CeO2–Al2O3, CeO2–SiO2, CeO2–TiO2 core-shell spheres: formation mechanisms and UV absorption†
Qing
Fang
and
Xin
Liang
*
The State Key Laboratory of Organic-Inorganic Composites, Beijing University of Chemistry Technology, Beijing 100029, P. R. China. E-mail: liangxin@mail.buct.edu.cn; Fax: (+86)10-64436787; Tel: (+86)10-64412054
First published on 10th May 2012
Abstract
In this work, a general colloid synthesis method for CeO2–Al2O3, CeO2–SiO2, and CeO2–TiO2 core-shell spheres was reported. The properties of these CeO2-based composites were greatly dependent on the additive species. A diffusion-controlled kinetic model was applied to explore the morphology and crystallinity evolution of these core-shell spheres. CeO2–Al2O3 spheres undergo a significant morphology evolution when the Ce/Al molar ratio is varied from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 to 1
0.2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. It also provided an effective way to observe the diffusion channel for mass transport in the diffusion process by dissolving the Al2O3 in CeO2–Al2O3 spheres in alkaline solution. Moreover, a significant composition and structure dependent nature was observed from the UV-vis absorption spectra of these CeO2–Al2O3, CeO2–SiO2, and CeO2–TiO2 core-shell spheres.
1. It also provided an effective way to observe the diffusion channel for mass transport in the diffusion process by dissolving the Al2O3 in CeO2–Al2O3 spheres in alkaline solution. Moreover, a significant composition and structure dependent nature was observed from the UV-vis absorption spectra of these CeO2–Al2O3, CeO2–SiO2, and CeO2–TiO2 core-shell spheres.
Introduction
CeO2-based mixed oxides have a far-ranging applications in UV-shielding,1 chemical-mechanical polishing for microelectronics,2 sensors,3 water treatment,4 and their crucial application as promoters of three-way catalysts due to their reversible redox and oxygen storage ability, arising from the facile cycling between Ce3+ and Ce4+.5 Researches have revealed that mixing CeO2 with other metal oxides is an effective way to obtain functional materials with desirable properties, such as higher oxygen storage ability and higher textural stability.6–8 Inspired by the intensive application potentials of CeO2-based materials, much effort has been made on the preparation of CeO2-based materials. Over the past few years, a remarkable process has been developed for the synthesis of ceria based mixed oxides, including CeO2–ZrO2,9 CeO2–PbO2,10 CeO2–CuO,11 CeO2–MnOx,12 CeO2–TiO2,13 CeO2–Al2O314 and CeO2–SiO2.15 Until now synthetic routes have been developed for preparation of CeO2-based mixed oxides such as co-precipitation, high-temperature calcinations, high-energy mechanical milling, surfactant-assisted approach, micro-emulsion, sol–gel technique, and chemical filing, etc.16–20 Synthesizing CeO2-based mixed oxides with well controlled morphologies is desirable for exploring their morphology–properties relationship and extending the applications. However, the phase- and morphology-controlled synthesis of CeO2-based mixed oxides is more difficult than the synthesis of pure CeO2. Compared with the synthesis of a single oxide, the synthesis of mixed oxides requires a fundamental understanding of the nature and the dynamic nucleation and growth processes of different components, as well as a subtle control of the multifarious factors that govern the morphology, shape and physical and chemical properties of the final products. Unfortunately, systematic and detailed studies on this point are limited. The fabrication of various CeO2-based mixed oxides with well-controlled morphology, chemical composition and desirable properties is still a great challenge.Herein we report on a general colloid synthesis for CeO2–Al2O3, CeO2–SiO2, and CeO2–TiO2 core-shell spheres. A diffusion-controlled kinetic model based on the nanoscale Kirkendall effect was applied to explore the formation process of these core-shell spheres. We show how thermodynamics and kinetic factors govern the morphology and crystallinity evolution of the CeO2-based oxides. It is shown that the morphologies of the CeO2–Al2O3 nanostructures could be easily adjusted by the Ce/Al molar ratio. The diffusion channel for mass transport in the formation process can be clearly observed by dissolving the Al2O3 in the CeO2–Al2O3 spheres in alkaline solution. The UV-vis absorption properties of these CeO2-based composites were demonstrated to depend on their composition and structure.
Results and discussion
The preparation of the CeO2–Al2O3, CeO2–SiO2, and CeO2–TiO2 samples followed a two-step colloid synthesis approach. Firstly, CeO2 spheres were formed through a reported solvothermal method.21 In the second step, the additive species (Al(III), Si(IV), Ti(IV)) were introduced into the synthetic system to fabricate a series of CeO2–Al2O3, CeO2–SiO2, and CeO2–TiO2 composite spheres under solvothermal condition. Aluminum nitrate, tetrabutyl silicate and tetrabutyl titanate were used as the source of additive species of Al(III), Si(IV) and Ti(IV), respectively. The X-ray powder diffraction patterns of the as-synthesized products with the molar ratio of Ce to the additive species of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 are shown in Fig. 1. The peak positions and relative peak intensities in the given XRD patterns are almost identical for CeO2–Al2O3, CeO2–SiO2, and CeO2–TiO2 mixed oxides. The diffraction peaks can be readily indexed to that of the face-centered cubic phase of CeO2 structure (PDF ICDD. 34-0394) with lattice constant a = 5.522 Å. No extra lines due to the additive oxides or new-formed mixed phases are found, which is similar to the results reported in the literature.22 Interestingly, it can be noted that the diffraction peaks in the given XRD patterns broaden on going from CeO2–Al2O3 to CeO2–SiO2 to CeO2–TiO2, implying differences in the crystallinity of these samples. Based on the half-height of the strongest (111) diffraction peaks in the XRD patterns, the average crystallite sizes of CeO2 in the as-prepared CeO2–Al2O3, CeO2–SiO2, and CeO2–TiO2 spheres are about 5.2 nm, 4.9 nm and 4.6 nm respectively, estimated by the Scherrer equation. It can be concluded the crystallinity of these mixed oxides shows a decreasing order: CeO2–Al2O3 > CeO2–SiO2 > CeO2–TiO2. Since the synthetic process is identical for these samples, it can be concluded that the crystallite size of the final products depends on the type of the introduced additive oxides.
0.3 are shown in Fig. 1. The peak positions and relative peak intensities in the given XRD patterns are almost identical for CeO2–Al2O3, CeO2–SiO2, and CeO2–TiO2 mixed oxides. The diffraction peaks can be readily indexed to that of the face-centered cubic phase of CeO2 structure (PDF ICDD. 34-0394) with lattice constant a = 5.522 Å. No extra lines due to the additive oxides or new-formed mixed phases are found, which is similar to the results reported in the literature.22 Interestingly, it can be noted that the diffraction peaks in the given XRD patterns broaden on going from CeO2–Al2O3 to CeO2–SiO2 to CeO2–TiO2, implying differences in the crystallinity of these samples. Based on the half-height of the strongest (111) diffraction peaks in the XRD patterns, the average crystallite sizes of CeO2 in the as-prepared CeO2–Al2O3, CeO2–SiO2, and CeO2–TiO2 spheres are about 5.2 nm, 4.9 nm and 4.6 nm respectively, estimated by the Scherrer equation. It can be concluded the crystallinity of these mixed oxides shows a decreasing order: CeO2–Al2O3 > CeO2–SiO2 > CeO2–TiO2. Since the synthetic process is identical for these samples, it can be concluded that the crystallite size of the final products depends on the type of the introduced additive oxides.
 | ||
| Fig. 1 XRD pattern of (a) CeO2–Al2O3, (b) CeO2–SiO2, (c) CeO2–TiO2. | ||
The morphologies of above products are characterized by TEM and HR-TEM images. As shown in Fig. 2a–d, the CeO2–Al2O3 showed core-shell spheres with a narrow size distribution. Careful observation revealed that the spheres were coated by thin shells and some of the spheres have hollow interiors. The core-shell structure of the CeO2–Al2O3 spheres was supported by the HRTEM image in Fig. 2c which clearly showed an amorphous shell and a crystalline core. As noted in Fig. 2c, the crystalline core shows clear and continuous crystal fringes with an interplanar spacing of about 0.313 nm which corresponds to the (111) lattice spacing of ceria cubic crystal structure. The corresponding fast Fourier transform (FFT) pattern in Fig. 2d is neither a typical polycrystalline ring diffraction pattern, nor the typical single crystal dot diffraction pattern, but a unique in-between diffraction pattern, which illustrated that the CeO2 in CeO2–Al2O3 nanospheres are small nanocrystals with similar crystal orientation. The as-prepared CeO2–SiO2 sample shows spherical core-shell morphology (Fig. 2e–h). It can be observed that the spheres also exhibit hollow interiors. TEM and HRTEM images of CeO2–TiO2 in Fig. 2i–k show the clear hollow structure of the CeO2–TiO2 spheres; the hollow characteristics are the most obvious of the three samples. HRTEM images show the crystal fringes in the CeO2–TiO2 sphere are noncontinuous in all directions, illustrating the poor crystallinity. The FFT analysis over a CeO2–TiO2 sphere shows a typical polycrystalline ring diffraction pattern which can be indexed to the (111) and (200) crystal planes of cubic CeO2. The diversification in the FFT patterns of these three samples indicates that the nanocrystals in CeO2–TiO2 are more disordered than those in the CeO2–SiO2 and CeO2–Al2O3 spheres, which is well consistent with the result from the XRD patterns.
 | ||
| Fig. 2 a–c) Representative TEM and HRTEM images of CeO2–Al2O3 nanospheres, d) the corresponding FFT-transformed pattern; e–g) representative TEM and HRTEM images of CeO2–SiO2 nanospheres, h) the corresponding FFT-transformed pattern; i–k) representative TEM image of CeO2–TiO2 nanospheres, l) the corresponding FFT-transformed pattern of CeO2–TiO2. | ||
Based on the above analysis from TEM, HRTEM, FFT patterns and XRD patterns of these samples, it can be concluded that the morphology and structure of these CeO2-based composites greatly depended on the additive species. The hollow characteristics of these mixed oxides spheres follow an increasing order: CeO2–Al2O3 < CeO2–SiO2 < CeO2–TiO2, while the crystallinity sequence of these mixed oxides is CeO2–Al2O3 > CeO2–SiO2 > CeO2–TiO2.
Since the synthetic approach is a two-step process, a diffusion-controlled model based on the nanoscale Kirkendall effect was applied to explore the formation process of the as-prepared CeO2-based mixed oxides qualitatively. According to Fan et al.,23 the development of core-shell structures with hollow interiors based on the Kirkendall effect undergoes two main stages. The initial stage is the generation of small Kirkendall voids via a bulk diffusion process; the second stage is dominated by surface diffusion of the core material along the pore surface. Based on this theory, the growth process of the CeO2-based mixed oxides was illustrated in Fig. 3. In this work, CeO2 nanospheres were used as starting materials. As the additive species would hydrolyse under solvothermal condition, a newly-formed amorphous layer (B shell layer) grows epitaxially on the surface of the CeO2 spheres (A core layer). Then the two species diffuse into each other due to the different diffusion rate. Accompanied with the diffusion, a CeO2–MO2 mixed layer (A–B layer) forms as a boundary layer, where rin(t) and rout(t) denote the positions of the inner and outer boundaries. The final morphology of the products is controlled by the outward and inward diffusion flux. For an extreme Kirkendall diffusion system, the out-diffusion of atoms is much faster than the in-diffusion, and typical hollow nanostructures would form. In the previous work, CeO2–ZrO2 nanocages have been successfully synthesized via the Kirkendall effect by using CeO2 nanospheres as a starting solid phase.21 Though similar CeO2 nanospheres were also used as starting materials in this work, the as-prepared CeO2–Al2O3, CeO2–TiO2, and CeO2–SiO2 have not shown typical hollow structures.
 | ||
| Fig. 3 The formation mechanism of as-prepared CeO2-based composites. | ||
The morphology evolution of these CeO2-based composites were analyzed by the diffusion-controlled model. We made a simplifying assumption that considering CeO2–MOx system as a quasi-binary system and considered that the diffusion within the CeO2 core and the shell layer was driven by the difference in chemical potential of atoms. According to Tu et al.,24 the growth rate of the outward diffusion interface can be deduced as eqn (1) based on the Gibbs–Thomson effect.
 | (1) |
In which γ1, γ2 are the interfacial energy of the inside and outside interfaces; Ω is the atomic volume of A in the product phase, ΔGr is the formation energy of A–B per atom. From eqn (1), it can be deduced that the out-diffusion rate could be greatly influenced by the formation energy ΔGr. For various kinds of CeO2-based composites, the formation energy is different. As a consequence, the growth rate of the outward diffusion interface varies in different CeO2–MOx (M = Si, Al, Ti) systems. Thus, it can be well understood that the morphologies of the as-obtained CeO2–MOx (M = Si, Al, Ti) samples depends on the type of the additive oxide, although the synthesis procedure is almost the same for the three composites. According to the hollow characteristics of these mixed oxides spheres, the outward diffusion rate of the Ce atoms in these diffusion systems would also follow an increasing sequence: CeO2–Al2O3 < CeO2–SiO2 < CeO2–TiO2. Since the out diffusion process refers to the diffusion of lattice atoms of CeO2 from the core layer, the crystallinity and mechanical strength of the core materials would deteriorate. When other conditions are constant, the Gibbs–Thomson effect can be completely influenced by different ΔGr. According to Tu et al.'s theory,24 the outward diffusion flux is promoted at this condition due to the Gibbs–Thomson effect. As a result, CeO2–TiO2 with the most hollow sphere characteristics formed at this condition. This deduction is consistent with the result as observed by XRD, HRTEM and FFT patterns that the crystallinity decreases in the as-prepared CeO2–Al2O3, CeO2–SiO2 and CeO2–TiO2 spheres.
Besides the consideration of the Gibbs–Thomas effect, another important viewpoint is that the driving force of the diffusion is the concentration gradient, and the diffusion is governed by Ficks' first law. According to Yin et al.,25,26 the outward growth rate of the core layer and the inward growth rate of the product layer are given by eqn (2, 3).
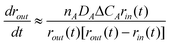 | (2) |
 | (3) |
Where DA, ΔCA and nA denote the diffusivity, concentration difference, and volume density of A in the inner layer, respectively. DB, ΔCB and nB denote the diffusivity, concentration difference, and volume density of B in the shell layer, respectively.
The two mechanisms consider the diffusion process from the point of thermodynamics and dynamics, respectively. To further investigate the diffusion process and the relationship between the two mechanisms in details, CeO2–Al2O3 was selected as a model system and a series of controlled experiments have been carried out.
As noted in Fig. 4, CeO2–Al2O3 mixed oxides underwent a remarkable morphology evolution as the Ce/Al molar ratio changes from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 02 to 1
02 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The representative TEM images of the products prepared by various Ce/Al molar ratios (1
1. The representative TEM images of the products prepared by various Ce/Al molar ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2, 1
0.2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3, 1
0.3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and 1
0.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) are shown in Fig. 4a–d, respectively. The shell thickness of CeO2–Al2O3 spheres derived from a Ce/Al molar ratio of 1
1) are shown in Fig. 4a–d, respectively. The shell thickness of CeO2–Al2O3 spheres derived from a Ce/Al molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 is very thin and almost no hollow structures were found in this condition. The samples derived from Ce/Al molar ratios 1
0.2 is very thin and almost no hollow structures were found in this condition. The samples derived from Ce/Al molar ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 and 1
0.3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 exhibit core-shell structures: the spherical cores were coated by flower-like shells. Hollow structures can be found in these two conditions. When the Ce/Al molar ratio is equal to 1
0.5 exhibit core-shell structures: the spherical cores were coated by flower-like shells. Hollow structures can be found in these two conditions. When the Ce/Al molar ratio is equal to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, an obvious core-shell structure was formed. As Fig. 4d shows, the shell thickness is fairly thick and estimated to be 40 nm. However, there are no hollow structures observed in this condition. Based on the observations, it is clear that the shell thickness of the core-shell structures and the incidence of hollow structures have a close relationship with the Ce/Al molar ratio. The shell thickness of the spheres increased significantly with the increase of the Ce/Al molar ratio. Moreover, the hollow structures can be created only at certain Ce/Al molar ratios: the hollowing takes place at the Ce/Al molar ratios 1
1, an obvious core-shell structure was formed. As Fig. 4d shows, the shell thickness is fairly thick and estimated to be 40 nm. However, there are no hollow structures observed in this condition. Based on the observations, it is clear that the shell thickness of the core-shell structures and the incidence of hollow structures have a close relationship with the Ce/Al molar ratio. The shell thickness of the spheres increased significantly with the increase of the Ce/Al molar ratio. Moreover, the hollow structures can be created only at certain Ce/Al molar ratios: the hollowing takes place at the Ce/Al molar ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 and 1
0.3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, while it is failure at Ce/Al molar ratios 1
0.5, while it is failure at Ce/Al molar ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 and 1
0.2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Many studies have investigated the relationship between different concentration ratios of the reactants and the morphological evolution of the products. In Ahmed's work,27 the particle size of copper-cobalt nanostructures can range from 40–200 nm by controlling the concentration of reducing agent. In Ahmed's another work,28 they have developed a vaterite form of calcium carbonate with rods, hexagonal plates and spherical particles.
1. Many studies have investigated the relationship between different concentration ratios of the reactants and the morphological evolution of the products. In Ahmed's work,27 the particle size of copper-cobalt nanostructures can range from 40–200 nm by controlling the concentration of reducing agent. In Ahmed's another work,28 they have developed a vaterite form of calcium carbonate with rods, hexagonal plates and spherical particles.
 | ||
Fig. 4 TEM images of CeO2–Al2O3 products obtained by different Ce/Al molar ratios (a) Ce/Al = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2, (b) Ce/Al = 1 0.2, (b) Ce/Al = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3, (c) Ce/Al = 1 0.3, (c) Ce/Al = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, (d) Ce/Al = 1 0.5, (d) Ce/Al = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
According to Tu et al.'s theory,24 the out-diffusion flux can be prevented due to the Gibbs–Thomson effect in the case of small values of r2 and r1. Since the initial concentration of Al3+ ions is very low in the case of Ce/Al 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2, only a very thin shell formed on the surface of the CeO2 spheres. As a result, the value of r2 would be very small in this condition. The outward diffusion flux is inhibited for this condition due to the Gibbs–Thomson effect. As a result, no hollow nanostructure formed for this condition.
0.2, only a very thin shell formed on the surface of the CeO2 spheres. As a result, the value of r2 would be very small in this condition. The outward diffusion flux is inhibited for this condition due to the Gibbs–Thomson effect. As a result, no hollow nanostructure formed for this condition.
While the above case with the high Ce/Al molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 is well explained by the Gibbs–Thomson effect, the case with a low Ce/Al molar ratio 1
0.2 is well explained by the Gibbs–Thomson effect, the case with a low Ce/Al molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 can be easily understood by Yin et al.'s theory. As the initial concentration of Al3+ ions in the synthetic solution is high in the case of a Ce/Al molar ratio of 1
1 can be easily understood by Yin et al.'s theory. As the initial concentration of Al3+ ions in the synthetic solution is high in the case of a Ce/Al molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the shell layer coated on CeO2 spheres becomes thicker and ΔCB becomes larger. According to eqn (3), derived from Ficks' first law, the inward diffusion growth rate becomes faster at higher Al3+ concentration. The in-diffusion is too quick, which is against the generation of voids and further prevents the formation of hollow structures. CeO2–Al2O3 nanospheres with thick shells finally formed at this condition. For the case of Ce/Al molar ratios 1
1, the shell layer coated on CeO2 spheres becomes thicker and ΔCB becomes larger. According to eqn (3), derived from Ficks' first law, the inward diffusion growth rate becomes faster at higher Al3+ concentration. The in-diffusion is too quick, which is against the generation of voids and further prevents the formation of hollow structures. CeO2–Al2O3 nanospheres with thick shells finally formed at this condition. For the case of Ce/Al molar ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 and 1
0.3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, the in-diffusion rate is not too fast. The condition is more suitable for hollow structure formation and some hollow nanostructures could be found at Ce/Al molar ratios 1
0.5, the in-diffusion rate is not too fast. The condition is more suitable for hollow structure formation and some hollow nanostructures could be found at Ce/Al molar ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 and 1
0.3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5. So it can be concluded that the two mechanisms do not work alone. The CeO2–Al2O3 system provides a sound experimental demonstration to show that the diffusion process is not governed by a single driving force, but a synergism of thermodynamics and dynamics.
0.5. So it can be concluded that the two mechanisms do not work alone. The CeO2–Al2O3 system provides a sound experimental demonstration to show that the diffusion process is not governed by a single driving force, but a synergism of thermodynamics and dynamics.
Interestingly, the CeO2–Al2O3 system also provided a route to observe the mass diffusion path. People have tried to observe the intermediate state to research the diffusion path in the Kirkendall effect by controlling the reaction time.29 As Al2O3 can easily be dissolved by alkaline solution, and CeO2 is stable in alkaline solution, the CeO2–Al2O3 system can be served as an ideal model to observe the mass transport channel. Fig. 5a–b show contrast TEM images of as-obtained CeO2–Al2O3 mixed oxides with Ce/Al = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 before and after being washed by NaOH solution. It can be seen from the Fig. 5b that the shell layer disappeared after washing and the surface of the interior spheres shows a radiating morphology, indicating the inter-diffusion between the Ce species and Al species. As Tu et al. proposed that the growth process of voids in Kirkendall diffusion systems involves surface diffusion via the pore surfaces as an essential transport path,23 the result indicates that the skeletal bridges which the Ce and Al atoms diffuse along are radial capillary-like. Fig. 5c shows a TEM image of the CeO2–Al2O3 core-shell spheres with Ce/Al = 1
0.5 before and after being washed by NaOH solution. It can be seen from the Fig. 5b that the shell layer disappeared after washing and the surface of the interior spheres shows a radiating morphology, indicating the inter-diffusion between the Ce species and Al species. As Tu et al. proposed that the growth process of voids in Kirkendall diffusion systems involves surface diffusion via the pore surfaces as an essential transport path,23 the result indicates that the skeletal bridges which the Ce and Al atoms diffuse along are radial capillary-like. Fig. 5c shows a TEM image of the CeO2–Al2O3 core-shell spheres with Ce/Al = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. After washing (Fig. 5d) the thick shell layer has disappeared and the remainders are spheres with hollow interiors, indicating the fast in-diffusion of Al atoms to the interior of the spheres. The radiating surface also proved the inter-diffusion of Al species and Ce species within each other.
1. After washing (Fig. 5d) the thick shell layer has disappeared and the remainders are spheres with hollow interiors, indicating the fast in-diffusion of Al atoms to the interior of the spheres. The radiating surface also proved the inter-diffusion of Al species and Ce species within each other.
 | ||
Fig. 5 Contrast TEM images of CeO2–Al2O3 products before and after being washed by NaOH solution: a) original samples with Ce/Al = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and (b) the corresponding sample after washing, the inset is the enlarged image of the edge of the spheres after washing; c) original samples with Ce/Al = 1 0.5 and (b) the corresponding sample after washing, the inset is the enlarged image of the edge of the spheres after washing; c) original samples with Ce/Al = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, d) the corresponding sample after washing. 1, d) the corresponding sample after washing. | ||
As CeO2 is semiconductor material with wide band gap and promising UV-blocking properties, the UV-visible absorption properties of these CeO2-based composites were studied carefully to investigate the structure-properties relationship of these materials. Typical UV-visible absorption spectra of CeO2–Al2O3, CeO2–SiO2, and CeO2–TiO2 core-shell spheres with the molar ratio Ce to the additive species of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3 are shown in Fig. 6. The spectral characteristics of the three kinds of CeO2-based composites are similar, but they have a little difference in the absorption edges. The strong absorption band in the UV region (200∼350 nm) can be well attributed to the typical absorption bands of CeO2: the absorption band at ∼278 nm corresponds to the Ce4+ ← O2− charge transfer and the absorption at ∼313 nm can be attributed to the inter-band transitions.30 For semiconductor materials, the direct band gap energy (Eg) can be estimated by using the following eqn (4):
0.3 are shown in Fig. 6. The spectral characteristics of the three kinds of CeO2-based composites are similar, but they have a little difference in the absorption edges. The strong absorption band in the UV region (200∼350 nm) can be well attributed to the typical absorption bands of CeO2: the absorption band at ∼278 nm corresponds to the Ce4+ ← O2− charge transfer and the absorption at ∼313 nm can be attributed to the inter-band transitions.30 For semiconductor materials, the direct band gap energy (Eg) can be estimated by using the following eqn (4):
| αhυ = C(hυ − Eg)1/2 | (4) |
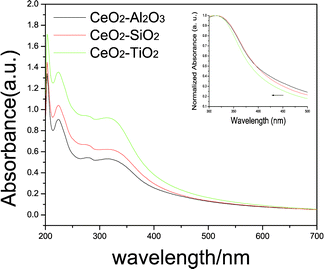 | ||
| Fig. 6 UV-vis absorption spectra of prepared CeO2–Al2O3, CeO2–SiO2, and CeO2–TiO2 mixed oxides; the insert is the comparison of the absorption edge of these mixed oxides. | ||
Where α is the absorption coefficient, hν is photon energy. C is the constant. According to the absorption spectra, the (αhν)2versus hν plots (Fig. S1†) can be derived to estimate the value of Eg. The as-calculated direct band gap energies (Eg) of CeO2–Al2O3, CeO2–SiO2, and CeO2–TiO2 spheres are 3.11 eV, 3.14 eV, and 3.21 eV respectively which is similar to the results reported in the literature.30 Since the decrease in particle size would create a blue shift in the absorption edge for a direct band gap semiconductor material,31 the variance of the absorption edge from CeO2–Al2O3 to CeO2–TiO2 would be caused by the smaller crystallite size of CeO2 in the CeO2–TiO2 matrix compared with that in the CeO2–SiO2 and CeO2–Al2O3 matrices.
In addition, the UV-vis absorption spectra of CeO2–Al2O3 mixed oxides with different Ce/Al molar ratios are shown in Fig. 7. As the Ce/Al molar ratio varies from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 to 1
0.2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, an obvious blue-shift of the UV absorption edges was observed. This blue-shift is attributed to the numerous defects induced by the formation of the boundary layer and the inter-diffusion process.32 The inter-diffusion was enhanced as the Ce/Al molar ratio varies from 1
1, an obvious blue-shift of the UV absorption edges was observed. This blue-shift is attributed to the numerous defects induced by the formation of the boundary layer and the inter-diffusion process.32 The inter-diffusion was enhanced as the Ce/Al molar ratio varies from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 to 1
0.2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. As a result, the spatial confinement is strengthened and the band-offset increases.
1. As a result, the spatial confinement is strengthened and the band-offset increases.
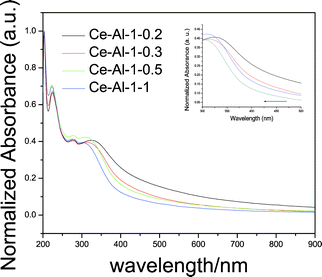 | ||
| Fig. 7 UV-visible absorption spectra of the prepared CeO2–Al2O3 products with different Ce/Al molar ratios; the insert is the comparison of the absorption edge of CeO2–Al2O3 with different Ce/Al molar ratios. | ||
Experimental
Chemicals
Tetraethyl orthosilicate and tetrabutyl titanate were bought from Beijing chemical company and used without further purification. Also, cerium nitrate and aluminum nitrate were bought from Tianjin fine chemical research laboratory and used at a high purity of over 99.9%.Synthesis
CeO2–Al2O3 mixed oxides were synthesized via a solvothermal method. 1 mmol Ce(NO3)3·6H2O (0.5 mol L−1) was dissolved in 30 mL glycol with magnetic stirring. The mixture was further stirred vigorously until it became homogeneous and then sealed in a Teflon-lined stainless steel autoclave. The autoclave was maintained at 180 °C for 8 h and then cooled to room temperature. 0.3 mmol Al(NO3)3·9H2O was added into the solution, then the solution was aged in the Teflon-lined autoclave at 180 °C for 3 h. A light purple precipitate was obtained by centrifuging, the solid product was recovered, sequentially washed with deionized water several times, and then dried in an electric oven at 100 °C for 12 h. The CeO2–Al2O3 mixed oxides with controlled shapes and sizes can be easily adjusted by changing the content of Al(NO3)3·9H2O (0.2, 0.3, 0.5, 1 mmol) while keeping the other conditions constant. The synthesis step of CeO2–SiO2, CeO2–TiO2 mixed oxides were prepared by the same synthesis procedure except for different foreign species sources (using tetraethyl orthosilicate as silicon source and tetrabutyl titanate as titanium source).Characterization
The crystal structure and phase purity of the product were characterized with X-ray powder diffraction (XRD; Bruker D8 diffractometer) with Cu Kα as the radiation source (λ = 0.15406 nm). The morphology and size of the particles were observed on a transmission electron microscope (TEM; JEM-3010) and with high-resolution transmission electron microscopy (HRTEM; FEI Titan 80-300 Cs-corrected TEM with resolution 0.08 nm at 300 kV). The UV-vis spectra were obtained on a Hitachi F-4500 UV-vis spectrophotometer. CeO2–Al2O3, CeO2–SiO2, and CeO2–TiO2 samples are dispersed in ethanol solution and prepared in the same concentration for UV-vis detection. Ethanol solution was used as reference.Conclusion
CeO2–Al2O3, CeO2–SiO2, and CeO2–TiO2 core-shell spheres have been fabricated via a simple colloid synthesis. CeO2–Al2O3 with different morphologies has been prepared by changing the aluminum content. A Kirkendall diffusion model was applied to analyse the formation process of these CeO2-based composites from the point of both thermodynamics and dynamics. It has been proved that the out-diffusion and in-diffusion are the key factors to determine the morphology of the products. The UV-vis absorption properties of these products also have a close relationship with the diffusion process. Such results would be helpful for understanding the formation mechanisms and applications of CeO2-based mixed oxides.Acknowledgements
This work was financially supported by NSFC (grant no. 21001015, 21121064), RFDP (grant no. 20100010120003) and the State Key Project of Fundamental Research for Nanoscience and Nanotechnology (2011CB932402)References
- S. Yabe, M. Yashimata, S. Momose, K. Tahira, S. Yashida, R. X. Li, S. Yin and T. Sato, Int. J. Inorg. Mater., 2001, 3, 1003 CrossRef CAS.
- X. D. Feng, D. C. Sayle, Z. L. Wang, M. S. Paras, B. Santora, A. C. Sutorik, T. X. T. Sayle, Y. Yang, Y. Ding, X. D. Wang and Y. S. Her, Science, 2006, 312, 1504 CrossRef CAS.
- P. Kumar, Y. Sun and R. O. Idem, Energy Fuels, 2007, 21, 3113 CrossRef CAS.
- L. S. Zhong, J. S. Hu, A. M. Cao, Q. Liu, W. G. Song and L. J. Wan, Chem. Mater., 2007, 19, 1648 CrossRef CAS.
- J. Kaspar, P. Fornasiero and M. Graziani, Catal. Today, 1999, 50, 285 CrossRef CAS.
- A. Trovarelli, Catal. Rev. Sci. Eng., 1996, 38, 439 CAS.
- G. Liu, J. A. Rodriguez, J. Hrbek, J. Dvorak and C. H. F. Peden, J. Phys. Chem. B, 2001, 105, 7762 CrossRef CAS.
- J. A. Rodriguez, J. C. Hanson, J. Y. Kim, G. Liu, A. Iglesias-Juez and M. Fernandez-Garcia, J. Phys. Chem. B, 2003, 107, 3535 CrossRef CAS.
- B. M. Reddy, A. Khan, Y. Yamada, T. Kobayashi, S. Loridant and J. C. Volta, Langmuir, 2003, 19, 3025 CrossRef CAS.
- Y. Zhang, A. Andersson and M. Muhammed, Appl. Catal., B, 1995, 6, 325 CrossRef CAS.
- L. Dong, Y. Hu, F. Xu, D. Lu, B. Xu, Z. Hu and Y. Chen, J. Phys. Chem. B, 2000, 104, 78 CrossRef CAS.
- S. Imamura, M. Shono, N. Okamoto, A. Hamada and S. Ishida, Appl. Catal., A, 1996, 142, 279 CrossRef CAS.
- J. Rynkowski, J. Farbotko, R. Touroude and L. Hilaire, Appl. Catal., A, 2000, 203, 335 CrossRef CAS.
- K. M. S. Khalil, J. Colloid Interface Sci., 2007, 307, 172 CrossRef CAS.
- F. Grasset, R. Marchand, A. M. Marie, D. Fauchadour and F. Fajardie, J. Colloid Interface Sci., 2006, 299, 726 CrossRef CAS.
- M. Alifanti, B. Baps, N. Blangenois, J. Naud, P. Grange and B. Delmon, Chem. Mater., 2003, 15, 395 CrossRef CAS.
- Z. L. Wang and X. Feng, J. Phys. Chem. B, 2003, 107, 13563 CrossRef CAS.
- Q. Yuan, Q. Liu, W. G. Song, W. Feng, W. L. Pu, L. D. Sun, Y. W. Zhang and C. H. Yan, J. Am. Chem. Soc., 2007, 129, 6698 CrossRef CAS.
- S. W. Yang and L. Gao, J. Am. Chem. Soc., 2006, 128, 9330 CrossRef CAS.
- S. Carrettin, P. Concepcion, A. Corma, J. M. L. Nieto and V. F. A. Puntes, Angew. Chem., Int. Ed., 2004, 43, 2538 CrossRef CAS.
- X. Liang, X. Wang, Y. Zhuang, B. Xu, S. M. Kuang and Y. D. Li, J. Am. Chem. Soc., 2008, 130, 2736 CrossRef CAS.
- Q. Yu, X. X. Wu, C. J. Tang, L. Qi, B. Liu, F. Gao, K. Q. Sun, L. Dong and Y. Chen, J. Colloid Interface Sci., 2011, 354, 341 CrossRef CAS.
- H. J. Fan, M. Knez, R. Scholz, D. Hesse, K. Nielsch, M. Zacharias and U. Gösele, Nano Lett., 2007, 7, 993 CrossRef CAS.
- K. N. Tu and U. Gösele, Appl. Phys. Lett., 2005, 86, 093111 CrossRef.
- Y. D. Yin, R. M. Rioux, C. K. Erdonmez, S. Hughes, G. A. Somorjai and A. P. Alivisatos, Science, 2004, 304, 711 CrossRef CAS.
- Y. D. Yin, C. K. Erdonmez, A. Cabot, S. Hughes and A. P. Alivisatos, Adv. Funct. Mater., 2006, 16, 1389 CrossRef CAS.
- J. Ahmed, A. Ganguly, S. Saha, G. Gupta, P. Trinh, A. M. Mugweru, S. E. Lofland, K. V. Ramanujachary and A. K. Ganguli, J. Phys. Chem. C, 2011, 115, 14526 CAS.
- J. Ahmed, M. Menaka and A. K. Ganguli, CrystEngComm, 2009, 11, 927 RSC.
- H. J. Fan, U. Gösele and M. Zacharias, Small, 2007, 3, 1660 CrossRef CAS.
- A. Bensalem, J. C. Muller and F. Bozon-Verduraz, J. Chem. Soc., Faraday Trans., 1992, 88, 153 RSC.
- N. Sabari Arul, D. Mangalaraj, P. C. Chen, N. Ponpandian and C. Viswanathan, Mater. Lett., 2011, 65, 2635 CrossRef.
- R. Xie, U. Kolb, J. Li, T. Basche and A. Mews, J. Am. Chem. Soc., 2005, 127, 7480 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2ra01331b |
| This journal is © The Royal Society of Chemistry 2012 |
