. M. Veca and S. Y. Xie, J. Am. Chem. Soc., 2006, 128, 7756 CrossRef CAS; (b) X. Wang, L. Cao, S. T. Yang, F. Lu, M. J. Meziani, L. Tian, K. W. Sun, M. A. Bloodgood and Y. P. Sun, Angew. Chem. Int. Ed., 2010, 49, 5310 CrossRef CAS.
- (a) R. Liu, D. Wu, S. Liu, K. Koynov, W. Knoll and Q. Li, Angew. Chem. Int. Ed., 2009, 48, 4598 CrossRef CAS; (b) A. B. Bourlinos, A. Stassinopoulos, D. Anglos, R. Zboril, M. Karakassides and E. P. Giannelis, Small, 2008, 4, 455 CrossRef CAS.
- H. Liu, T. Ye and C. Mao, Angew. Chem. Int. Ed., 2007, 46, 6473 CrossRef CAS.
- (a) Q. L. Zhao, Z. L. Zhang, B. H. Huang, J. Peng, M. Zhang and D. W. Pang, Chem. Commun., 2008, 5116 RSC; (b) H. Zhu, X. Wang, Y. Li, Z. Wang, F. Yang and X. Yang, Chem. Commun., 2009, 5118 RSC; (c) D. Pan, J. Zhang, Z. Li, C. Wu, X. Yan and M. Wu, Chem. Commun., 2010, 46, 3681 RSC.
- H. Li, X. He, Z. Kang, H. Huang, Y. Liu, J. Liu, J. Liu, S. Lian, C. H. A. Tsang, X. Yang and S. T. Lee, Angew. Chem. Int. Ed., 2010, 122, 4532 CrossRef.
- (a) D. Pan, J. Zhang, Z. Li and M. Wu, Adv. Mater., 2010, 22, 734 CrossRef CAS; (b) V. Gupta, N. Chaudhary, R. Srivastava, G. D. Sharma, R. Bhardwaj and S. Chand, J. Am. Chem. Soc., 2011, 133, 9960 CrossRef CAS.
- Y. Li, Y. Hu, Y. Zhao, G. Shi, L. Deng, Y. Hou and L. Qu, Adv. Mater., 2011, 23, 776 CrossRef CAS.
- (a) L. Li and X. Yan, J. Phys. Chem. Lett., 2010, 1, 2572 CrossRef CAS; (b) X. Yan, X. Cui and L. Li, J. Am. Chem. Soc., 2010, 132, 5944 CrossRef CAS.
- (a) R. Liu, D. Wu, X. Feng and K. Müllen, J. Am. Chem. Soc., 2011, 133, 15221 CrossRef CAS; (b) X. Feng, W. Pisula and K. Müllen, Pure Appl. Chem., 2009, 81, 2203 CrossRef CAS.
- (a) X. Sun, Z. Liu, K. Welsher, J. T. Robinson, A. Goodwin, S. Zaric and H. Dai, Nano Res., 2008, 1, 203 CrossRef CAS; (b) X. Wang, H. Bai and G. Shi, J. Am. Chem. Soc., 2011, 133, 6338 CrossRef CAS.
- J. Lu, P. S. E. Yeo, C. K. Gan, P. Wu and K. P. Loh, Nat. Nanotech., 2011, 6, 247 CrossRef CAS.
- T. Gokus, R. R. Nair, A. Bonetti, M. Böhmler, A. Lombardo, K. S. Novoselov, A. K. Geim, A. C. Ferrari and A. Hartschuh, ACS Nano, 2009, 3, 3963 CrossRef CAS.
- G. Eda, Y. Y. Lin, C. Mattevi, H. Yamaguchi, H. A. Chen, I. S. Chen, C. W. Chen and M. Chhowalla, Adv. Mater., 2010, 22, 505 CrossRef CAS.
- J. Shen, Y. Zhu, C. Chen, X. Yang and C. Li, Chem. Commun., 2011, 47, 2580 RSC.
- Q. Mei, K. Zhang, G. Guan, B. Liu, S. Wang and Z. Zhang, Chem. Commun., 2010, 46, 7319 RSC.
- J. Shen, Y. Zhu, X. Yang, J. Zong, J. Zhang and C. Li, New J. Chem., 2012, 36, 97 RSC.
- S. Zhu, J. Zhang, C. Qiao, S. Tang, Y. Li, W. Yuan, B. Li, L. Tian, F. Liu, R. Hu, H. Gao, H. Wei, H. Zhang, H. Sun and B. Yang, Chem. Commun., 2011, 47, 6858 RSC.
- K. A. Ritter and J. W. Lyding, Nat. Mater., 2009, 8, 235 CrossRef CAS.
- H. Zheng, Q. Wang, Y. Long, H. Zhang, X. Huang and R. Zhu, Chem. Commun., 2011, 47, 10650 RSC.
- L. Bao, Z. L. Zhang, Z. Q. Tian, L. Zhang, C. Liu, Y. Lin, B. Qi and D. W. Pang, Adv. Mater., 2011, 23, 5801 CrossRef CAS.
- S. Zhao, M. Shao and S. T. Lee, ACS Nano, 2012 DOI:10.1021/nn2040395.
Footnote |
| † Electronic Supplementary Information (ESI) available: See DOI: 10.1039/c2ra20182h/ |
| This journal is © The Royal Society of Chemistry 2012 |
