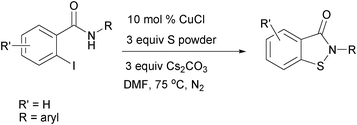
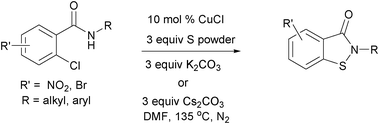
Copper-catalysed one-pot synthesis of N-substituted benzo[d]isothiazol-3(2H)-ones via C-S/N-S bond formation†
Rajesh
Paul
and
Tharmalingam
Punniyamurthy
*
Department of Chemistry, Indian Institute of Technology Guwahati, Guwahati 781039, India. E-mail: tpunni@iitg.ernet.in; Fax: +91 0361 2690762; Tel: +91 0361 2582309
First published on 13th June 2012
Abstract
One-pot synthesis of N-substituted benzo[d]isothiazol-3(2H)-ones has been described using copper-catalysis from N-substituted 2-halobenzamides and sulfur powder via C-S/N-S bond formation with high yield.
N-Substituted benzo[d]isothiazol-3(2H)-ones and their analogues have attracted considerable interest in biological and chemical sciences due to their promising antibacterial and antifungal properties.1,2 They have also been studied as potential antithrombotic agents,3a antipsychotic agents3c as well as models for the development of metallothionein inspired molecular pincers.3g Furthermore, they are being investigated as potential inhibitors of phosphomannose3h and NADPH-oxidase.3h In addition, benzo[d]isothiazol-3(2H)-ones have been utilized for the development of glutamate receptor subtype-2.3i As a result, a number of routes leading to the target heterocycles have been reported,3,4 but although they have been proven to be useful protocols, they generally require stoichiometric amount of the reagents and some of them involve highly toxic and corrosive agents such as chlorine gas.4a Development of catalytic methods for the construction of the benzoisothiazol-3(2H)-one structural framework has thus been important in organic synthesis.
The recent advances in cross-coupling reactions using transition-metal-catalysis have led to the development of effective methods for the construction of carbon-heteroatom bonds.5,6 Among them, copper-based catalytic systems are attractive because they are cheap, readily available and less toxic.6–9 Herein, we wish to report a new one-pot synthesis of N-substituted benzo[d]isothiazol-3(2H)-ones from N-substituted 2-halobenzamides and sulfur powder using copper-catalyzed C-S cross-coupling reaction followed by N-S bond formation. The procedure is general and the target compounds could be obtained in high yield.
First, the optimization of the reaction conditions was performed with N-benzyl-2-iodobenzamide as a model substrate using different solvents, copper and sulfur sources at varied temperatures (Table 1). The reaction occurred to give the desired N-benzyl-benzo[d]isothiazol-3(2H)-one in 78% conversion when the substrate was stirred with 10 mol % copper source, 3 equiv. sulfur powder and 3 equiv. K2CO3 for 6 h at 75 °C in DMF under nitrogen. In a set of copper sources screened, CuCl, CuBr, CuI and Cu(OAc)2·H2O, CuCl gave the best result (entry 1). DMF was found to be the solvent of choice, whereas the reaction in DMSO gave the target product in 70% conversion. In contrast, the process in toluene showed no reaction. Sulfur powder was found to be the superior sulfur source compared to thiourea. Lowering of the reaction temperature (70 °C) or quantity of the catalyst (5 mol %) or sulfur source (2 equiv) or base (2 equiv) led to the formation of the target molecule in < 60% conversion. Control experiments confirmed that the desired heterocycle was not formed in the absence of the copper source.
| entry | catalyst | solvent | sulfur source | temp. (°C) | product (%)ab |
|---|---|---|---|---|---|
| a Determined by 1H NMR spectroscopy. b N-Benzyl-2-iodobenzamide (0.5 mmol), copper source (10 mol %), sulfur source (1.5 mmol) and K2CO3 (1.5 mmol) were stirred at 75 °C for 6 h in DMF (1 mL) under N2. c Catalyst (5 mol %) used. n.d. = not detected. | |||||
| 1 | CuCl | DMF | S powder | 75 | 78 |
| 2 | CuCl | DMSO | S powder | 75 | 70 |
| 3 | CuCl | toluene | S powder | 75 | n.d. |
| 4 | CuBr | DMF | S powder | 75 | 70 |
| 5 | CuI | DMF | S powder | 75 | 65 |
| 6 | Cu(OAc)2·H2O | DMF | S powder | 75 | 45 |
| 7 | CuCl | DMF | thiourea | 75 | 51 |
| 8 | CuCl | DMF | S powder | 70 | 60 |
| 9 | — | DMF | S powder | 75 | n.d. |
| 10 | CuCl | DMF | S powder | 75 | 40c |
Next, the scope of the procedure was investigated for the synthesis of a series of N-alkyl-benzo[d]isothiazol-3(2H)-ones (Table 2). 2-Iodobenzamides with N-butyl, N-cyclohexyl, N-isopropyl, N-(2-phenylethyl) and N-(3,4-dimethoxyphenethyl) substituents proceeded with 83–93% yield (entries 1–5). Similarly, 2-iodo-N-(2-(octyloxy)ethyl)benzamide and N,N′-(ethane-1,2-diyl)-bis(2-iodobenzamide) could be transformed into the corresponding N-alkyl-benzo[d]isothiazol-3(2H)-ones in 90% and 81% yield, respectively (entries 6 and 7). Furthermore, N-benzyl-2-iodobenzamides with 5-methoxy and 5-octyloxy substituents underwent reactions with 67% and 72% yield, respectively (entries 8 and 9), while the substrate with 5-nitro substituent on benzamide ring gave the target molecule in 85% yield (entry 10).
|
|
||||
|---|---|---|---|---|
| entry | product | time (h) | yield (%)a,b | |
| a N-Substituted 2-iodobenzamide (0.5 mmol), CuCl (10 mol %), sulfur powder (1.5 mmol) and K2CO3 (1.5 mmol) were stirred at 75 °C in DMF (1 mL) under N2. b Isolated yield. c CuCl (20 mol %), sulfur powder (3 mmol) and K2CO3 (3 mmol) were used. | ||||
|
||||
| 1 | R = n-butyl | 9 | 93 | |
| 2 | R = cyclohexyl | 8 | 95 | |
| 3 | R = isopropyl | 8 | 90 | |
| 4 | R = 2-phenylethyl | 8 | 87 | |
| 5 | R = 3,4-dimethoxy-phenethyl | 8 | 83 | |
| 6 |

|
10 | 90 | |
| 7 |

|
12 | 81c | |

|
||||
| 8 | R′ = OME | R = benzyl | 16 | 67 |
| 9 | R′ = OC8H17 | R = benzyl | 16 | 72 |
| 10 | R′ = NO2 | R = benzyl | 8 | 85 |
Furthermore, the synthesis of N-arylbenzo[d]isothiazol-3(2H)-ones was studied (Table 3). These reactions were found to be more effective using Cs2CO3 instead of K2CO3, leading to the target compounds in high yield. Thus, 2-iodobenzamides with N-phenyl, N-(2-methoxyphenyl), N-(2-methylphenyl), N-(4-chlorophenyl), N-(4-methoxyphenyl), N-(4-methylphenyl) and 4-((phenyldiazenyl)phenyl) substituents enabled the reactions to afford the corresponding N-arylbenzo[d]isothiazol-3(2H)-ones in 63–90% yield (entries 1–3, 5–7 and 9). Under these conditions, the substrates with N-(3-nitrophenyl) and N-(4-nitrophenyl) substituents were decomposed and the target compounds were not obtained (entries 4 and 8). However, 2-iodobenzamides with N-(2,4-dimethylphenyl), N-(2,6-dimethylphenyl) and N-(3,4-dimethylphenyl) substituents gave the target molecule in 86–92% yield (entries 10–12). Recrystallization of N-(4-methoxy)phenylbenzo-[d]isothiazol-3(2H)-one in CHCl3 gave single crystals whose structure was determined using a X-ray analysis (Fig. 1).
![ORTEP diagram of N-(4-methoxyphenyl)benzo-[d]isothiazol-3(2H)-one. Thermal ellipsoids are drawn at a 40% probability level. Hydrogen atoms have been omitted for clarity.](/image/article/2012/RA/c2ra20724a/c2ra20724a-f1.gif) | ||
| Fig. 1 ORTEP diagram of N-(4-methoxyphenyl)benzo-[d]isothiazol-3(2H)-one. Thermal ellipsoids are drawn at a 40% probability level. Hydrogen atoms have been omitted for clarity. | ||
|
|
|||
|---|---|---|---|
| entry | product | time (h) | yield (%)ab |
| a N-Substituted 2-iodobenzamide (0.5 mmol), CuCl (10 mol %), sulfur powder (1.5 mmol) and Cs2CO3 (1.5 mmol) were stirred at 75 °C in DMF (1 mL) under N2. b Isolated yield. c CuCl (99.99%) was used. n.d. = not detected. | |||
| 1 |

|
14 | 90, 91c |

|
|||
| 2 | R = OMe | 10 | 84 |
| 3 | R = Me | 12 | 88 |
| 4 |

|
10 | n.d. |

|
|||
| 5 | R = Cl | 18 | 85 |
| 6 | R = OMe | 10 | 89 |
| 7 | R = Me | 12 | 93 |
| 8 | R = NO2 | 10 | n.d. |
| 9 | R = N2Ph | 21 | 63 |
| 10 |

|
14 | 92 |
| 11 |

|
14 | 91 |
| 12 |
|
14 | 86 |
To reveal the reactivity of the other 2-halobenzamides, the reactions of 2-bromobenzamides were next studied (Table 4). These substrates underwent reactions at 100 °C to afford the target compounds in moderate to good yield (entries 1–7). Thus, N-butyl-2-bromobenzamide gave 60% yield, while the substrates with 5-nitro and 5-bromo substituents on the benzamide ring afforded the target molecules in 78% and 90% yield, respectively (entries 1–3). The substrate with a 5-methoxy substituent on the benzamide ring proceeded with a 45% yield (entry 4). Similarly, N-aryl-2-bromobenzamides containing N-(4-chlorophenyl) and N-(4-methylphenyl) substituents afforded the target compounds in moderate yield (entries 5 and 6), whereas N-(4-methylphenyl)-2-bromo-5-nitrobenzamide gave a 71% yield (entry 7).
|
|
||||
|---|---|---|---|---|
| entry | product | time (h) | yield (%)ab | |
| a N-Substituted 2-bromobenzamide (0.5 mmol), CuCl (10 mol %), sulfur powder (1.5 mmol) and K2CO3 (1.5 mmol) were stirred at 100 °C in DMF (1 mL) under N2. b Isolated yield. c Cs2CO3 (1.5 mmol) was used. | ||||
| 1 |

|
9 | 60 | |
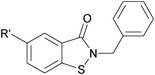
|
||||
| 2 | R′ = NO2 | 10 | 78 | |
| 3 | R′ = Br | 10 | 90 | |
| 4 | R′ = OMe | 18 | 45 | |
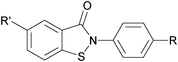
|
||||
| 5c | R′ = H | R = Cl | 18 | 50 |
| 6c | R′ = H | R = OMe | 10 | 54 |
| 7c | R′ = NO2 | R = Me | 10 | 71 |
Finally, the reactions of the 2-chlorobenzamides were studied (Table 5).6j These substrates enabled the reaction to proceed at 135 °C to give desired N-substituted benzo[d]isothiazol-3(2H)-ones in moderate yield (Table 5). For example, 2-chlorobenzamides with N-alkyl substituents, N-benzyl-2-chloro-5-nitrobenzamide and N-benzyl-2-chloro-5-bromobenzamide gave the target molecules in 35% and 21% yield, respectively (entries 1 and 2), whereas N-(4-methoxyphenyl)-2-chloro-5-nitrobenzamide and N-(4-methylphenyl)-2-chloro-5-nitrobenzamide reacted with 45% and 30% yields, respectively (entries 4 and 5). In contrast, the substrate with a 5-methoxy group on the benzamide ring showed no reaction and the formation of the target compound was not observed.
|
|
||||
|---|---|---|---|---|
| entry | product | time (h) | yield (%)a,b | |
| a N-Substituted 2-chlorobenzamide (0.5 mmol, CuCl (10 mol %), sulfur powder (1.5 mmol) and K2CO3(1.5 mmol) were stirred at 135 °C in DMF (1 mL) under N2. b Isolated yield. c Cs2CO3 (1.5 mmol) used. d CuCl (10 mol % and 1,2- bis(diphenylphosphinoethane) (10 mol %) was used. n.d. = not detected. | ||||
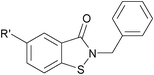
|
||||
| 1 | R′ = NO2 | 28 | 35 | |
| 2 | R′ = Br | 28 | 21 | |
| 3 | R′ = OMe | 28 | n.d. | |

|
||||
| 4c | R′ = NO2 | R = Me | 28 | 30 |
| 5c | R′ = NO2 | R = OMe | 24 | 45,48d |
Conclusions
In conclusion, copper-catalyzed one-pot synthesis of N-substituted benzo[d]isothiazol-3(2H)-ones has been described from N-substituted 2-halobenzamides and sulfur powder via C-S/N-S bond formation. Further study towards the elucidation of the mechanism is currently underway in our laboratory.Acknowledgements
We thank Department of Science and Technology, New Delhi and Council of Scientific and Industrial Research, New Delhi for financial Support.References
- (a) For some examples, see: F. Gialdi, R. Ponci and A. Baruffini, Farmaco, Ed. Sci., 1961, 16, 509 CAS; (b) R. Ponci, A. Baruffini, M. Croci and F. Gialdi, Mycopathologia, 1964, 24, 163 CrossRef; (c) F. Gialdi, R. Ponci and P. Caccialanza, Mycopathologia, 1964, 24, 163 CrossRef CAS; (d) R. Ponci, F. Gialdi and A. Baruffini, Farmaco, Ed. Sci., 1964, 19, 254 CAS; (e) R. Ponci, T. Vitali, F. Mossini and L. Amorett, Farmaco, Ed. Sci., 1967, 22, 989 CAS; (f) T. Vitali, L. Amorett and V. Plazzi, Farmaco, Ed. Sci., 1968, 23, 1075 CAS; (g) M. Davis, Adv. Heterocycl. Chem., 1972, 14, 58 CrossRef.
- R. F. Chapman and B. J. Peart, in Comprehensive Heterocyclic Chemistry II; ed. I. Shinkai, Pergamon: Oxford, 1996, vol. 3, pp. 371–372 Search PubMed.
- (a) K. H. Baggaley, P. D. English, J. A. Jennings, B. Morgan, B. Nunn and A. W. R. Tyrell, J. Med. Chem., 1985, 28, 1661 CrossRef CAS; (b) R. Okachi, H. Niino, K. Kitaura, K. Mineura, Y. Nakamizo, Y. Murayama, T. Ono and A. Nakamizo, J. Med. Chem., 1985, 28, 1772 CrossRef CAS; (c) M. H. Norman, G. C. Rigdon, F. Navas III and B. R. Cooper, J. Med. Chem., 1994, 37, 2552 CrossRef CAS; (d) J. A. Loo, T. P. Holler, J. Sanchez, R. Gogliotti, L. Maloney and M. D. Reily, J. Med. Chem., 1996, 39, 4313 CrossRef CAS; (e) J. A. Turpin, Y. Song, J. K. Inman, M. Huang, A. Wallqvist, A. Maynard, D. G. Covell, W. G. Rice and E. Apella, J. Med. Chem., 1999, 42, 67 CrossRef CAS; (f) S. Ranganathan, K. M. Muraleedharan, P. Bharadwaj, D. Chatterji and I. Karle, Tetrahedron, 2002, 58, 2861 CrossRef CAS; (g) A. Henke and J. Srogl, Chem. Commun., 2010, 6819 RSC; (h) J. D. Lambeth, S. M. Smith, T. Kawahara, J. Min, J. Snyder, A. Sun and G. Thota, PCT Int. Appl., 2011, WO2011062864A2 Search PubMed; (i) R. P. Dhanya, S. Sidique, D. J. Shefflar, H. H. Nickols, A. Herath, L. Yang, R. Dahl, R. Ardecky, S. Semenova, A. Markou, P. J. Conn and N. D. P. Cosford, J. Med. Chem., 2011, 54, 342 CrossRef CAS.
- (a) For examples, see: E. W. McClelland and A. J. Gait, J. Chem. Soc., 1926, 921 RSC; (b) L. Katz and W. Schroeder, J. Org. Chem., 1954, 19, 103 CrossRef CAS; (c) N. Kamigata, S. Hasimoto, M. Kobayashi and H. Nakanishi, Bull. Chem. Soc. Jpn., 1985, 58, 3131 CrossRef CAS; (d) J. C. Grivas, J. Org. Chem., 1975, 40, 2029 CrossRef CAS; (e) Y. Uzida and S. Kozuka, Bull. Chem. Soc. Jpn., 1982, 55, 1183 CrossRef; (f) A. Correa, I. Tellitu, E. Dominguez and R. Sanmartin, Org. Lett., 2006, 8, 4811 CrossRef CAS; (g) H. Suzuki and H. Abe, Synth. Commun., 1996, 26, 3413 CrossRef CAS; (h) W. Kim, J. Donnaldson and K. S. Gates, Tetrahedron Lett., 1996, 37, 5337 CrossRef CAS; (i) T. Sano, T. Takagi, Y. Gama, I. Shibuya and M. Shimizu, Synthesis, 2004, 1585 CAS; (j) F. Wang, C. Chen, G. Deng and C. Xi, J. Org. Chem., 2012, 77, 4148 CrossRef CAS.
- (a) For some recent reviews on carbon-heteroatom cross-coupling reactions, see: I. P. Beletskaya, Pure Appl. Chem., 2005, 77, 2021 CrossRef CAS; (b) P. Bichler and J. A. Love, Topics in Organometallic Chemistry, 2010, 31, 30 CrossRef; (c) D. S. Surry and S. L. Buchwald, Chem. Sci., 2010, 1, 13 RSC; (d) I. P. Beletskaya and V. P. Ananikov, Chem. Rev., 2011, 111, 1596 CrossRef CAS; (e) R. Jana, T. P. Pathak and M. S. Sigman, Chem. Rev., 2011, 111, 1417 CrossRef CAS; (f) B. M. Rosen, K. W. Quasdorf, D. A. Wilson, N. Zhang, A.-M. Resmerita, N. K. Garg and V. Percec, Chem. Rev., 2011, 111, 1346 CrossRef CAS; (g) A. Casitas, A. E. King, T. Parella, M. Costas, S. S Stahl and X. Ribas, Chem. Sci., 2010, 1, 326 RSC; (h) F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 6954 CrossRef CAS; (i) C. Wang and F. Glorius, Angew. Chem., Int. Ed., 2009, 48, 5240 CrossRef CAS; (j) J. F. Hartwig, in Comprehensive Coordination Chemistry II, ed. J. A. McCleverty and T. J. Meyer, Elsevier, Oxford, 2004, vol. 9, pp.369–398 Search PubMed; (k) J. F. Hartwig, Nature, 2008, 455, 314 CrossRef CAS; (l) J. F. Hartwig, in Handbook of Organopalladium Chemistry for Organic Synthesis, ed. E. I. Negishi, John Wiley & Sons, Inc.Hoboken, N. J., 2002, vol.1, pp. 1097–1106 Search PubMed; (m) J. F. Hartwig, Acc. Chem. Res., 1998, 31, 852 CrossRef CAS; (n) R. J. Lundgren and M. Stradiotto, Angew. Chem., Int. Ed., 2010, 49, 9322 CrossRef CAS; (o) R. J. Lundgren, D. K. Hesp and M. Stradiotto, Synlett, 2011, 2443 CAS; (p) P. G. Alsabeh, R. J. Lundgren, L. E. Longobardi and M. Stradiotto, Chem. Commun., 2011, 47, 6936 RSC.
- (a) For some examples on C-S cross-coupling reactions, see: C. Palomo, M. Oiarbide, R. Lopez and E. Gomez-Bengoa, Tetrahedron Lett., 2000, 41, 1283 CrossRef CAS; (b) P. S. Herradura, K. A. Pendola and R. K. Guy, Org. Lett., 2000, 2, 2019 CrossRef CAS; (c) F. Y. Kwong and S. L. Buchwald, Org. Lett., 2002, 4, 3517 CrossRef CAS; (d) C. G. Bates, R. K. Gujadhur and D. Venkataraman, Org. Lett., 2002, 4, 2803 CrossRef CAS; (e) C. Savarin, J. Srogl and L. S. Liebeskind, Org. Lett., 2002, 4, 4309 CrossRef CAS; (f) C. G. Bates, P. Saejueng, M. Q. Doherty and D. Venkataraman, Org. Lett., 2004, 6, 5005 CrossRef CAS; (g) L. Rout, T. K. Sen and T. Punniyamurthy, Angew. Chem., Int. Ed., 2007, 46, 5583 CrossRef CAS; (h) E. Sperotto, G. P. M. van Klink, J. G. de Vries and G. van Koten, J. Org. Chem., 2008, 73, 5625 CrossRef CAS; (i) L. Rout, P. Saha, S. Jammi and T. Punniyamurthy, Eur. J. Org. Chem., 2008, 640 CrossRef CAS; (j) S. Jammi, S. Sakhthivel, L. Rout, T. Mukherjee, S. Mandal, R. Mitra, P. Saha and T. Punniyamurthy, J. Org. Chem., 2009, 74, 1971 CrossRef CAS; (k) T. Ramana, P. Saha, M. Das and T. Punniyamurthy, Org. Lett., 2010, 12, 84 CrossRef CAS; (l) S. Ranjit, R. Lee, D. Heryodi, C. Shen, J. E. Wu, P. Zhang, K. W. Huang and X. Liu, J. Org. Chem., 2011, 76, 8999 CrossRef CAS; (m) X.-Y. Zhang, X.-Y. Zhang and S.-R. Guo, J. Sulfur Chem., 2011, 32, 23 CrossRef CAS; (n) L.-F. Niu, Y. Cai, C. Liang, X.-P. Hu and P.-F. Xu, Tetrahedron, 2011, 67, 2878 CrossRef CAS.
- R. A. Sheldon, Pure Appl. Chem., 2000, 72, 1233 CrossRef CAS.
- Y. Jiang, Y. Qin, S. Xie, X. Zhang, J. Dong and D. Ma, Org. Lett., 2009, 11, 5250 CrossRef CAS.
- (a) For some examples, see: H. Deng, Z. Li, F. Ke and X. Zou, Chem.–Eur. J., 2012, 18, 4840 CrossRef CAS; (b) L.-L. Sun, C. L. Deng, R.-Y. Tang and X.-G. Zhang, J. Org. Chem., 2011, 76, 7546 CrossRef CAS; (c) D. Ma, X. Lu, L. Shi, H. Zhang, Y. Jiang and X. Liu, Angew. Chem., Int. Ed., 2011, 50, 1118 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: experimental procedure, CCDC 876574 (N-(4-methoxyphenyl)benzo-[d]isothiazol-3(2H)-one) and NMR spectra (1H and 13C). See DOI: 10.1039/c2ra20724a/ |
| This journal is © The Royal Society of Chemistry 2012 |