FeCl3/Montmorillonite K10 as an efficient catalyst for solvent-free aza-Michael reaction between amine and α,β-unsaturated compounds
V. R.
Choudhary
*,
D. K.
Dumbre
and
S. K.
Patil
Chemical Engineering and Process Development Division, National Chemical Laboratory, Pune, 411 008, India. E-mail: vr.choudhary@ncl.res.in; vrc0001@yahoo.co.in; Fax: +91-20-25902612; Tel: +91-20-25902318
First published on 13th June 2012
Abstract
A highly efficient, inexpensive and greener protocol for aza-Michael addition reaction of different aromatic and aliphatic/cyclic amines to α,β-unsaturated compounds using a FeCl3/MontK10 catalyst under solvent-free conditions has been developed. The Michael addition products are obtained in good to excellent yields. The catalyst was insensitive to moisture and it also showed excellent reusability in the reaction. Its high activity is attributed mostly to redox properties of FeCl3.
Introduction
The aza-Michael addition reaction is one of the most important methodologies in synthetic organic chemistry for the preparation of β-aminocarbonyl compounds, which are biologically important products and are used as the key intermediates for the synthesis of β-amino alcohols, antibiotics, β-amino acids, chiral auxiliaries, and other nitrogen-containing compounds.1,2 The aza-Michael addition of nitrogen nucleophiles including amides, carbamates, sulfonamides, and amines to electron-deficient alkenes in the presence of a strong base or acid catalyst has attracted considerable attention as an alternative route for the synthesis of β-amino carbonyl compounds.3–5 Moreover, it is extensively used in the fine chemicals and pharmaceutical sectors.6,7A number of methods, using stoichiometric or catalytic amounts of Lewis acids, such as metal chloride,8–15 chlorates,16,17 nitrates,18–20 acetates11,21 and triflates,22–24 have been reported for the aza-Michael addition reaction. However, most of these methods often involve the use of strong acid catalysts, causing polymerization and/or demanding an aqueous workup for the catalyst separation. Particularly, the use of stoichiometric amounts of Lewis acids causes waste disposal problems. Generally, most of the Lewis acid catalysts are highly moisture sensitive, demanding moisture-free reaction conditions and they also cannot be reused in the reaction. Recently a few methods using easily separable and reusable solid catalysts, such as sulfated zirconia,25 AlCl3/SiO2,26 SiO2-SO3H,27 Cu-Al-hydrotalcite28 and polystyrenesulfonic acid29 have also been reported. Use of molecular iodine,30 ionic liquids31 and Cu (acac)2 immobilized in an ionic liquid32 was also suggested for the aza-Michael addition. However, most of the earlier methods for the aza-Michael addition reaction suffer from limitations, such as use of undesirable solvents, difficulty in handling moisture sensitive Lewis acid catalysts and/or harsh reaction conditions. These drawbacks have a negative impact on the environment.
Therefore, it is of both scientific and practical interest to develop an environmentally benign moisture-insensitive catalyst which shows high activity under mild reaction conditions.
In this communication, we report a simple and effective greener protocol for the aza-Michael addition reaction of aromatic and aliphatic amines to α,β-unsaturated compounds using a moisture-insensitive MontK10 supported FeCl3 catalyst in the absence of any solvent (Scheme 1). The catalyst showed high activity in the reaction under mild conditions. It can be easily separated from the reaction mixture simply by filtration and it can be reused several times without significant loss of its activity.
 | ||
| Scheme 1 Aza-Michael addition of aromatic and aliphatic/cyclic amines to α,β-unsaturated compounds. | ||
In order to optimize the catalyst parameters and reaction conditions to achieve high product yield, we first studied the influence of catalyst parameters (viz. FeCl3 loading on MontK10 and catalyst pretreatment temperature) and use of different solvents on the catalyst performance in the aza-Michael reaction between aniline and methyl acrylate.
Results and discussion
Results showing the influence of FeCl3 loading on MontK10 in the catalyst (preheated at 120 °C) in the aza-Michael addition of aniline to methyl acrylate, under solvent-free condition are presented in Fig. 1. At the lower FeCl3 loadings, the product yield increased sharply with increasing the FeCl3 loading up to the loading of 1.0 mmol g−1 and then it leveled off. Further work was therefore carried out using the catalyst with FeCl3 loading of 1.0 mmol g−1. | ||
| Fig. 1 Influence of the FeCl3 loading in FeCl3/MontK10 catalyst (preheated at 120 °C) in the solvent-free reaction of aniline with methyl acrylate | ||
Fig. 2 shows the influence of the pretreatment temperature of the catalyst (FeCl3 loading = 1.0 mmol g−1) on its performance in the reaction. The product yield was found to decrease continuously with increasing the pretreatment temperature. This is expected because of the sintering and modification of the catalyst due to the reaction between FeCl3 and the surface hydroxyl groups of the catalyst support. The catalyst (pre-treated at 120 °C) was therefore used in the following work.
 | ||
| Fig. 2 Influence of the preheating temperature of the FeCl3/MontK10 (FeCl3 loading = 1.0 mmol g−1) catalyst on the product yield in the aza-Michael reaction of aniline with methyl acrylate | ||
Product yields in the aza-Michael addition of aniline to methyl acrylate over the catalyst with FeCl3 loading of 1.0 mmol g−1 (catalyst pretreatment T = 120 °C) in the presence of different solvents and also in the absence of any solvent were measured. The catalyst showed the best performance in the absence of any solvent. The yield of methyl 3-(phenylamino)-propanoate formed in the aza-Michael reaction of aniline with methyl acrylate over the catalyst in the absence of any solvent was found to be 93% and in the presence of different solvents it was 35% (toluene), 25% (ethanol), 30% (water) and 50% (acetonitrile). These results reveal that the catalyst showed the best performance in the absence of any solvent. This is mostly expected because of the competitive adsorption of solvent molecules on the catalyst surface, which blocks some of the active sites of the catalyst. In the case of water as a solvent, the dissolution of FeCl3 and formation of a separate aqueous layer might have drastically affected the catalyst activity. Hence all the further aza-Michael addition reactions of different amines were carried out in the absence of any solvent.
Results for the aza-Michael addition of different aromatic and aliphatic/cyclic amines to α,β-unsaturated compounds over the FeCl3 (1.0 mmol g−1) MontK10 catalyst (preheated at 120 °C) under solvent-free conditions are presented in Table 1. From the results, the following important observations can be made.
| Entry | Amine | α,β-unsaturated compound | Product | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| a 5th reuse of the catalyst. b When amine saturated with water was used in the reaction. c When the reaction was carried out under totally moisture-free conditions. | |||||
| 1 |

|

|

|
3 | 93 |
| 2 |

|

|

|
3 | 88 |
| 3 |
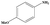
|
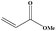
|
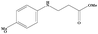
|
3.5 | 88 |
| 4 |

|

|
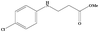
|
3.5 | 90 |
| 5 |

|
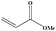
|
|
4 | 80 |
| 6 |

|

|

|
5 | 80 |
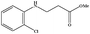
| 7 |

|
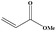
|

|
3.5 | 90 |
| 8 |

|

|
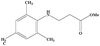
|
7 | 50 |
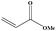
| 9 |

|
|

|
2.5 | 80 |
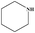
| 10 |
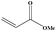
|
|

|
2 | 89 |
| 11 |

|

|

|
3 | 90 |
| 12 |

|

|

|
3 | 88 |
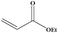
| 13 |

|
|

|
2 | 88 |
| 14 |
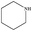
|

|
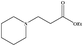
|
2 | 84 |
| 15 |

|
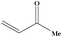
|

|
2 | 93 |
| 16 |

|
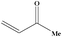
|

|
1.5 | 89 |
| 17 |

|
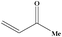
|

|
4 | 80 |
| 18 |

|

|

|
2 | 65 |
| 19 |

|

|

|
5 | 85 |
| 20 |

|

|
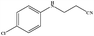
|
6 | 88 |
| 21 |
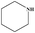
|

|

|
2 | 80 |
| 22 |

|

|

|
3 | 93a |
| 23 |

|

|
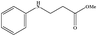
|
3 | 92b |
| 24 |

|

|
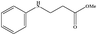
|
3 | 94c |
• The catalyst showed very good performance in the aza-Michael addition of the different amines to α,β-unsaturated compounds. The product yield in the reaction was good to excellent in a short reaction period (1–7 h), depending upon the nature of the amine to be added and the α,β-unsaturated compound.
• The product yield was lower particularly for ortho- or meta- substituted anilines (entries 5, 6, 8, 18, 19) or when methyl acrylate was replaced by the other α,β-unsaturated compound in the reaction.
• The addition of aniline to methyl acrylate gave an excellent product yield of 93%. However, even in the addition of cyclic amines, the product yield was quite good (entries 10–12, 14, 21). In general, the addition of amines to acrylonitrile or cyclohexenone was found to give lower product yields (entries 18–21).
• The catalyst showed excellent reusability without a significant loss in its activity in the reaction (entries 1 and 22).
• While using the catalyst in the reaction, no special precautions were taken to avoid its exposure to atmospheric moisture, yet the catalyst showed very good performance in the reaction. When the reaction was carried out in totally moisture-free conditions, the product yield was just slightly increased (entries 1 and 24). Also when water-saturated aniline was used in the reaction, the product yield was slightly decreased (entries 1 and 23). These observations clearly reveal that the catalyst is almost insensitive to moisture for its performance in the reaction.
It is interesting to note that when anhydrous FeCl3 (without supporting it on Mont-K10) was used in the aza-Michael reaction of aniline with methyl acrylate, the product yield was significantly lower (65%). This fact clearly indicates the beneficial effect of the use of the Mont-K10 support for the catalyst, not only for its ease of separation and reuse but also for achieving better catalytic activity in the reaction.
In earlier studies using sulphated zirconia,25 AlCl3/SiO2,26 SiO2–SO3H,27 Cu–Al-hydrotalcite,28 as a solid catalyst, for the aza-Michael reaction (with excess of unsaturated compound), a comparable product yield was obtained.
All the above observations indicate that the FeCl3 (1.0 mmol g−1) MontK10 is a highly promising catalyst for the aza-Michael addition of different amines to α,β-unsaturated compounds. It is moisture-insensitive and also shows excellent reusability in the reaction.
Although, the aza-Michael addition has been well investigated using different types of catalysts (viz. Lewis/Brønsted acids25–27,33 or basic metal compounds28,33), the FeCl3/Mont-K10 catalyst is an unique Lewis acid catalyst for the reaction. The novelty of the latter catalyst lies in the fact that its Lewis acid sites are not responsible for its high catalytic activity in the aza-Michael reaction. Unlike the conventional Lewis acid catalysts, the aza-Michael activity of the FeCl3/Mont-K10 is not influenced significantly by the presence of moisture in the reaction mixture (Table 1). Obviously, the reaction over this catalyst is not expected to follow the Lewis acid catalyzed Michael addition.33 Very likely, the redox properties of FeCl3 (Fe3+ + e− ↔ Fe2+) rather than its Lewis acidity seem to be responsible for the observed high activity of the FeCl3/Mont-K10 catalyst in the reaction. Similar observations have also been made earlier for the Friedel-Craft type benzylation and acylation reactions over Fe-containing catalysts.34–36 This reveals that the aza-Michael addition can be catalyzed not only by acid/base catalysts but also by catalysts with redox properties.
Unlike the conventional Lewis acid catalysts, the FeCl3/Mont-K10 catalyst requires no special precautions for its handing to avoid its exposure to atmospheric moisture and also for drying substrates. Because of its moisture insensitivity, the catalyst does not lose its activity in its several reuses in the reaction. Moreover, this catalyst showed higher activity in the aza-Michael reaction than that shown by the catalysts reported earlier25–28 [under somewhat similar reaction conditions, the amount of catalyst (mg per mmol of amines) used for getting comparable product yield in the aza-Michael reaction was 50 (sulfated ZrO2),25 200 (AlCl3/SiO2),26 84 (Silica-Sulfuric acid),27 50 (Cu–Al-hydrotalcite)28 but only 9 mg mmol−1 of amine for the FeCl3/Mont-K10 catalyst]. The high catalytic activity of the FeCl3/Mont-K10 is mostly attributed to its redox properties.
Conclusions
In summary, Mont K10 supported FeCl3 (1.0 mmol g−1) is a highly active and moisture-insensitive catalyst useful for the solvent-free aza-Michael addition of different aromatic and aliphatic/cyclic amines to α,β-unsaturated compounds. The catalyst is environmentally friendly, it can be easily separated and it also shows excellent reusability in the reaction. Its performance in the reaction is strongly influenced by its preheating temperature. The high activity of the catalyst is attributed mostly to its redox properties rather than its Lewis acidity.Experimental section
a) Catalyst preparation and characterization
The solid catalyst used in this investigation was FeCl3/MontK10 (FeCl3 = 1.0 mmol g−1) and surface area = 150 m2 g−1, particle size = 0.15 μm. The MontK10 supported FeCl3 catalysts with different FeCl3 loadings (0–2.0 mmol g−1) were prepared by impregnating MontK10 clay with anhydrous FeCl3 from its acetonitrile solution and evaporating the solvent in a vacuum oven at 120 °C for 8 h. The resulting catalyst was finely powdered. The catalyst was used in the reaction without taking any precautions for avoiding adsorption of atmospheric moisture on the catalyst surface. Unless otherwise mentioned, the catalyst pre-treatment temperature was 120 °C and the FeCl3 loading on MontK10 was 1.0 mmol g−1.The catalyst was characterized for its surface area by the single-point N2 adsorption method (using a surface area analyzer; Quanta Chrome, USA) and for particle/crystal size by SEM (using a Stereo Scan 440 made in Cambridge UK).
b) General procedure for catalytic aza-Michael reaction
The catalytic aza-Michael reaction was carried out in a magnetically stirred round bottom flask (capacity: 25 cm3), with the following reaction conditions: reaction mixture = 2.5 mmol amine + 5 mmol α,β-unsaturated compound + catalyst (10 wt% w.r.t. amine), bath T = 60 °C and reaction time = 1–7 h. The reaction was monitored by thin layer chromatography. After completion of the reaction the catalyst was separated by filtration and the filtrate was treated with water followed by the extraction of organic compounds with ethyl acetate to get the crude product, which was subsequently purified by column chromatography on a silica gel column, using petroleum ether/ethyl acetate as an eluent. The reaction product was isolated by column chromatography. The catalyst was further washed with acetone, dried and reused. All of the products are known and were confirmed from their NMR and by comparison with those reported in the literature. The catalyst was further washed with acetone, dried and reused.Acknowledgements
V. R. C. and D. K. D. are grateful to the National Academy of Sciences (India) for the NASI Sr. Scientist Platinum Jubilee Fellowship and Project Assistantship, respectively.References
- M. Misra, R. Luthra and K. Singh, Comprehensive Natural Products Chemistry, ed., Barton, D. H. R., Nakanishi, K., Meth-Cohn, O., Pergamon, Oxford, 1999, Vol. 4, p. 25 Search PubMed.
- P. Traxler, U. Trinks, E. Buchduger, H. Mett, T. Meyer, M. Muller, U. Regenass, J. Rosel and N. Lydon, J. Med. Chem., 1995, 38, 2441–2447 CrossRef CAS.
- E. Juaristi, Enantioselective Synthesis of β-Amino Acids, Wiley-VCH, New York, 1997 Search PubMed.
- M. E. Jung, Comprehensive Organic Synthesis, Pergamon, New York/NY, 1991, 4, pp. 30–41 Search PubMed.
- P. Perlmutter, Conjugate Addition Reactions in Organic Synthesis, Pergamon, New York, 1992, p. 114 Search PubMed.
- G. Cardillo and C. Tomasini, Chem. Soc. Rev., 1996, 25, 117–128 RSC.
- E. J Corey and G. A. Reichard, Tetrahedron Lett., 1989, 30, 5207–5210 CrossRef.
- J. Cabral, P. Laszlo, L. Mahe, M. T. Montaufier and S. L. Randriamahefa, Tetrahedron Lett., 1989, 30, 3969–3971 CrossRef CAS.
- T. P. Loh and L.L. Wei, Synlett, 1998, 975–978 CrossRef CAS.
- G. Bartoli, M. Bosco, E. Marcantoni, M. Pertrini, L. Sambri and E. Torregiani, J. Org. Chem., 2001, 66, 9052–9055 CrossRef CAS.
- L.W. Xu, L. Li and C. G. Xia, Helv. Chim. Acta, 2004, 87, 1522–1526 CrossRef CAS.
- G. Smitha and C. S. Reddy, Catal. Commun., 2007, 8, 434–436 CrossRef CAS.
- M. Perez and R. Pleixats, Tetrahedron, 1995, 51, 8355–8362 CrossRef CAS.
- R. Alleti, S. O. Woon, M. Perambuduru, C. V. Ramena and V. P. Reddy, Tetrahedron Lett., 2008, 49, 3466–3470 CrossRef CAS.
- N. Azizi, R. Baghi, H. Ghafuri, H. Bolourtchian and M. Hashemi, Synlett, 2010, 3, 379–382 CrossRef.
- L. Fadini and A. Tongi, Chem. Commun., 2003, 30–32 RSC.
- N. Azizi and M. R. Saidi, Tetrahedron, 2004, 60, 383–388 CrossRef CAS.
- N. Srivastava and B. K. Banik, J. Org. Chem., 2003, 68, 2109–2111 CrossRef CAS.
- M. J. Bhanushali, N. S. Nandurkar, S. R. Jagtap and B. M. Bhanage, Catal. Commun., 2008, 9, 1189–1195 CrossRef CAS.
- R. Varala, N. Sreelatha and S. R. Adapa, Synlett, 2006, 10, 1549–1553 Search PubMed.
- R. Trivedi, P. Lalitha and S. Roy, Synth. Commun., 2008, 38, 3556–3566 CrossRef CAS.
- S. Matsubara, M. Yoshioka and K. Utimoto, Chem. Lett., 1994, 827–830 CrossRef CAS.
- G. Jenner, Tetrahedron Lett., 1995, 36, 233–236 CrossRef CAS.
- R. Varala, M. M. Alam and S. R. Adapa, Synlett, 2003, 5, 720–723 Search PubMed.
- B. M. Reddy, M. K. Patil and B. T. Reddy, Catal. Lett., 2008, 126, 413–418 CrossRef CAS.
- M. R. Saidi, Y. Pourshojaei and F. Aryanasab, Synth. Commun., 2009, 39, 1109–1119 CrossRef CAS.
- Y. Wang, Y-Q. Yuan and S.-R. Guo, Molecules, 2009, 14, 4779–4789 CrossRef CAS.
- M. L. Kantam, B. Neelima and C. V. Reddy, J. Mol. Catal. A: Chem., 2005, 241, 147–150 CrossRef CAS.
- V. Polshettiwar and R. S. Varma, Tetrahedron Lett., 2007, 48, 8735–8738 CrossRef CAS.
- K. J. Borah, M. Phukan and R. Borah, Synth. Commun., 2010, 40, 2830–2836 CrossRef CAS.
- A. Ying, L. Liu, G. F. Wu, G. Chen, X.-Z. Chen and W. Ye, Tetrahedron Lett., 2009, 50, 1653–1657 CrossRef CAS.
- M. L. Kantam, B. Neelima, C. V. Reddy and R. Charavarti, Ind. Eng. Chem. Res., 2007, 46, 8614–8619 CrossRef CAS.
- B. D. Mather, K. Viswanathan, K. M. Miller and T. E. Long, Prog. Polym. Sci., 2006, 31, 487–531 CrossRef CAS.
- V. R. Choudhary, S. K. Jana and A. S. Mamman, Microporous Mesoporous Mater., 2002, 56, 65–71 CrossRef CAS.
- V. R. Choudhary and S. K. Jana, J. Mol. Catal. A: Chem., 2002, 180, 267–276 CrossRef CAS.
- V. R. Choudhary and S. K. Jana, Appl. Catal., A, 2002, 224, 51–62 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
