Crystallization condition-controlled assembly of oxygen-bridged tetranuclear and hexanuclear Ni(II) clusters: syntheses, structures and properties†
Shizheng
Liu
a,
Suna
Wang
ab,
Fan
Cao
a,
Haiying
Fu
a,
Dacheng
Li
a and
Jianmin
Dou
*a
aShandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, School of Chemistry and Chemical Engineering, Liaocheng University, Liaocheng, P. R. China. E-mail: jmdou@lcu.edu.cn; Tel: +86-0635-8239298
bState Key Laboratory of Coordination Chemistry, Nanjing University, Nanjing, P. R. China
First published on 21st December 2011
Abstract
Crystallization of a Schiff base with nickel acetate in different solvents yields a tetranuclear (dicubane-like [Ni4O4] core with two missing vertices) and a hexanuclear cluster ([Ni6O6] core with two nickel(II) ions attached to a [Ni4O4] cubane through oxygen bridges). Metal centers within these clusters exhibit ferromagnetic and antiferromagnetic coupling interactions, respectively.
During the past few decades, polynuclear compounds of paramagnetic metal ions have received considerable interests because of their potential application in the fields of molecular magnetic materials, especially those that can function as single-molecule magnets (SMMs).1–4 Properties of these compounds is generally governed by the spin values of metal ions, the linkages of polydentate ligands and especially the arrangements of the metal ions through these bridges. A series of ligands, especially those that could provide alkoxo or phenoxo donor groups, such as 2-(hydroxymethyl)pyridine (hmp),4a 2-(2-hydroxyethyl)pyridine (hep),4b Schiff base4c,d and salicylaldoxime as well as its various derivatives (Rsao),4e have been proved effective to generate compounds with good magnetic properties. The assembly process is largely dependent upon the crystallization conditions, such as temperature, solvent, template, pH, guest, concentration and so on.
Recently, we have investigated a linear Mn4 cluster and a novel Mn9 cluster based on hmp and Mesao ligands, respectively. Both exhibit single molecular magnetic properties.3e,f Along with our work in this field, we selected a Schiff base ligand, N,N′-bis(2-hydroxyphenyl-1-ethylidene)-1,3-diamino-2-hydroxypropane (H3L1), which possesses both alkoxo and phenoxo donor groups. To the best of our knowledge, only several CuII, CoII/CoIII and NiII compounds have been reported assembled from this ligand very recently.5 In addition, nickel(II), which is known to have a large single-ion zero-field splitting (ZFS) and easily give rise to high spin molecules with high anisotropy depending on the coordination geometry and symmetry, often leads to ferromagnetic coupling.6 Herein, we obtained a tetranuclear, [Ni4(L1)2(MeO)2(MeOH)2] (1), and a hexanuclear cluster, [Ni6(L1)2(L2)2(OAc)2]·3DMF (2) (H2L2 = N-2-hydroxyphenyl-1-ethylidene-1,3-diamino-2-hydroxypropane) through the reaction of this Schiff base ligand and nickel acetate in different crystallization conditions.‡
Reaction of Ni(OAc)2·6H2O with H3L1 in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio in DMF gave a green solution from which green crystals of 1 and red crystals of 2 were obtained through the diffusion of CH3OH and slow evaporation, respectively.
2 molar ratio in DMF gave a green solution from which green crystals of 1 and red crystals of 2 were obtained through the diffusion of CH3OH and slow evaporation, respectively.
The single-crystal X-ray diffraction analysis indicates that 1 crystallizes in the monoclinic P21/c space group. As shown in Fig. 1, the core of 1 is composed of four NiII ions, two triply-deprotonated Schiff base ligands L13−, two μ3–MeO− anions and two MeOH ligands. Ni1 and Ni1A adopt six-coordinated octahedron geometry with two μ3–MeO− oxygens, one phenoxo oxygen, one imine nitrogen and one MeOH oxygen, respectively. Ni2 and Ni2A, however, are surrounded by one alkoxo oxygen, two phenoxo oxygens, one μ3–MeO− oxygen and one imine nitrogen, respectively. The geometry could be described as much distorted square pyramidal with the Addison parameter τ of 0.523.7 Two η2:η1:η2:η1:η1:μ3 Schiff base ligands and two μ3–MeO− anions bridge four NiII ions into a defected face-shared dicubane-like [Ni4O4] core with two missing vertices. The NiII ions are located at the four corners. The intracluster Ni⋯Ni distances of Ni1⋯Ni2, Ni1⋯NiA and Ni2⋯Ni2A are 3.016(1), 3.100(1) and 5.155(5) Å, respectively. All the doubly bridged Ni–O–Ni angles are in the range of 95.02(2)–97.46(3)°. It should be noted that, although this kind of tetranuclear arrangement is quite common, this tetranuclear structure may be the second example in which five and six-coordinated NiII ions are involved since the first and similar example has been reported very recently.5b
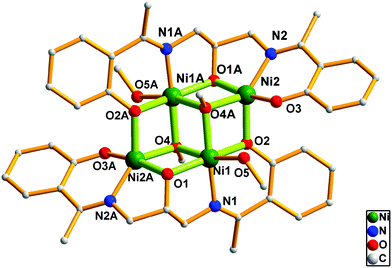 | ||
| Fig. 1 View of the molecular structure of 1. Hydrogen atoms have been omitted for clarity. Symmetry codes: A, −x, 1−y, −z. | ||
Compound 2 crystallizes in the orthorhombic Pbcn space group and is a novel hexanuclear cluster which features a novel [Ni4O4] core with two NiII ions attached to a [Ni4O4] cubane through oxygen bridges (Fig. 2). Interestingly, the Schiff base ligand in this case undergoes partial hydrolysis of the imine bond during the crystallization and results in another Schiff base ligand, N-2-hydroxyphenyl-1-ethylidene-1,3-diamino-2-hydroxypropane (H2L2). The hydrolysis of Schiff base in the presence of a metal ion has been reported, while the probable reasons for the above reaction are still unknown.8 Considering the same reaction, this hydrolysis may take place in a different crystallization process. The addition of MeOH and the slow evaporation of DMF at room temperature may prevent/promote the probability of this hydrolysis process.
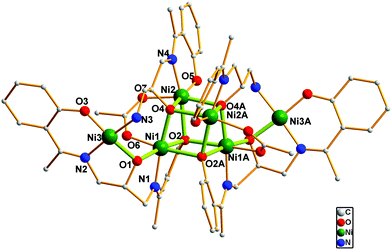 | ||
| Fig. 2 View of the molecular structure of 2. Hydrogen atoms have been omitted for clarity. Symmetry codes: A, 1−x, y, 0.5−z. | ||
The asymmetric unit of 2 consists of three crystallographically independent NiII ions, one triply-deprotonated Schiff base ligands (L13−), one doubly-deprotonated Schiff base ligand (L22−) and one syn–syn bisdentate acetate ion as well as one and a half DMF lattice molecules. Three metal centers exhibit different coordination environment. Ni1 and Ni2 adopt six-coordinated distorted octahedron geometry. The former is surrounded by one imine nitrogen, one μ2-alkoxo oxygen and two μ3-phenoxo oxygen of two L13−, one μ3-alkoxo oxygen of L22− as well as one acetate oxygen. The latter, however, is coordinated with one μ3-phenoxo oxygen of L13−, one imine nitrogen, one η1-phenoxo oxygen and two μ3-alkoxo oxygens of two L22− as well as one acetate oxygen. Ni3 is four-connected and the square plane geometry is furnished by one amine nitrogen of L22−, one μ2-phenoxo oxygen, one imine nitrogen, one η1-phenoxo oxygen of L13−. As a result, two Schiff base ligands L13− and L22− act as η3:η1:η2:η1:η1: μ4 and η1:η1:η3:η1: μ4 bridges to connect six Ni(II) centers, forming a novel hexanuclear [Ni6O6] cluster in which two NiII ions is bridged with the [Ni4O4] cube through η2-alkoxo oxygen atoms. Within the cube subunit the Ni⋯Ni distances and the oxygen doubly-bridged Ni–O–Ni angles are in the range of 2.973(2)–3.335(2) Å and 88.67(18)–103.1(2)°, respectively. The Ni2⋯Ni3 separation across the η2-alkoxo oxygen of L22− ligand is 3.312(2) Å with the Ni–O–Ni angle of 114.3(3)°. The family of Ni6 compounds is growing rapidly with various topologies, such as lines, hexagons, open books, cycles with chair conformation, Chinese-lantern-like objects and face-shared distorted dicubanes with the corners of the cubane missing and so on.9 It should be noted that only one compound has very recently been reported to have a similar metal arrangement9g and this compound is also the first hexanuclear cluster involving both six- and four-coordinated NiII ions.
The temperature dependence of magnetic susceptibilities of 1 and 2 was measured on powdered polycrystalline samples in the temperature range of 2.5–300 K in a magnetic field of 2kOe and shown as χMT vs. T plot in Fig. 3 and 4, respectively. Interestingly, the NiII ions within these two compounds exhibit ferromagnetic and antiferromagnetic coupling interactions, respectively.
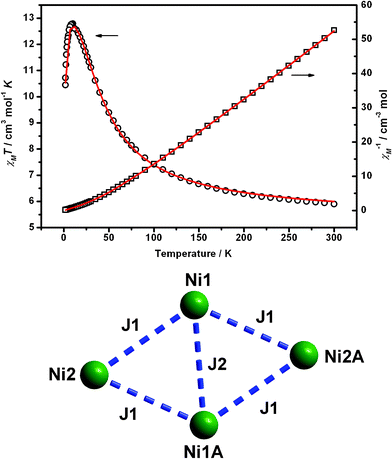 | ||
| Fig. 3 Top: Plot of χMT vs. T of 1 in an applied field of 2kOe. The solid lines represent the best-fit calculations. Bottom: The magnetic exchange interactions between the metal centers. | ||
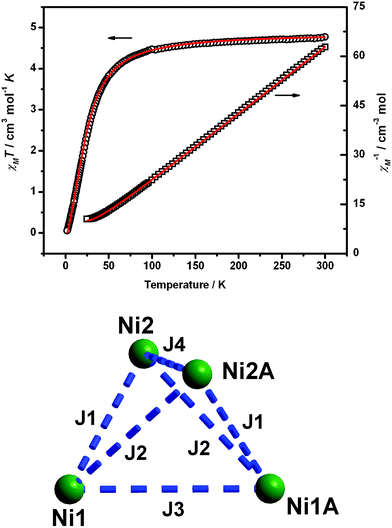 | ||
| Fig. 4 Top: Plot of χMT vs. T of 2 in an applied field of 2kOe. The solid lines represent the best-fit calculations. Bottom: The magnetic exchange interactions between the metal centers. Ni3 and Ni3A are omitted due to the diamagnetic properties. | ||
The χMT of 1 is equal to 5.89 cm3 mol−1 K at 300 K, much larger than the spin-only value for four isolated S = 1 NiII center with g = 2.20 (4.8 cm3 mol−1 K). Upon cooling, the value increases gradually and reaches the maximum of 12.78 cm3 mol−1 K at 9.0 K, which is consistent with 12.1 cm3 mol−1 K based on ground state spin ST = 4 and g = 2.20, indicating ferromagnetic exchange between NiII ions is dominant in this compound. Below this temperature, the value of χMT sharply drops to 10.53 cm3 mol−1 K at 1.8 K, which may be attributed to the weak intertetramer antiferromagnetic interactions and/or significant zero-field splitting as well as Zeeman effects. The interactions between adjacent tetranuclear clusters are very weak, and only the intratetramer coupling interactions are considered when trying to simulate the experimental data. Two different coupling constants were considered: (i) J1, along the sides of the rhomb, exchange mediated by phenolate oxygen and alkoxo oxygen bridges; and (ii) J2, along the short diagonal, exchange resulting from two alkoxo bridges. When the ZFS (D) is taken into account, the Heisenberg spin Hamiltonian was written as: H = -2J1(S1S2 + S1AS2 + S1S2A + S1AS2A)-2J2S1S1A-D[Sz2-S(S + 1)/3)]. The best fits with the parameters of g = 2.314(2), J1 = + 5.87(8) cm−1, J2 = + 12.3(4) cm−1 and |D| = 0.056(1) cm−1 are consistent with the ferromagnetic interactions deduced from the plot (see ESI†).
The χMT of 2 at room temperature is 4.77 cm3 mol−1 K, which is in good agreement with the expected value for the presence of four isolated nickel(II) metal ions with S = 1 and g = 2.20. Upon cooling, the value remains almost constant until about 100 K. From then on, χMT decreases sharply and reaches the minimum of 0.08 cm3 mol−1 K at 2.5 K, indicating antiferromagnetic exchange in this compound. The rapid decrease in the low temperature may also be attributed to the zero-field splitting, Zeeman effects or both. Due to the diamagnetic properties of planar coordinated Ni3 and Ni3A ions, only the coupling interactions between the four Ni(II) centers within the cubane were considered: (i) J1, exchange mediated by one syn–syn acetate and two alkoxo bridges, (ii) J2, exchange resulting from two alkoxo bridges, and finally (iii) J3 and J4, exchange across two alkoxo bridges. As a consequence, the following Heisenberg spin Hamiltonian has been used to calculate the magnetic susceptibility: H = −2J1(S1S2 + S1AS2A) − 2J2(S1S2A + S1AS2) − 2J3S1S1A − 2J4S2S2A. The magnetic anisotropy and weak inter-compound interactions are completely neglected in this approximate model. The data were fitted by MAGPAG program.10 In order to avoid overparameterization of the simulation, we simplified the problem by considering J2, J3 and J4 are equal and obtained the best fits with J1 = 0.66, J2 = J3 = J4 = −0.51 cm−1 and g = 2.20(1).
The magneto-structural corrections for double oxo bridged NiII compounds have been well established.11 The coupling is largely dependent upon the Ni–O–Ni angle: the magnetic coupling is expected to be ferromagnetic when the angles are close to 90°, and a change from ferromagnetic to antiferromagnetic exchange coupling corresponds to a Ni–O–Ni angle of about 98–99°. An inspection of the structure of these compounds allows us to account for the observed ferromagnetic/antiferromagnetic coupling. All Ni–O–Ni angles within the tetramer of 1 are in the range of 95.02–97.46°. In 2, however, most Ni–O–Ni angles are above 99° (in the range of 100.6–103.1°) except the triply bridged Ni1 and Ni2 metals with the Ni–O–Ni angles in the range of 88.6–90.7°. Of course, the magnitude of the exchange coupling is also influenced by the environment of the Ni centers, such as the Ni–O distances, the dihedral angle in the Ni2O2 unit and so on.
In summary, crystallization of a Schiff base ligand with Ni(II) ions under different conditions led to the formation of a tetranuclear and a hexanuclear cluster, respectively. The former exhibits a defect dicubane-like core with two missing vertices, while the latter features a [Ni6O6] core with two Ni(II) ions attached to a [Ni4O4] cubane through oxygen atoms. Although the bridges are all the oxygen atoms, two compounds demonstrate overall different ferromagnetic and antiferromagnetic coupling interactions between the Ni(II) ions. Solvent plays an important role on the resulting structures and consequently the magnetic coupling interactions.
This work was supported by the National Natural Science Foundation of China (grant No. 20671048 and 21041002) and the Shandong Taishan Scholar Fund.
References
- (a) R. Sessoli, D. Gatteschi, A. Caneschi and M. A. Novak, Nature, 1993, 365, 141 CrossRef CAS; (b) L. K. Thompson, O. Waldmann and Z. Q. Xu, Coord. Chem. Rev., 2005, 249, 2677 CrossRef CAS; (c) H. Miyasaka, A. Saitoh and S. Abe, Coord. Chem. Rev., 2007, 251, 2622 CrossRef CAS; (d) C. E. Kostakis and A. K. Powell, Coord. Chem. Rev., 2009, 253, 2686 CrossRef; (e) T. Taguchi, W. Wernsdorfer, K. A. Abboud and G. Christou, Inorg. Chem., 2010, 49, 199 CrossRef CAS.
- (a) J. K. Tang, I. Hewitt, N. T. Madhu, G. Chastanet, W. Wernsdorfer, C. E. Anson, C. Benelli, R. Sessoli and A. K. Powell, Angew. Chem., Int. Ed., 2006, 45, 1729 CrossRef CAS; (b) C. J. Milios, A. Vinslava, P. A. Wood, S. Parsons, W. Wernsdorfer, G. Christou, S. P. Perlepes and E. K. Brechin, J. Am. Chem. Soc., 2007, 129, 8 CrossRef CAS; (c) P. H. Lin, T. J. Burchell, R. Clérac and M. Murugesu, Angew. Chem., Int. Ed., 2008, 47, 8848 CrossRef CAS; (d) D. E. Freedman, D. M. Jenkins, A. T. Lavarone and J. R. Long, J. Am. Chem. Soc., 2009, 130, 2884 CrossRef; (e) T. Taguchi, W. Wernsdorfer, K. A. Abboud and G. Christou, Inorg. Chem., 2010, 49, 199 CrossRef CAS.
- (a) S. Wang, J. L. Zuo, H. C. Zhou, H. J. Choi, Y. X. Ke, J. R. Long and X. Z. You, Angew. Chem., Int. Ed., 2004, 43, 5940 CrossRef CAS; (b) Y. Z. Zhang, W. Wernsdorfer, F. Pan, Z. M. Wang and S. Gao, Chem. Commun., 2006, 3302 RSC; (c) M. H. Zebg, M. X. Yao, H. Liang, W. X. Zhang and X. M. Chen, Angew. Chem., Int. Ed., 2007, 46, 1832 CrossRef; (d) M. Morimoto, H. Miyasaka, M. Yamashita and M. Irie, J. Am. Chem. Soc., 2009, 131, 9823 CrossRef CAS; (e) D. C. Li, H. S. Wang, S. N. Wang, Y. P. Pan, C. J. Li, J. M. Dou and Y. Song, Inorg. Chem., 2010, 49, 3688 CrossRef CAS; (f) S. N. Wang, L. Q. Kong, H. Yang, Z. T. He, Z. Jiang, D. C. Li, S. Y. Zeng, M. J. Niu, Y. Song and J. M. Dou, Inorg. Chem., 2011, 50, 2705 CrossRef CAS.
- (a) J. Zhang, P. Teo, R. Pattacini, A. Kermagoret, R. Welter, G. Rogez, T. S. A. Hor and P. Braunstein, Angew. Chem., Int. Ed., 2010, 49, 4443 CrossRef CAS; (b) V. Mereacre, Y. H. Lan, R. Clérac, A. M. Ako, J. Hewitt lan, W. Wernsdorfer, G. Buth, C. E. Anson and A. K. Powell, Inorg. Chem., 2010, 49, 5293 CrossRef CAS; (c) A. Ray, S. Banerjee, G. M. Rosair, V. Gramlich and S. Mitra, Struct. Chem., 2008, 19, 459 CrossRef CAS; (d) Y. Lan, G. Novitchi, R. Clérac, J. K. Tang, N. T. Madhu, I. J. Hewitt, C. E. Anson, S. Brooker and A. K. Powell, Dalton Trans., 2009, 1721 RSC; (e) C. J. Milios, S. Piligkos and E. K. Brechin, Dalton Trans., 2008, 1809 RSC.
- (a) S. Basak, S. Sen, G. Rosair, C. Desplanches, E. Garribba and S. Mitra, Aust. J. Chem., 2009, 62, 366 CrossRef CAS; (b) S. Banerjee, M. Nandy, S. Sen, S. Mandal, G. M. Rosair, A. M. Z. Slawin, C. J. Gómez García, J. M. Clemente-Juan, E. Zangrando, N. Guidolin and S. Mitra, Dalton Trans., 2011, 40, 1652 RSC.
- (a) R. Boca, Coord. Chem. Rev., 2004, 248, 757 CrossRef CAS; (b) J. Krzystek, A. Ozarowski and J. Telser, Coord. Chem. Rev., 2006, 250, 2308 CrossRef CAS; (c) C. Papatriantafyllopoulou, L. F. Jones, T. Nguyen, N. Matamoros-Salvador, L. Cunha-Silva, F. A. A. Paz, J. Rocha, M. Evangelisti, E. K. Brechin and S. P. Perlepes, Dalton Trans., 2008, 3153 RSC; (d) B. Biswas, U. Pieper, T. Weyhermüller and P. Chaudhuri, Inorg. Chem., 2009, 48, 6781 CrossRef CAS.
- A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn and G. C. Verschoor, J. Chem. Soc., Dalton Trans., 1984, 1349 RSC.
- (a) G. Das, R. Shukla, S. Mandal, R. Singh and P. K. Bharadwaj, Inorg. Chem., 1997, 36, 323 CrossRef CAS; (b) B. Sarkar, M. S. Ray, M. G. B. Drew, A. Figuerola, C. Diaz and A. Ghosh, Polyhedron, 2006, 25, 3084 CrossRef CAS; (c) P. Mukherjee, M. G. B. Drew and A. Ghosh, Eur. J. Inorg. Chem., 2008, 3372 CrossRef CAS; (d) S. Naiya, B. Sarkar, Y. Song, S. Ianelli, M. G. B. Drewe and A. Ghosh, Inorg. Chim. Acta, 2010, 363, 2488 CrossRef CAS.
- (a) C. H. Chien, J. C. Chang, C. Y. Yeh, G. H. Lee, J. M. Fang, Y. Song and S. M. Peng, Dalton. Trans., 2006, 3429 Search PubMed; (b) C. Papatriantafyllopoulou, G. Aromi, A. J. Tasiopoulos, V. Nastopoulos, C. P. Raptopoulou, S. J. Teat, A. Escuer and S. P. Perlepes, Eur. J. Inorg. Chem., 2007, 2761 CrossRef CAS; (c) M. Mikuriya, K. Tanaka, N. Inoue, D. Yoshioka and J. W. Lim, Chem. Lett., 2003, 32, 126 CrossRef CAS; (d) V. Ovcharenko, E. Fursova, G. Romanenko, I. Eremenko, E. Tretyakov and V. Ikorskii, Inorg. Chem., 2006, 45, 5338 CrossRef CAS; (e) A. N. Georgopoulou, C. P. Raptopoulou, V. Psycharis, R. Ballesteros, B. Abarca and A. K. Boudalis, Inorg. Chem., 2009, 48, 3167 CrossRef CAS; (f) S. T. Zheng, D. Q. Yuan, H. P. Jia, J. Zhang and G. Y. Yang, Chem. Commun., 2007, 1858 RSC; (g) H. L. C. Feltham, R. Clérac and S. Brooker, Dalton Trans., 2009, 2965 RSC.
- (a) J. J. Borras-Almenar, J. M. Clemente-Juan, E. Coronado and B. S. Tsukerblat, Inorg. Chem., 1999, 38, 6081 CrossRef CAS; (b) J. J. Borrás-Almenar, J. M. Clemente-Juan, E. Coronado and B. Tsukerblat, J. Comput. Chem., 2001, 22, 985 CrossRef.
- (a) A. P. Ginsberg, J. A. Bertrand, R. I. Kaplan, C. E. Kirkwood, R. L. Martin and R. C. Sherwood, Inorg. Chem., 1970, 10, 240 CrossRef; (b) M. A. Halcrow, J. S. Sun, J. C. Huffman and G. Christou, Inorg. Chem., 1995, 34, 4167 CrossRef CAS; (c) J. M. Clemente-Juan, E. Coronado, J. R. Galan-Mascaros and C. J. Gomez-Garcia, Inorg. Chem., 1999, 38, 55 CrossRef CAS; (d) U. Kortz, Y. P. Jeannin, A. Teze, G. Herve and S. Isber, Inorg. Chem., 1999, 38, 3670 CrossRef CAS; (e) P. Mukherjee, M. G. B. Drew, C. J. Gómez-García and A. Ghosh, Inorg. Chem., 2009, 48, 5848 CrossRef CAS; (f) B. Biswas, U. Pieper, T. Weyhermuller and P. Chaudhuri, Inorg. Chem., 2009, 48, 6781 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional figures of the compounds. CCDC reference numbers 795063–795064. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra01219g |
| ‡ Syntheses of 1 and 2: H3L1 (0.1903 g, 0.58 mmol) and three-ethyl amine (3 ml) were dissolved in 50 ml DMF. The solution was refluxed for 30 min under stirring. Then, Ni(OAc)2·4H2O (0.2489 g, 1 mmol) was added. This mixture was refluxed for 4 h under stirring. The green crystals of 1 were grown by slow diffusion of methanol into DMF solution. The red crystals of 2 were obtained from the DMF solution by slow evaporation at room temperature after one month. Crystal structure for 1: C42H52N4O10Ni4, Mr = 1007.72, monoclinic, space group P21/c, a = 13.0082(15), b = 12.0689(12), c = 13.7934(17) Å, β = 107.5830(10), V = 2064.3(4) Å3, Z = 2, Dc = 1.621 g cm−3, F(000) = 1048, R1 = 0.0431, wR2 = 0.1028 for 3643 reflections with I > 2σ(I), GOF = 1.000. 2: C73H93N11O17Ni6, Mr = 1748.84, orthorhombic, space group Pbcn, a = 27.556(3), b = 16.8879(16), c = 17.3304(19) Å, V = 8065.0(14) Å3, Z = 4, Dc = 1.440 g cm−3, F(000) = 3648, R1 = 0.0829, wR2 = 0.2091 for 7131 reflections with I > 2σ(I), GOF = 1.000. |
| This journal is © The Royal Society of Chemistry 2012 |
