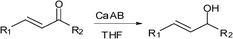
Calcium amidoborane, a new reagent for chemoselective reduction of α,β-unsaturated aldehydes and ketones to allylic alcohols†
Weiliang
Xu
b,
Ruimin
Wang
a,
Guotao
Wu
a and
Ping
Chen
*a
aDalian Institute of Chemical Physics, Dalian, China. E-mail: pchen@dicp.ac.cn; Fax: +86 411-84379583; Tel: +86 411-84379905
bNational University of Singapore, Singapore
First published on 25th April 2012
Abstract
Calcium amidoborane (Ca(NH2BH3)2, CaAB) is a new member of the borohydride family, which possesses interesting dehydrogenation features. CaAB also exhibits superior performance in chemoselective reduction. Our experimental results show that CaAB can reduce α,β-unsaturated carbonyl compounds chemoselectively to the corresponding allylic alcohols at ambient temperature.
Introduction
To utilize H2 as a fuel for transportation, a safe and efficient storage media is needed. One of the prospective solid storage media is the chemical hydrides system.1 Ammonia Borane (NH3BH3, AB) has been extensively studied as one of the chemical hydrides in the past years due to its high hydrogen content of 19.6 wt% and low dehydrogenation temperature at around 100 °C.2 The driving force for AB dehydrogenation is the co-existence of protic H(N) and hydridic H(B) in its structure.2a Recently, metal amidoborane (MAB, [M]n+[NH2BH3]n−), which is the product of cationic substitution of protic H on AB by an alkali or alkaline earth element, was developed and received increasing interests in the field of hydrogen storage.3 Compared with AB, MAB has better performances in terms of hydrogen purity, dehydrogenation kinetics and thermodynamics.3aDevelopment of various hydride reagents and application of those hydrides in organic synthesis continue to be of interest to organic chemistry. AB was reported to reduce ketones and aldehydes in 1980.4 Recently, AB was also found to be able to reduce C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups5 and polarized C
N groups5 and polarized C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups6 at 60 °C or room temperature through a double hydrogen transfer process. Ramachandran et al.7 reported reductive amination using AB as the reducing reagent. Although AB and MAB share some common features, such as the co-existence of protic H(N) and hydridic H(B) as well as the presence of B–N dative bonding, MAB differs from AB in the presence of M…H–B interaction,3g and the electron-rich boron in MAB.3a Both of the two factors lead to weaker B–H bonding in MAB. It is, therefore, of particular interest to figure out the difference in reducing capability between MAB and AB in selective reduction.
C groups6 at 60 °C or room temperature through a double hydrogen transfer process. Ramachandran et al.7 reported reductive amination using AB as the reducing reagent. Although AB and MAB share some common features, such as the co-existence of protic H(N) and hydridic H(B) as well as the presence of B–N dative bonding, MAB differs from AB in the presence of M…H–B interaction,3g and the electron-rich boron in MAB.3a Both of the two factors lead to weaker B–H bonding in MAB. It is, therefore, of particular interest to figure out the difference in reducing capability between MAB and AB in selective reduction.
The chemoselective reduction of α,β-unsaturated carbonyl compounds is one of the essential tasks to define the reducing characteristics of new hydride reagents.8 Reduction of α,β-unsaturated aldehydes and ketones by NaBH4 generally gives three possible products, i.e. allylic alcohol through 1,2-reduction, saturated carbonyl compound after 1,4-reduction and saturated alcohol after 1,4-reduction followed by 1,2-reduction, depending on the steric hindrance of the carbonyl and reaction conditions.9 The most reliable reagent for the 1,2-reduction of α,β-unsaturated carbonyl compounds is the Luche reagent, which is a mixture of NaBH4 and stoichiometric lanthanide chlorides, such as CeCl3 in methanol.10 One of the disadvantages of that system is the toxic cerium byproducts. Other reagents, such as NaBH4–CaCl2,11a NaBH4–guanidine chloride,11b NaBH4–pentaflurophenol,11c and NaBH4–H3PW12O40,11d are also employed to achieve high selectivity for 1,2-reduction. Another type of reducing reagent in 1,2-reduction is lithium aminoborohydride (LiNRR'BH3), such as lithium pyrrolidinoborohydride, which has been intensively investigated by Singaram's group.12 The reduction is a two-step process, including hydroboration and hydrolysis. Recently we found that lithium amidoborane (LiNH2BH3, LiAB for short) is capable of chemoselectively reducing α,β-unsaturated ketones to the corresponding allylic alcohols at ambient temperature.13 Compared with lithium aminoborohydride, LiAB has two protic Hs bonded with N. The protic H(N) and hydridic H(B) in LiAB transfer to the O and C sites of carbonyl group, respectively, through a double hydrogen transfer process.
Calcium amidoborane (Ca(NH2BH3)2, or CaAB for short) is a typical alkaline earth amidoborane. It releases 4 equiv. H2 at temperatures below 250 °C,3f,3h which is somehow higher than that for the decomposition of LiAB. Notably, CaAB can be conveniently obtained by reacting CaH2 with AB in THF (reaction 1).3g
 | (1) |
However, to the best of our knowledge, the reducing ability of CaAB in organic reductions has yet been reported. Due to its similar structure to LiAB but distinct dehydrogenation properties, we propose that CaAB should exhibit some interesting features in the reduction of α,β-unsaturated carbonyl compounds.
Results and discussion
In an attempt at reducing chalcone by CaAB, we observed that CaAB exhibited superior selectivity, leading to the formation of the corresponding allylic alcohol. Based on this finding, a series of α,β-unsaturated aldehydes and ketones were chosen to react with CaAB in THF at ambient temperature. The results are listed in the Table 1.| Entry | Substrate | t/min | Yield %b |
|---|---|---|---|
| a The ratio of substrate to CaAB was 4 to 1 and the concentration of CaAB was 0.05 M. b Isolated overall yields. | |||
| 1 |
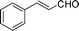
|
15 | 88 |
| 2 |
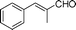
|
15 | 80 |
| 3 |
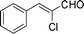
|
15 | 85 |
| 4 |
|
15 | 87 |
| 5 |
|
15 | 84 |
| 6 |
|
15 | 88 |
| 7 |
|
15 | 93 |
| 8 |
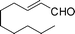
|
15 | 82 |
| 9 |
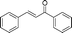
|
60 | 93 |
| 10 |
|
60 | 89 |
| 11 |
|
60 | 96 |
| 12 |
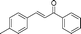
|
60 | 83 |
| 13 |
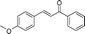
|
60 | 98 |
| 14 |
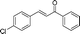
|
60 | 89 |
| 15 |
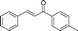
|
60 | 95 |
| 16 |
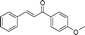
|
60 | 94 |
| 17 |
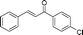
|
60 | 96 |
| 18 |
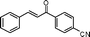
|
60 | 84 |
| 19 |
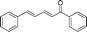
|
60 | 98 |
| 20 |
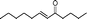
|
60 | 92 |
| 21 |
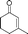
|
60 | 93 |
We chose THF as the solvent because CaAB was synthesized by reacting CaH2 and AB in THF. The CaAB solution was directly used without solvent removal. Protic solvents, such as methanol, are unsuitable due to the solvolysis of CaAB. Other solvents, such as chloroform, diethyl ether and toluene, were also excluded due to low CaAB solubility. The molar ratio of carbonyl compound to CaAB was 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. α,β-Unsaturated aldehydes and ketones were chemoselectively reduced by CaAB in-between 15 to 60 min. In all the cases, conversion rates of carbonyl compounds were all above 99% as measured by gas chromatography (GC). The final product of CaAB is amorphous and does not dissolve in THF and other common organic solvents available in our lab. Therefore, it is difficult to determine its structure by XRD.
1. α,β-Unsaturated aldehydes and ketones were chemoselectively reduced by CaAB in-between 15 to 60 min. In all the cases, conversion rates of carbonyl compounds were all above 99% as measured by gas chromatography (GC). The final product of CaAB is amorphous and does not dissolve in THF and other common organic solvents available in our lab. Therefore, it is difficult to determine its structure by XRD.
An interesting experimental phenomenon is that alcohol products were directly formed in THF without further hydrolysis, which is significantly different from those of borohydrides and aminoborohydride.9–12 We speculate that the protic hydrogens on the N atom in CaAB would be involved in the reaction. In order to prove the participation of protic H(N) of CaAB in the reduction, Ca(ND2BH3)2 (CaA(D)B) was employed to react with cinnamaldehyde (Table 1, entry 1) in THF. A singlet at δ = 1.25ppm attributed to O–D was observed in the 2H NMR spectrum, evidencing the transfer of deuterium on N to the O of carbonyl group upon reduction (see Supporting information†). In a related experiment of reacting Ca(NH2BD3)2 (CaAB(D)) and cinnamaldehyde in THF, a deuterated product at the carbon end of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, which was in 85% isolated yield, was obtained (see Supporting information†), showing the transfer of the deuterium on B of CaAB to the C of carbonyl group in the reduction. These experimental results confirm that both N–H and B–H in CaAB participate in the reduction process and transfer directly to the O and C sites of carbonyl group, respectively (Scheme 1).
O bond, which was in 85% isolated yield, was obtained (see Supporting information†), showing the transfer of the deuterium on B of CaAB to the C of carbonyl group in the reduction. These experimental results confirm that both N–H and B–H in CaAB participate in the reduction process and transfer directly to the O and C sites of carbonyl group, respectively (Scheme 1).
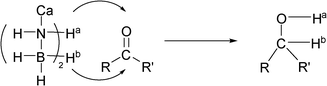 | ||
| Scheme 1 The overall reaction model for CaAB with a carbonyl compound. | ||
Comparing with LiAB, CaAB shows similar reactivity in reducing α,β-unsaturated ketones. However, in the case of reducing α,β-unsaturated aldehydes, CaAB differs from LiAB significantly. As mentioned previously, under the 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of conjugated aldehydes and CaAB, high isolated yields of allylic alcohols were achieved after reduction, implying that CaAB transferred all four protic hydrogens to O sites of carbonyl groups to form four equivalent alcohols. However, when LiAB reacted with two equivalent conjugated aldehydes, only ca. 50% of allylic alcohols were obtained. High isolated yields up to 98% can be achieved only after hydrolysis of the reduction product with aqueous HCl solution. A few examples are shown in Table 2. From the experimental results the following reaction is likely to occur in the case of LiAB reducing an α,β-unsaturated aldehyde:
1 molar ratio of conjugated aldehydes and CaAB, high isolated yields of allylic alcohols were achieved after reduction, implying that CaAB transferred all four protic hydrogens to O sites of carbonyl groups to form four equivalent alcohols. However, when LiAB reacted with two equivalent conjugated aldehydes, only ca. 50% of allylic alcohols were obtained. High isolated yields up to 98% can be achieved only after hydrolysis of the reduction product with aqueous HCl solution. A few examples are shown in Table 2. From the experimental results the following reaction is likely to occur in the case of LiAB reducing an α,β-unsaturated aldehyde:
LiNH2BH3 + 2R–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CHO → R–C C–CHO → R–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH2OH + R–C C–CH2OH + R–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH2–O–BH C–CH2–O–BH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH(Li) NH(Li) |
| Entry | Substrate | t/min | Conver. rate %b | Yield% w/o hydrolysisc | Yield % with hydrolysisc |
|---|---|---|---|---|---|
| a The ratio of substrate and LiAB was 2 to 1 and the concentration of LiAB was 0.083 M. b Detected by GC. c Isolated overall yields. | |||||
| 1 |
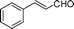
|
15 | >99 | 52 | 97 |
| 2 |
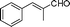
|
15 | >99 | 53 | 98 |
| 3 |
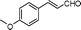
|
15 | >99 | 52 | 90 |
The formation of [R–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH2O–BH
C–CH2O–BH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH(Li)] is evidenced by the observation of white precipitate from the solution and 11B MAS NMR. For example, the chemical shift of B in C6H5–C
NH(Li)] is evidenced by the observation of white precipitate from the solution and 11B MAS NMR. For example, the chemical shift of B in C6H5–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH2O–BH
C–CH2O–BH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH(Li), which is the product obtained after LiAB reacting with 2 equiv. cinnamaldehyde, is 14 ppm. It is attributed to the N
NH(Li), which is the product obtained after LiAB reacting with 2 equiv. cinnamaldehyde, is 14 ppm. It is attributed to the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B–O environment.14 The transfer of the second H(N) to O appears to be difficult in this case (in comparison with that of CaAB), which is probably due to two possible reasons. The first explanation is the poor solubility of R–C
B–O environment.14 The transfer of the second H(N) to O appears to be difficult in this case (in comparison with that of CaAB), which is probably due to two possible reasons. The first explanation is the poor solubility of R–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH2O–BH
C–CH2O–BH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH(Li) in THF (we observed rapid precipitation from the solution upon reaction), which slows down the protic H transfer due to the lack of solvent mediation. The second reason is that a stronger O–B or N–H bonding would be formed. We suppose that the H–N(Li) bond strength in R–C
NH(Li) in THF (we observed rapid precipitation from the solution upon reaction), which slows down the protic H transfer due to the lack of solvent mediation. The second reason is that a stronger O–B or N–H bonding would be formed. We suppose that the H–N(Li) bond strength in R–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH2O–BH
C–CH2O–BH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH(Li) would be stronger than that of H–N(Ca), i.e., the electronegativity of Li is smaller than Ca, therefore, more electrons are transferred to N from Li than from Ca. As a consequence, H shares more electrons to N and thus a stronger covalent bond is established in R–C
NH(Li) would be stronger than that of H–N(Ca), i.e., the electronegativity of Li is smaller than Ca, therefore, more electrons are transferred to N from Li than from Ca. As a consequence, H shares more electrons to N and thus a stronger covalent bond is established in R–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH2O–BH
C–CH2O–BH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH(Li).3h Although detailed investigation is needed to give more clear picture of the reaction, the difference between LiAB and CaAB in reducing aldehydes should be essentially resulted from the difference in chemical nature of Li and Ca.
NH(Li).3h Although detailed investigation is needed to give more clear picture of the reaction, the difference between LiAB and CaAB in reducing aldehydes should be essentially resulted from the difference in chemical nature of Li and Ca.
Conclusion
In summary, CaAB is a versatile and efficient reagent for the chemoselective reduction of α,β-unsaturated aldehydes and ketones to allylic alcohols in good to excellent yields without a hydrolysis step. With the unique properties, i.e. relatively high thermal stability, high reducing capability, double hydrogen transfer and chemoselectivity, CaAB would be of considerable interest to organic synthesis. Continuous efforts in exploring CaAB in other reductions are ongoing.Experimental section
General remarks
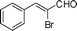
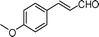
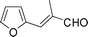
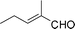
Most solvents and some reagents were purchased commercially and used without further purification: THF (Honywell, HPLC, dried over NaH), diethyl ether (Honeywell, HPLC), hexane (Beijing HuaGong, AR), EtOAc (Beijing HuaGong, AR), ammonia borane (Sigma-Aldrich, 97%), calcium hydride (Alfa, 95%) , cinnamaldehyde (entry 1, Table 1, Aladin, 97%), α-methylcinnamaldehyde (entry 2, Table 1, Aladin, 95%), α-chlorocinnamaldehyde (entry 3, Table 1, Aladin, 97%), α-bromocinnamaldehyde (entry 4, Table 1, Alfa, 98%), 4-methoxycinnamaldehyde (entry 5, Table 1, Alfa, 98%), 2-methyl-3-(2-furyl)propenal (entry 6, Table 1, Alfa, 97%), 2-methyl-2-pentenal (entry 7, Table 1, Alfa, 97%), trans-2-decenal(entry 8, Table 1, Alfa, 95%), chalcone (entry 9, Table 1, Alfa, 97%), 4′-methoxychalcone (entry 16, Table 1, Alfa, 97%), 3-methyl-2-cyclohexen-1-one (entry 21, Table 1, Alfa, 98%). Other conjugated ketones were synthesized by corresponding aldehydes and ketones. The procedure is described in the supporting information (S2 and S3†). NMR spectra were recorded on a Bruker DRX-500 instrument. Chemical shifts, quoted in ppm, are relative to the internal or external standard (only for 2H NMR): singlet δ = 0 ppm of TMS for 1H NMR; the middle of CDCl3 triplet δ = 77 ppm for 13C NMR; singlet δ = 7.26 ppm of CDCl3 for 2H NMR. IR spectra were obtained by Varian 3100 FTIR spectrophotometer using the Resolution Pro program. GC results were detected by RAMIN 2060 series with HP-5 column. MS analyses were performed on an Agilent 6890-5973 GC-MS.Synthesis of CaAB, CaA(D)B and CaAB(D)
1 mmol NH3BH3, ND3BH3 or NH3BD3 was firstly dissolved in 10 ml THF in a metal jar in a glove box. Then, 0.5 mmol CaH2 was quickly added into the solution and the jar cap was closed. The system was stirred at room temperature. After one equivalent of H2 was released detected by pressure gauge, clear 0.05 M CaAB solution was obtained, characterized by 11B NMR. The solution can be directly used in the reducing reaction without further purification.General experimental procedure for reducing α,β-unsaturated ketones with CaAB
5 ml 0.05 M CaAB solution (THF as solvent) was added to 1 ml 1 M substrate solution (THF as solvent) at room temperature in a closed glass bottle under argon gas protection. A FT-IR spectrometer was used to monitor the consumption of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group and the formation of the OH group. After the reaction, THF was evaporated. Then 10 ml diethyl ether was added to the glass bottle to extract the alcohol three times. The diethyl ether solution further underwent centrifugation to remove suspended substances. Next, diethyl ether was evaporated to leave a transparent liquid residue, which was further purified by column chromatography (silica gel, 200–300 mesh, hexane/EtOAc (v/v, 10/1) as an eluent). Alcohol products were characterized by 1H NMR, 13C NMR, FT-IR and GC-MS.
O group and the formation of the OH group. After the reaction, THF was evaporated. Then 10 ml diethyl ether was added to the glass bottle to extract the alcohol three times. The diethyl ether solution further underwent centrifugation to remove suspended substances. Next, diethyl ether was evaporated to leave a transparent liquid residue, which was further purified by column chromatography (silica gel, 200–300 mesh, hexane/EtOAc (v/v, 10/1) as an eluent). Alcohol products were characterized by 1H NMR, 13C NMR, FT-IR and GC-MS.
Product characterization
Acknowledgements
We appreciate the advice from Prof. Yong-Gui Zhou of Dalian Institute of Chemical Physics. We are also grateful to the financial supports from the CAS Hundred Talents Project (KGCX2-YW-806), Knowledge Innovation Program of CAS (KJCX2-YW-H21).References
- (a) P. Chen and M. Zhu, Mater. Today, 2008, 11, 36–43 CrossRef CAS; (b) L. Schlapbach and A. Zuttel, Nature, 2001, 414, 353–358 CrossRef CAS.
- (a) A. Staubitz, A. P. M. Robertson and I. Manners, Chem. Rev., 2010, 110, 4079–4124 CrossRef CAS; (b) T. He, Z. T. Xiong, G. T. Wu, H. L. Chu, C. Z. Wu, T. Zhang and P. Chen, Chem. Mater., 2009, 21, 2315–2318 CrossRef CAS; (c) D. W. Himmelberger, L. R. Alden, M. E. Bluhm and L. G. Sneddon, Inorg. Chem., 2009, 48, 9883–9889 CrossRef CAS; (d) F. H. Stephens, V. Pons and R. T. Baker, Dalton Trans., 2007, 2613–2626 RSC; (e) F. H. Stephens, R. T. Baker, M. H. Matus, D. J. Grant and D. A. Dixon, Angew. Chem., Int. Ed., 2007, 46, 746–749 CrossRef CAS; (f) Q. Xu and M. Chandra, J. Power Sources, 2006, 163, 364–370 CrossRef CAS.
- (a) Y. S. Chua, P. Chen, G. Wu and Z. Xiong, Chem. Commun., 2011, 47, 5116–5129 RSC; (b) Y. S. Chua, G. T. Wu, Z. T. Xiong, T. He and P. Chen, Chem. Mater., 2009, 21, 4899–4904 CrossRef CAS; (c) Z. T. Xiong, C. K. Yong, G. T. Wu, P. Chen, W. Shaw, A. Karkamkar, T. Autrey, M. O. Jones, S. R. Johnson, P. P. Edwards and W. I. F. David, Nat. Mater., 2008, 7, 138–141 CrossRef CAS; (d) Z. T. Xiong, Y. S. Chua, G. T. Wu, W. L. Xu, P. Chen, W. Shaw, A. Karkamkar, J. Linehan, T. Smurthwaite and T. Autrey, Chem. Commun., 2008, 5595–5597 RSC; (e) Z. Xiong, G. Wu, Y. S. Chua, J. Hu, T. He, W. Xu and P. Chen, Energy Environ. Sci., 2008, 1, 360–363 RSC; (f) J. Spielmann, G. Jansen, H. Bandmann and S. Harder, Angew. Chem., Int. Ed., 2008, 47, 6290–6295 CrossRef; (g) H. V. K. Diyabalanage, R. P. Shrestha, T. A. Semelsberger, B. L. Scott, M. E. Bowden, B. L. Davis and A. K. Burrell, Angew. Chem., Int. Ed., 2007, 46, 8995–8997 CrossRef; (h) H. Wu, W. Zhou and T. Yildirim, J. Am. Chem. Soc., 2008, 130, 14834–14839 CrossRef CAS; (i) D. Kim, N. Singh, H. Lee and K. Kim, Chem.–Eur. J., 2009, 15, 5598–5604 CrossRef CAS.
- (a) G. C. Andrews, Tetrahedron Lett., 1980, 21, 697–700 CrossRef CAS; (b) G. C. Andrews and T. C. Crawford, Tetrahedron Lett., 1980, 21, 693–696 CrossRef CAS; (c) B. L. Allwood, H. Shahriarizavareh, J. F. Stoddart and D. J. Williams, J. Chem. Soc., Chem. Commun., 1984, 1461–1464 RSC.
- X. H. Yang, L. L. Zhao, T. Fox, Z. X. Wang and H. Berke, Angew. Chem., Int. Ed., 2010, 49, 2058–2062 CrossRef CAS.
- X. Yang, T. Fox and H. Berke, Chem. Commun., 2011, 47, 2053–2055 RSC.
- P. V. Ramachandran, P. D. Gagare, K. Sakavuyi and P. Clark, Tetrahedron Lett., 2010, 51, 3167–3169 CrossRef.
- (a) D. Ward and C. Rhee, Can. J. Chem., 1989, 67, 1206–1211 CrossRef CAS; (b) S. Kadin, J. Org. Chem., 1966, 31, 620–622 CrossRef CAS; (c) S. Chaikin and W. Brown, J. Am. Chem. Soc., 1949, 71, 122–125 CrossRef CAS; (d) F. Johnson, Chem. Rev., 1968, 68, 375 CrossRef CAS; (e) A. W. Johnstone, A. H. Wilby and I. D. Entwistle, Chem. Rev., 1985, 85, 129 CrossRef.
- (a) H. Blaser, C. Malan, B. Pugin, F. Spindler, H. Steiner and M. Studer, Adv. Synth. Catal., 2003, 345, 103–151 CrossRef CAS; (b) J. S. Cha, Bull. Korean Chem. Soc., 2007, 28, 2162–2190 CrossRef CAS; (c) J. S. Cha, Bull. Korean Chem. Soc., 2011, 32, 1808–1846 CrossRef CAS.
- (a) A. L. Gemal and J. L. Luche, J. Am. Chem. Soc., 1981, 103, 5454–5459 CrossRef CAS; (b) J. L. Luche, J. Am. Chem. Soc., 1978, 100, 2226–2227 CrossRef CAS.
- (a) H. Fujii, K. Oshima and K. Utimoto, Chem. Lett., 1991, 1847–1848 CrossRef CAS; (b) A. Heydari, A. Arefi and M. Esfandyari, J. Mol. Catal. A: Chem., 2007, 274, 169–172 CrossRef CAS; (c) J. Fuller, S. Williamson and B. Singaram, J. Fluorine Chem., 1994, 68, 265–268 CrossRef CAS; (d) A. Heydari, S. Khaksar, J. Akbari, M. Esfandyari, M. Pourayoubi and M. Tajbakhsh, Tetrahedron Lett., 2007, 48, 1135–1138 CrossRef CAS.
- J. C. Fuller, E. L. Stangeland, C. T. Goralski and B. Singaram, Tetrahedron Lett., 1993, 34, 257–260 CrossRef CAS.
- W. Xu, Y. G. Zhou, R. Wang, G. Wu and P. Chen, Org. Biomol. Chem., 2012, 10, 367–371 CAS.
- M. Wörle, H. Meyer zu Altenschildesche and R. Nesper, J. Alloys Compd., 1998, 264, 107–114 CrossRef.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra01291j/ |
| This journal is © The Royal Society of Chemistry 2012 |