Nanoscopic NaCl crystals as water-soluble porogens for polymer membranes
Sven
Range
and
Matthias
Epple
*
Inorganic Chemistry and Center for Nanointegration Duisburg-Essen (CeNIDE), University of Duisburg-Essen, Universitaetsstr, 5-7, D-45117 Essen, Germany. E-mail: matthias.epple@uni-due.de; Fax: +49 201 1832621; Tel: +49 201 2402
First published on 26th June 2012
Abstract
Nanoscopic NaCl was prepared by elimination from sodium malonate and phenacyl chloride with a particle diameter of 100 nm. The NaCl crystals were added to either poly(D,L)lactide or polysulfone (dissolved in dichloromethane) as water-soluble porogens. The dispersion was applied to silicon wafers by dip-coating. After drying in air, the NaCl crystals were removed by washing with water, leaving behind a porous membrane with pore diameters around 100 nm. These free-standing membranes were prepared with a thickness from 1 to 10 μm. They can be used to separate small molecules from larger molecules like proteins.
Introduction
Polymer membranes are important for many separation processes, e.g. in ultrafiltration.1 Conventional thin polymer membranes are produced with uniform pore size mainly by lithography or track-etch techniques.2,3Porous inorganic materials can be easily prepared by removable templates.4–6 One way to induce a specific membrane permeability in polymers is the application of porogens with defined size.7,8 Goedel and coworkers have demonstrated how monodisperse silica spheres with diameters between 100 and 1000 nm can be used as porogens which can then be dissolved by treatment with hydrofluoric acid (HF). Such membranes have a thickness of 10–100 nm and a very ordered pore structure.9–11 However, these leaching conditions are rather harsh and may damage some membrane materials. This can be avoided with water-soluble templates.
We have recently shown how nanoscopic sodium chloride crystals can be prepared by salt elimination reactions from sodium malonate and a chlorinated organic molecule. They can be transferred into a stable suspension of particles with diameters between 100–300 nm.12 These nanocrystals are ideally suited as porogens because they are not soluble in organic solvents (in which synthetic polymers can often be dissolved) but are easily soluble in water.
Here we demonstrate for the first time the application of such nanoparticles as water-soluble porogens for free-standing membranes with defined nanoscopic pore sizes.
Experimental
Synthesis
All syntheses were carried out under an oxygen- and water-free (<0.1 ppm) argon atmosphere, either inside a glove-box (Braun Unilab) or using standard Schlenk techniques. Toluene and hexane (J. T. Baker, p.a.) were dried and distilled over a potassium–sodium alloy. Dichloromethane was used in p.a. quality (Acros) and thoroughly dried over phosphorus pentaoxide. Ethanol (VWR, AnalaR Normapur) was dried with diethylphthalate and sodium. Phenacyl chloride (Fluka, >98%), diethylmalonate (Aldrich, 99%), and sodium metal (Fluka, purum) were used as received. Fluorescein-isothiocyanate-labelled bovine serum albumin (FITC-BSA) was obtained from Sigma-Aldrich (Germany).Poly(D,L)lactide (PDLLA) was obtained from Boehringer Ingelheim (Resomer R208). Polysulfone (PSU) was obtained from Sigma-Aldrich (average Mn ∼22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1).
000 g mol−1).
Sodium diethylmalonate was prepared from malonic acid diethylester and sodium and characterized by elemental analysis and 1H-NMR spectroscopy. Nanoscopic sodium chloride was prepared as follows: a 500 mL two-necked round bottom flask connected to a vacuum/argon line through a take-off was charged with 50 mL dry ethanol and 100 mL dry toluene. Sodium malonate (10.0 g, 54.9 mmol) was added and dissolved at room temperature. 8.49 g (54.9 mmol) Phenacyl chloride dissolved in 50 mL toluene was rapidly added with stirring at room temperature. The reaction occurred immediately as visible by the change in colour of the reaction mixture from colourless to light yellow. Sodium chloride was isolated by centrifugation and washed with 40 mL of dry toluene, recovered by centrifugation (2540 g). This was repeated until the NaCl was colourless (about five times). The nanoscopic NaCl was then either redispersed in dichloromethane for dynamic light scattering and scanning electron microscopy or dried in vacuum. The yield was 2.72 g (46.5 mmol; 84%). NaCl was identified by X-ray powder diffraction. Elemental analysis gave 3.7% C and 0.4% H, indicating some remaining organic material around the crystals (probably the alkylation product according to Fig. 1).
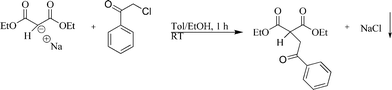 | ||
| Fig. 1 The reaction of sodium diethylmalonate with phenacyl chloride leads to nanoscopic, dispersible NaCl crystals. | ||
Membrane preparation
4.90 mmol of the polymer (0.50 g polylactide; 2.31 g polysulfone; referring to the molecular weight of the monomer) was dissolved in 10 mL of dichloromethane. NaCl prepared as described above was added in different amounts (see Fig. 4), and the dispersion was stirred for 30 min. For the preparation of the membranes, we used dip-coating. A silicon wafer (20·50 mm2) was immersed perpendicular with the short side 20 mm deep into the dispersion with 85 mm min−1. The wafer was kept in the dispersion immersion for a defined period (1, 10, 30, 60 s, respectively) and then pulled out from the dispersion with 50 mm min−1.The polymer layer on the silicon wafer was rapidly dried in air. Then, the wafer was rinsed for three minutes with water to remove the NaCl porogen. The membrane was removed from the silicon wafer by immersion for 5 min in warm water, followed by peeling the layer off carefully with tweezers. It was possible to vary the membrane thickness by the immersion time.
Diffusion experiments
The diffusion experiments were carried out in lid-closed U-tubes which prevented water evaporation during the experiments. The membrane was carefully mounted between two silicon O-rings to avoid any damage. To ensure that the membrane was intact after mounting, one side of the U-tube was filled with 50 mL deionized water and left stand for one hour. If no water in the second half of the U-tube was observed, it was assumed that the membrane was still intact. Then, the other half of the U-tube was filled with 50 mL of the solution containing the molecules to diffuse through the membrane (effective membrane cross section: permeation area: 0.785 cm2). The diffusion experiments were carried out for 144 h. Aliquots were taken from both sides during that period and analysed by quantitative UV spectroscopy. The detection limit was about 0.3 mg L−1 for all compounds used.Characterisation techniques
Elemental analyses (C, H, N) were made on a Euro Vector EA instrument by combustion analysis. Sodium was determined with a Thermo Electron, M-Series atomic absorption spectrometer. 1H-NMR spectra were measured in a CDCl3 (99.8%) solution on a Bruker DPX 300 MHz spectrometer. X-Ray powder diffraction was carried out on a Bruker AXS D8 Advance diffractometer with Cu-Kα radiation (1.54 Å). The samples were mounted on a glass sample holder. Scanning electron microscopy (SEM) images of the Au/Pd-sputtered samples were recorded in high vacuum with an FEI ESEM Quanta 400 FEG instrument. Dynamic light scattering (DLS) was carried out with a Zetasizer nanoseries instrument (Malvern Nano-ZS, laser: λ = 532 nm). UV spectroscopy was performed with a Cary 300 BiO UV-visible spectrophotometer in 1 cm quartz microcuvettes.Results and discussion
The nanoscopic NaCl crystals were prepared according to Fig. 1. Dynamic light scattering of the NaCl crystals dispersed in pure dichloromethane gave a particle diameter of 114 nm (z-average; PDI 0.08), indicating well-dispersed and non-agglomerated NaCl crystals. The thin organic layer around the inorganic crystals obviously helped to disperse them in the organic medium. The NaCl crystals were uniform in size and shape with a diameter of about 100 nm (Fig. 2). For membrane preparation, the NaCl crystals were dispersed in a solution of the corresponding polymer (either PDLLA or PSU) in dichloromethane. Subsequently, the polymer–NaCl dispersion was transferred by dip-coating onto a silicon wafer.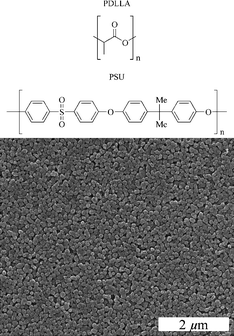 | ||
| Fig. 2 Structure of the two polymers used, PDLLA and PSU (top), and a SEM image of the nanoscopic NaCl crystals that we used as porogens (bottom). | ||
The composition of the polymer membranes including the NaCl was determined by detaching the membrane, weighing it and dissolving the NaCl with water. The content of sodium permitted the determination of the content of NaCl. For the PDLLA membrane that was prepared from 0.5 g PDLLA and 0.5 g NaCl, we found a weight ratio of PDLLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaCl of 67
NaCl of 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33. This shows that the content of NaCl in the membrane was lower than in the dispersion used for the preparation (50
33. This shows that the content of NaCl in the membrane was lower than in the dispersion used for the preparation (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50). Taking into account the densities of NaCl (2.17 g cm−3) and PDLLA (1.21 g cm−3), the volume ratio of PDLLA
50). Taking into account the densities of NaCl (2.17 g cm−3) and PDLLA (1.21 g cm−3), the volume ratio of PDLLA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaCl is 78
NaCl is 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22, i.e. the theoretical porosity after complete removal of NaCl is 22 vol%. For a PSU membrane that was prepared from 2.31 g PSU and 0.2 g NaCl we found a weight ratio of PSU
22, i.e. the theoretical porosity after complete removal of NaCl is 22 vol%. For a PSU membrane that was prepared from 2.31 g PSU and 0.2 g NaCl we found a weight ratio of PSU![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaCl of 94
NaCl of 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, i.e. again less than in the dispersion (91
6, i.e. again less than in the dispersion (91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9). With a density of PSU of 1.24 g cm−3, a theoretical porosity of 4% after the complete removal of NaCl is computed.
9). With a density of PSU of 1.24 g cm−3, a theoretical porosity of 4% after the complete removal of NaCl is computed.
The NaCl crystals were leached out with water so that a porous membrane on the silicon support remained. The detachment from the support of the membrane was carried out by rinsing with warm water (about 50–60 °C) which softened the polymer so that the membranes could be pulled off from the support with a pair of tweezers. The glass transition temperature is about 50 °C for PDLLA13 and 193 °C for PSU,14 respectively. However, it is well known that the glass transition temperature depends on the polymer’s morphology, its water content and its thermal history, therefore the material may well soften below these glass transition temperatures which refer to the pure state.
Fig. 3 shows typical scanning electron micrographs of PDLLA membranes before and after the extraction of NaCl. Before the extraction, the embedded NaCl crystals were clearly visible. After extraction, well-defined uniform pores remained in the polymer with diameters and shapes close to the NaCl porogen size.
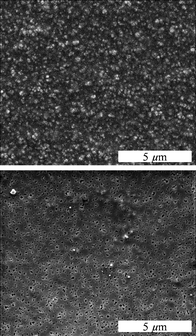 | ||
| Fig. 3 The view from above a PDLLA membrane prepared with NaCl crystals (0.5 g NaCl and 0.5 g PDLLA) before (top) and after (bottom) extraction of the NaCl crystals with water. | ||
An X-ray powder diffractogram showed the presence of crystalline NaCl in the polymer–salt membrane before the extraction with water and the quantitative removal after the extraction (Fig. 4). The same was observed for the PSU membrane before and after removal of NaCl.
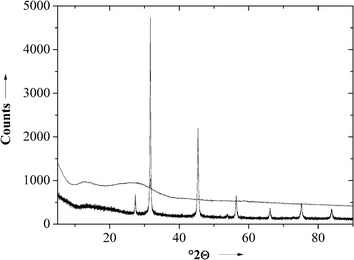 | ||
| Fig. 4 X-ray powder diffractogram of a PDLLA membrane before (bottom) and after (top) extraction of NaCl. | ||
By varying the added amount of sodium chloride to the polymer membranes, the area fraction of the pores varied between 1 and 13%, based on the analysis of the scanning electron micrographs (Fig. 5).
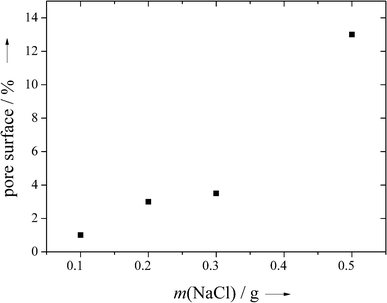 | ||
| Fig. 5 The fraction of the pore surface area in PDLLA as a function of the amount of NaCl porogen added to 0.5 g PDLLA. Note that the pore surface is not directly related to the porosity as shrinking may occur during the extraction of the salt (see text). | ||
Fig. 6 shows two side-views of the porous structure of the membranes after removal of the template. In the upper image the distribution of the pores on the membrane is easily visible.
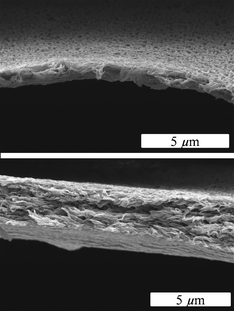 | ||
| Fig. 6 Side-views of a PDLLA membrane after extraction of NaCl (0.5 g NaCl and 0.5 g PDLLA) with water (coating time 10 s). | ||
The thickness of the membranes was measured by SEM before and after extraction of NaCl with warm water as described above. The PDLLA membrane shrunk by about 25% whereas the thickness of the PSU membrane remained constant, probably due to the higher glass transition temperature. Taking into account the computed theoretical porosity of the PDLLA membrane of about 22% (see above), we can conclude that the pores have shrunken considerably. However, they are still there as evidenced by the SEM images (Fig. 3 and 6).
By changing the immersion time in the NaCl–polymer dispersion, the thickness of the membrane could be adjusted to between 1 and 10 μm (Fig. 7; measured by SEM after the extraction of NaCl). It was possible to make even thinner membranes, but below 1 μm the membranes were not mechanically stable and difficult to handle.
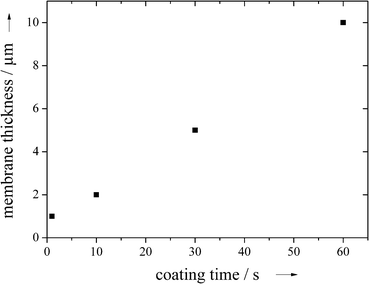 | ||
| Fig. 7 The thickness of the PDLLA membrane after extraction of NaCl as a function of the dip coating immersion time (0.5 g NaCl and 0.5 g PDLLA). | ||
Membranes with a thickness of 2 μm were then used for diffusion experiments. In terms of manageability, the membranes were easy to work with and were sufficiently mechanically stable to stretch in the diffusion apparatus.
Table 1 shows the results of the diffusion experiments. The PDLLA membrane was well suited to separate the small from the medium-sized molecules. In contrast, the PSU membrane was permeable to small molecules and only the protein was retained. We ascribe this difference to the different wettability of the two polymers by water and possibly to a different pore connectivity, especially after the partial collapse of the PDLLA membrane. Preferential adsorption of the protein to the polysulfone membrane may also play a role.15,16
| Molecule | PDLLA membrane (thickness 2 μm) | Polysulfone membrane (thickness 500 nm) |
|---|---|---|
| KMnO4 | 2.3 × 10−13 m2 s−1 | 5.1 × 10−14 m2 s−1 |
| Uranine | impermeable | 3.3 × 10−14 m2 s−1 |
| Rhodamine | impermeable | 4.0 × 10−14 m2 s−1 |
| FITC-labelled BSA | impermeable | impermeable |
The porosity of the membranes after extraction of the salt is of the order of a few percent as computed above. It must also be assumed that the membrane permeability is not directly related to the pore size but rather to the small voids which connect the pores. This explains why a BSA molecule with a size of several nm cannot penetrate the membrane.
Fig. 8 shows the quantification of the diffusion of KMnO4 through the membrane. From such data, the effective diffusion coefficients in Table 1 were computed. For these computations, we have applied Fick's first law by computing the flux through the membrane with an area of 0.785 cm2 and the given thickness as determined by scanning electron microscopy. Scanning electron micrographs after the diffusion experiments showed that the membrane morphology did not change during the experiment.
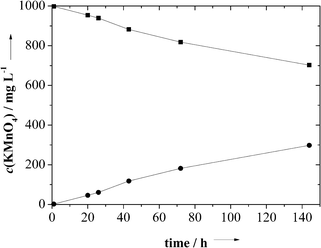 | ||
| Fig. 8 The result of the diffusion experiment of KMnO4 through a PDLLA membrane (thickness 2 μm; from 0.5 g PDLLA and 0.5 g NaCl) with starting concentrations of 0 and 1000 mg L−1 on the two sides of the membrane, respectively. | ||
Conclusions
Porous membranes of polylactide and polysulfone were prepared with water-soluble NaCl nanocrystals as porogens. This method leads to uniform pores in the polymer. The membrane thickness and the porosity can be varied by the preparation conditions. The membranes are free-standing and can be used for the separation of small and large molecules. The membrane material should be variable in a wide range as NaCl is insoluble in organic solvents where most polymers have good solubility. The remaining thin organic layer on the NaCl crystals leads to dispersed NaCl crystals and subsequently to dispersed pores in the polymeric matrix (i.e. the membrane). However, if the glass transition temperature of the polymer is exceeded, the membrane may shrink, resulting in a lower pore size and porosity.References
- M. Ulbricht, Polymer, 2006, 47, 2217–2262 CrossRef CAS.
- M. Yoshida, M. Asano, T. Suwa, N. Reber, R. Spohr and R. Katakai, Adv. Mater., 1997, 9, 757–758 CrossRef CAS.
- K. Ohlrogge and K. Ebert, Membranen, Wiley-VCH, Weinheim, 2006 Search PubMed.
- O. D. Velev, T. A. Jede, R. F. Lobo and A. M. Lenhoff, Nature, 1997, 389, 447–448 CrossRef CAS.
- D. Walsh, L. Arcelli, V. Swinerd, J. Fletcher and S. Mann, Chem. Mater., 2007, 19, 503–508 CrossRef CAS.
- E. S. Toberer and R. Seshadri, Chem. Commun., 2006, 3159–3165 RSC.
- S. A. Johnson, P. J. Ollivier and T. E. Mallouk, Science, 1999, 283, 963–965 CrossRef CAS.
- P. Jiang, K. S. Hwang, D. M. Mittleman, J. F. Bertone and V. L. Colvin, J. Am. Chem. Soc., 1999, 121, 11630–11637 CrossRef CAS.
- H. Xu and W. A. Goedel, Angew. Chem., 2003, 115, 4845–4848 CrossRef.
- H. Xu and W. A. Goedel, Angew. Chem., 2003, 115, 4842–4844 CrossRef.
- F. Yan and W. A. Goedel, Chem. Mater., 2004, 16, 1622–1626 CrossRef CAS.
- T. Annen and M. Epple, Dalton Trans., 2009, 9731–9734 RSC.
- E. Zuza, J. M. Ugartemendia, A. Lopez, E. Meaurio, A. Lejardi and J. R. Sarasua, Polymer, 2008, 49, 4427–4432 CrossRef CAS.
- B. Krause, R. Mettinkhof, N. F. A. van der Vegt and M. Wessling, Macromolecules, 2001, 34, 874–884 CrossRef CAS.
- K. S. Kim, K. H. Lee, K. Cho and C. E. Park, J. Membr. Sci., 2002, 199, 135–145 CrossRef CAS.
- A. Nabe, E. Staude and G. Belfort, J. Membr. Sci., 1997, 133, 57–72 CrossRef.
| This journal is © The Royal Society of Chemistry 2012 |
