Lineariifolianoids A–D, rare unsymmetrical sesquiterpenoid dimers comprised of xanthane and guaiane framework units from Inula lineariifolia†
Jiang-Jiang
Qin
ac,
Ying
Huang
a,
Dan
Wang
b,
Xiang-Rong
Cheng
a,
Qi
Zeng
a,
Shou-De
Zhang
a,
Zhen-Lin
Hu
b,
Hui-Zi
Jin
*a and
Wei-Dong
Zhang
*ab
aSchool of Pharmacy, Shanghai Jiao Tong University, Shanghai 200240, P. R. China. E-mail: wdzhangy@hotmail.com; kimhz@sjtu.edu.cn; Fax: +86 (0)21 34205989; Tel: +86 (0)21 34205989
bDepartment of Phytochemistry, School of Pharmacy, Second Military Medical University, Shanghai 200433, P. R. China
cDepartment of Pharmaceutical Sciences, School of Pharmacy, Texas Tech University Health Sciences Center, Amarillo, TX 79106, USA
First published on 6th January 2012
Abstract
Lineariifolianoids A–D (1–4), a rare class of unsymmetrical sesquiterpenoid dimers with xanthane and guaiane frameworks have been isolated for the first time from Inula lineariifolia. Notably, lineariifolianoid D (4) features the first exo-adduct of such dimers through a plausible Diels–Alderase catalyzed biogenetic pathway. Moreover, the absolute configuration of japonicone A (5) has been revised by the Flack method. The inhibitory effects of the new isolates on TNF-α-mediated L929 cytotoxicity have also been evaluated and only the exo-adduct lineariifolianoid D (4) displays the inhibitory property.
Sesquiterpenoid dimers have been reported to be a characteristic class of ingredients of the genus Inula (Asteraceae family), and exhibit more ‘drug-like’ and ‘biologically friendly’ molecular features compared with their monomers and they have recently received considerable interest.1 In continuation of the study on the intriguing bioactive sesquiterpenoid dimers from this family, four new sesquiterpenoid dimers, lineariifolianoids A–D (1–4) (Fig. 1), have been isolated for the first time from I. lineariifolia Turcz., widely distributed in China and referred to as the traditional Chinese medicine “JinFeiCao”.2 Notably, lineariifolianoids A–D (1–4) represent a rare unsymmetrical sesquiterpenoid dimers class, comprising of xanthane and guaiane units with the linkage mode C–11–C–3′. So far, to our knowledge, only three cases of xanthane–guaiane dimers while with a linkage of C–11–C–4′ were reported two decades ago and their absolute configurations have yet to be determined.3 Below are disclosed the details of the isolation, structure elucidation, and inhibitory effect evaluation on TNF-α-mediated L929 cytotoxicity of the new lineariifolianoids. Moreover, the revision of japonicone A's absolute structure has also been discussed.
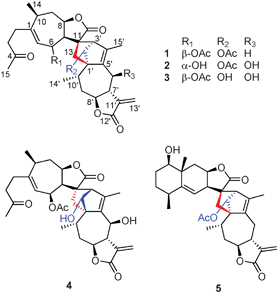 | ||
| Fig. 1 The structures of compounds 1–5. | ||
Lineariifolianoid A (1) was obtained as colorless monoclinic crystal, giving an [M + Na]+ ion in the HRESIMS at m/z 617.2746, appropriate for a molecular formula of C34H42O9 with14 degrees of unsaturation. The IR spectrum showed the presence of hydroxyl (3437 cm−1), carbonyl (1751 cm−1), and olefinic (1637 cm−1) functionalities. The 13C NMR spectrum of 1 exhibited 34 carbon signals in agreement with the HRESIMS data (Table S1, ESI†). The 1H and 13C NMR spectra also suggested the presence of two acetoxy groups (δH 2.01, δC 169.9 and 21.0; δH 2.01, δC 170.0 and 21.1). Their positions were determined by HMBC correlations (Fig. 2). A further analysis of 1D and 2D NMR data of the remaining 30 carbon signals revealed that they belonged to two different sesquiterpenoid moieties, A1 and B1 (Fig. 2). Compared with the NMR data of related japonicone A (5) isolated from I. japonica, an identical guaianolide framework A1 with a characteristic α-methylene lactone functionality can be authenticated by olefinic carbons C–11′ (δC 139.4), C–13′ (δC 119.5), and exocyclic olefinic protons H–13′a (δH 6.25) and H–13′b (δH 5.52).1c1H–1H COSY and HMBC correlations (Fig. 2) enabled the determination of the structure of the B1 fragment, by comparison with inuchinenolide A,4 previously isolated from the same plant.5 The NMR data of moiety B1 mainly differed in the chemical shifts of C–11 and C–13 from inuchinenolide A, suggesting that the two moieties were connected through these units. The key HMBC correlations from H–2′ to C–11 and C–13, H–3′ to C–7 and C–13, and H2–13 to C–2′ and C–5′ indicated the presence of a bridged ring norbornene system.1
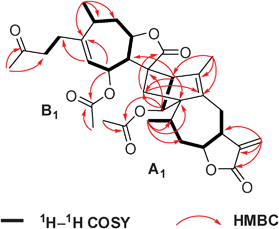 | ||
| Fig. 2 Key 1H–1H COSY and HMBC correlations of 1. | ||
The relative configuration of 1 was determined by NOESY NMR experiment (Fig. S1, ESI†) and further confirmed by single X-ray crystallography study (Fig. 3). These studies suggested that 1 was an endo-cylcoadduct of xanthane and guaiane from a Diels–Alder reaction.6 The strong NOESY correlations of H–7 with H–6, H–8, and H–10; and of H–8 with H–10 were observed in moiety B1, indicating that H–6, H–7, H–8, and H–10 adopted the same orientation, arbitrarily designated as α-orientation. In addition, the correlations of H–2′ with H–3′ and H3–14′; of H–3′ with H–7 and H–8; and of H–8′ with H–6′α and H3–14′ concluded that H–2′, H–3′, H–8′ and H3–14′ were also α-orientated. On the other hand, the correlations of H–7′ with H–6′β and H–9′β revealed the trans-fused lactone ring of moiety A1. The relative configuration of 1 established by NOESY NMR experiments was in good agreement with that determined by X-ray diffraction study with copper radiation (CCDC 806236) (Fig. 3). The absolute configuration of 1 was determined to be 6S, 7R, 8R, 10S, 11R, 1′S, 2′S, 3′R, 7′R, 8′S, and 10′S, respectively, which were deduced from the Flack parameter, 0.04 (14), refined using 2359 Friedel pairs.7
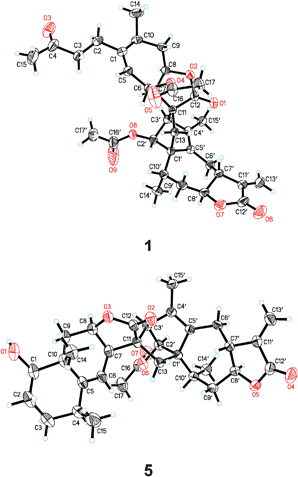 | ||
| Fig. 3 Single X-ray crystallographic structures of 1 and 5 showing their absolute configurations. | ||
Surprisingly, the obtained experimental data was inconsistent with the structurally related sesquiterpenoid dimer, japonicone A (5), whose absolute configuration was previously established by the modified Mosher method.1b In order to solve the contradiction between 1 and 5, an X-ray crystallographic analysis of 5 (CCDC 823557) (Fig. 3) as an anomalous dispersion with copper radiation was performed [Flack parameter, 0.10 (14)]. The careful analysis led to the exactly opposite of the reported result and accordingly the revised absolute configuration of 5, 1R, 4S, 7R, 8R, 10R, 11S, 1′S, 2′S, 3′R, 7′R, 8′S, and 10′S was proposed.
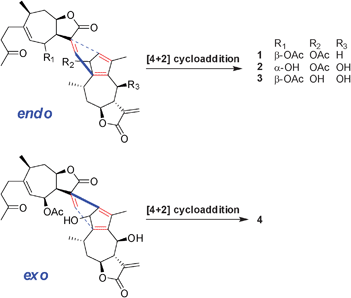
Lineariifolianoids B–D (2, 3 and 4) have the same molecular formula C32H40O9, established from their HRESIMS at m/z 591.2566, 591.2587, and 591.2576 [M + Na]+, respectively, possessing 13 degrees of unsaturation. Comparison of their 1D and 2D NMR data with those of 1 led to establishing their structures (Table S1 and Fig. S3–S5, ESI†). The NMR spectra of the three compounds suggested the presence of only one acetoxy group, and their positions were determined by the HMBC experiments (Fig. S3–S5, ESI†). In addition, the presence of oxygenated methines resonating at δC 69.7, 69.9, and 68.4 (C–6′) in the respective guaianolide substructures A2, A3, and A4 (see Fig. S3–S5, ESI†) of 2, 3, and 4, along with the absence of a methylene at δC 26.0 (C–6′) of 1, indicated that they all had an additional hydroxyl substituent at C–6′. The conclusions were further drawn by analyses of their 2D NMR data. Compared with 1, similar NOESY patterns allowed us to determine the relative configurations of 2 and 3 (Fig. S2 and 4, respectively, ESI†). They were also the dimers of the endo-adducts of xanthane and guaiane. The important NOESY correlations of H–8′ with H–6′ of the two compounds suggested the hydroxyl at C–6′ was β-orientation, while in the 1H NMR spectrum of 2 (Table S1, ESI†), the coupling constant between H–6 and H–7 changed to 9.6 Hz (but 0 Hz in 1), indicating that the hydroxyl at C–6 was α-orientation. Detailed comparison analyses of 2D NMR data of 3 and 4 (Fig. S4 and S5, respectively, ESI†) also suggested that 4 had the same planar structure as 3. However, the significant differences were also observed in the NMR resonances of their corresponding norbornene structures (Table S1, ESI†), mainly from C–11 to C–13 and from C–1′ to C–4′. These observations implied that the xanthane and guaiane units of 4 were cycloadded in an exo fashion. The absence of the important correlations of H–3′ with H–7 and H–8 in the NOESY spectrum of 4 (Fig. 4) further confirmed the above supposition. Thus, compound 4 was identified as the first exo-adduct with xanthane and guaiane units.
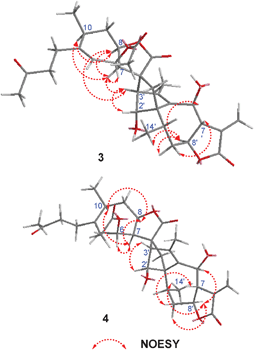 | ||
| Fig. 4 Key NOESY correlations of 3 and 4. | ||
It has been documented that the sesquiterpenoid dimers, a group of naturally occurring metabolites with C30 cores, almost always originate biosynthetically from two identical or different sesquiterpenoid units. The Diels–Alder reaction, as a powerful biological tool, has exhibited its versatility during the formation of such natural products.6b In the previous investigation, all the sesquiterpenoid dimers from the Inula genus have been reported to be formed by endo-selective Diels–Alder reactions, which are attributed to the most stable transition states.1,8 In contrast, the unusual natural exocycloaddition catalyzed by Diels–Alderase in the Inula genus has not been reported despite the fact that they have been occasionally observed from other plants.9
As a rare unsymmetrical sesquiterpenoid dimers class, comprised of xanthane and guaiane units with the linkage mode C–11–C–3′, lineariifolianoids A–D (1–4) are presumably formed by [4 + 2] cycloaddition of xanthane and guaiane catalyzed by Diels–Alderase as proposed in Scheme 1. Lineariifolianoids A–C (1–3) are constructed by a commonly observed endo-selective Diels–Alder reaction; nevertheless, lineariifolianoid D (4) is biosynthesized in an unusual exo fashion and serves as the first exo-adduct of such xanthane–guaiane dimers from a natural source.
 | ||
| Scheme 1 Plausible biogenetic pathway of Lineariifolianoids A–D (1–4). | ||
In the previous study, japonicone A was identified as a novel TNF-αantagonist by directly binding to TNF-α and therefore blocking TNF-α-mediated activities in cell-based assays. To determine whether lineariifolianoids have similar activities, in preliminary investigations, we accordingly tested the inhibitory effects on the TNF-α-sensitive L929 cells.10 The obtained results showed that the only exo-adduct, lineariifolianoid D (4), inhibited TNF-α-mediated cytotoxicity in a dose-dependent manner from 2.5 to 10 μM, but the three endo-adducts 1–3 didn't exhibit such property (Fig. S6, ESI†).
In conclusion, a rare unsymmetrical sesquiterpenoid dimer class, comprising of xanthane and guaiane units with the linkage mode C–11–C–3′, have been isolated and elucidated for the first time. Notably, an unusual exo-Diels–Alder adduct, lineariifolianoid D (4) comprising of xanthane–guaiane dimers from the Asteraceae family is discovered for the first time. Such a configuration is also critical for the observed TNF-α-mediated cytotoxicity. Moreover, we have revised the structure of japonicone A. Further structural and biological investigations of these intriguing compounds are being carried out in our laboratory and the results will be reported in due course.
The work was supported by NSFC (30725045), the Special Program for New Drug Innovation of the Ministry of Science and Technology, China (2009ZX09311-001, 2008ZX09101-Z-029, 2009ZX09103-375), National comprehensive technology platforms for innovative drug R&D, 2009ZX09301-007, Shanghai Leading Academic Discipline Project (B906), and in part by the Scientific Foundation of Shanghai Committee of Science and Technology (08DZ1971302, 09DZ1975700, 09DZ1971500, 09DZ1972200).
References
- (a) J. J. Qin, L. Y. Wang, J. X. Zhu, H. Z. Jin, J. J. Fu, X. F. Liu, H. L. Li and W. D. Zhang, Chem. Commun., 2011, 47, 1222–1224 RSC; (b) J. J. Qin, H. Z. Jin, J. X. Zhu, J. J. Fu, X. J. Hu, X. H. Liu, Y. Zhu, S. K. Yan and W. D. Zhang, Planta Med., 2010, 76, 278–283 CrossRef CAS; (c) J. J. Qin, H. Z. Jin, J. J. Fu, X. J. Hu, Y. Wang, S. K. Yan and W. D. Zhang, Bioorg. Med. Chem. Lett., 2009, 19, 710–713 CrossRef CAS; (d) H. Z. Jin, D. Lee, J. H. Lee, K. Lee, Y. S. Hong, D. H. Choung, Y. H. Kim and J. J. Lee, Planta Med., 2006, 72, 40–45 CAS.
- Y. Ling, in Flora of China, ed. Y. Ling, Science Press, Beijing, 1979, vol. 75, pp. 265–266 Search PubMed.
- (a) J. Jakupovic, C. Zdero, M. Grenz, F. Tsichritzis, L. Lehmann, S. M. Hashemi-Nejad and F. Bohlmann, Phytochemistry, 1989, 28, 1119–1131 CrossRef CAS; (b) C. Zdero and F. Bohlmann, Phytochemistry, 1989, 28, 3105–3120 CrossRef CAS.
- K. Ito and T. Iida, Phytochemistry, 1981, 20, 271–273 CrossRef CAS.
- L. Y. Nie, J. J. Qin, Y. Huang, L. Yan, Y. B. Liu, Y. X. Pan, H. Z. Jin and W. D. Zhang, J. Nat. Prod., 2010, 73, 1117–1120 CrossRef CAS.
- (a) F. Fringuelli and A. Taticchi, in The Diels–Alder Reaction: Selected Practical Methods, ed. F. Fringuelli and A. Taticchi, John Wiley & Sons, West Sussex, 2002, chp. 1, 14–15 Search PubMed; (b) H. Oikawa and T. Tokiwano, Nat. Prod. Rep., 2004, 21, 321–352 RSC.
- H. D. Flack, Acta Crystallogr., Sect. A: Found. Crystallogr., 1983, A39, 876–881 CrossRef CAS.
- (a) B. N. Su, Y. Takaishi, M. Tori, S. Takaoka, G. Honda, M. Itoh, Y. Takeda, O. K. Kodzhimatov and O. Ashurmetov, Tetrahedron Lett., 2000, 41, 1475–1479 CrossRef CAS; (b) B. Fu, B. N. Su, Y. Takaishi, G. Honda, M. Ito, Y. Takeda, O. K. Kodzhimatov and O. Ashurmetov, Phytochemistry, 2001, 58, 1121–1128 CrossRef CAS.
- (a) C. Zdero, F. Bohlmann and H. M. Niemeyer, Phytochemistry, 1991, 30, 1597–1601 CrossRef CAS; (b) M. Li, J. S. Zhang and M. Q. Chen, J. Nat. Prod., 2001, 64, 971–972 CrossRef CAS; (c) S. B. Singh, D. L. Zink, B. Heimbach, O. Genilloud, A. Teran, K. C. Silverman, R. B. Lingham, P. Felock and D. J. Hazuda, Org. Lett., 2002, 4, 1123–1126 CrossRef CAS.
- F. Denizot and R. J. Lang, Immunol. Methods, 1986, 89, 271–277 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, plant material, crystallographic data (1 and 5), cytotoxicity assay (1–4), and 1D and 2D NMR spectra of 1–4. CCDC reference numbers 806237 and 823557. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra01014c |
| This journal is © The Royal Society of Chemistry 2012 |
