Stabilized cycling performance of silicon oxide anode in ionic liquid electrolyte for rechargeable lithium batteries
Jin-Woo
Song
a,
Cao Cuong
Nguyen
b and
Seung-Wan
Song
*ab
aGraduate School of Green Energy Technology, Chungnam National University, Daejeon, 305-764, South Korea
bDept. of Fine Chemical Engineering & Applied Chemistry, Chungnam National University, Daejeon, 305-764, South Korea. E-mail: swsong@cnu.ac.kr; Fax: +82 42 822 6637; Tel: +82 42 821 7008
First published on 11th January 2012
Abstract
Control of electrode–electrolyte interfacial stability and the composition of the solid electrolyte interphase (SEI) layer is a promising approach for improved cycling performance of silicon-based anode material for rechargeable lithium batteries. Here we demonstrate that a room temperature ionic liquid electrolyte effectively passivates the surface of the SiO1.3 electrode and significantly enhances cycling ability in contrast to the commercial liquid electrolyte. The SiO1.3 electrode, prepared by pulsed laser deposition, showed 88% capacity retention in the ionic liquid electrolyte of 1 M LiTFSI/Py13TFSI delivering 1058–930 mA h g−1 over 200 cycles. Results from infrared and X-ray photoelectron spectroscopic analyses suggest that the presence of organic SEI compounds consisting of the pyrrolidinium cation and TFSI anion and their decomposition products on the oxygen-abundant SiO1.3 surface confers interfacial stability and cycling stability.
1. Introduction
Scaling-up of rechargeable lithium batteries toward electric vehicle and large format energy storage systems demands the development of higher energy density electrode materials with a more stable cycling ability than conventional ones. Silicon (Si) has been recognized as an interesting alternative anode material to graphite due to its high theoretical capacity (3579 mA h g−1) for Li15Si4 at room temperature1 and appropriate operation voltage above lithium. Severe structural volume change during alloying/dealloying with lithium ions however causes the cracking of Si particles and subsequent mechanical failure of the electrode structure and electrical disconnection between LixSi particles, and the LixSi particles and current collector. Those failure modes result in a rapid capacity fade and short cycle life, which need to be overcome for a practical use.1,2Alternatively, silicon monoxide (SiO) attracts a particular attention, since it provides improved cycling ability compared to Si. Recent reports showed that the specific discharge capacity of SiO was ∼600 mA h g−1,3–6 which is still higher than that of graphite. It was also reported that carbon composites with commercial SiO or carbon coating on the SiO particles improved the cycling performance.7,8 Commercial SiO material is best considered to be composed of homogeneously dispersed angstrom-scale domains of a half Si and another half SiO2. Intermediates (e.g., Li2O) that in-situ form during the reaction of the SiO and Li may behave as a buffer accommodating the volume change in the LixSi.3–6 Greater cycling ability can be achieved when more oxygen is included to silicon oxide. SiO2 with the highest oxygen content is however an insulator and has shown limited lithium storage capacity (<500 mA h g−1)9 compared to SiO. An oxygen concentration level of more than one mole but far smaller than two moles, (e.g., SiO1+y) is estimated to be effective in enhancing the cycling stability.
We have been able to prepare the SiO1.3 film electrode by pulsed laser deposition (PLD). Even with the oxygen content more than one, the film electrode shows a large initial discharge (lithiation) capacity of 1058 mA h g−1. Film electrode prepared with pulsed laser deposition (PLD), which generally possesses a strong physical interfacial adherence to an electronically conductive substrate, is a “pure” material. Film electrode as a model system permits the observation of the material's intrinsic electrochemical processes while inhibiting a peel-off event of the film during extended cycling in lithium cells. Since the film electrode generally possesses enlarged surface to volume ratio, electrochemical studies of film electrode combined with spectroscopic analysis provides clearer insight into electrode–electrolyte interfacial reaction and convenient analyses of the solid electrolyte interphase (SEI) composition without complications from carbon and polymeric binder additives that are necessary in bulk materials.10–15 Interfacial processes of the Si film electrode with the conventional carbonate-based liquid electrolyte (EL) have been established as the continued formation of the LiPF6-derived surface species with cycling is one of the causes responsible for the particle disconnection and performance fade.12–14
Electrode–electrolyte interfacial control for the formation of a stable SEI layer is a promising approach to obtain cycle life and safety of batteries.16 Room temperature ionic liquid (IL) electrolyte is of particular interest for an alternative electrolyte to conventional carbonate-based liquid electrolyte (EL) because of their non-flammability, and high electrochemical and thermal stabilities17,18 that are necessary for the establishment of non-flammable batteries. It was reported that room temperature ILs, consisting particularly of pyrrolidinium or piperidinium cation, and bis(trifluoromethanesulfonyl)imide (TFSI) or bis(fluorosulfonyl)imide (FSI) anion, are cathodically stable with various battery anodes (e.g., Li, graphite, etc.)19–25 including Si.26,27 Studies on the interfacial compatibility between silicon oxide and IL and the formation of the SEI layer, and their impacts on the cycling performance are yet to be reported. Studies of the SEI formation and composition in the IL should permit a basic understanding of the interfacial reaction and cycling behavior of silicon oxide. This would pave the way for the enhancement of the cycling performance, lifetime and safety of rechargeable lithium batteries employing an silicon oxide anode for wider use in future batteries.
Here we report the enhanced cycling performance of the SiO1.3 film electrode in a pyrrolidinium-based IL. The reasons for the cycling stability in the IL are discussed in the aspect of interfacial reaction behavior and the SEI composition on the SiO1.3 electrode.
2. Experimental
The 70 nm thick films of the SiO1.3 on a stainless steel substrate were prepared using PLD at 400 °C with a 30 min deposition in 5 mtorr of O2 back pressure, using a KrF excimer laser with an energy density of ∼3–4 mJ cm−2 at 10 Hz impinging on the targets that consisted of Si and SiO2 in an equal molar ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The surface Si valence of the as-prepared film (pristine) was determined by X-ray photoelectron spectroscopic (XPS) analysis. The spectra were recorded with MultiLab 2000 with Al Kα X-ray source at an output power of 200 W under a pressure of 2.0 × 10−9 mbar. The irradiated spot size was about 320 μm and the pass energy was 20 eV. The binding energy was calibrated based on the C 1s level at 284.7 eV. The Si 2p spectrum was fitted after correcting the baseline using the Shirley method until the goodness-of-fit (χ2) was minimized. Structural information was obtained from Raman spectroscopy. The spectra were recorded using a Raman microscope (Nanofinder 30, Tokyo Instrument Co.), using the 632 nm line of a He–Ne laser at 1 mW. Backscattering optics geometry with a double-notch filter and a standard CCD detector were used to collect, process, and analyze the Raman signal. The size of the laser beam at the sample was <200 nm. Particle morphology of the active materials was examined using field emission scanning electron microscopic (SEM, JEOL JSM-7000F) imaging at 5 kV.
1). The surface Si valence of the as-prepared film (pristine) was determined by X-ray photoelectron spectroscopic (XPS) analysis. The spectra were recorded with MultiLab 2000 with Al Kα X-ray source at an output power of 200 W under a pressure of 2.0 × 10−9 mbar. The irradiated spot size was about 320 μm and the pass energy was 20 eV. The binding energy was calibrated based on the C 1s level at 284.7 eV. The Si 2p spectrum was fitted after correcting the baseline using the Shirley method until the goodness-of-fit (χ2) was minimized. Structural information was obtained from Raman spectroscopy. The spectra were recorded using a Raman microscope (Nanofinder 30, Tokyo Instrument Co.), using the 632 nm line of a He–Ne laser at 1 mW. Backscattering optics geometry with a double-notch filter and a standard CCD detector were used to collect, process, and analyze the Raman signal. The size of the laser beam at the sample was <200 nm. Particle morphology of the active materials was examined using field emission scanning electron microscopic (SEM, JEOL JSM-7000F) imaging at 5 kV.
Lithium
cells containing the SiO1.3 film model electrode as a working electrode were examined for their charge (lithiation) and discharge (delithiation) ability using constant current cycling at 35 μA cm−2 (∼1.7 C rate) between 0.1 and 1.5 V vs.Li/Li+ in 1 M LiPF6/ethylene carbonate (EC):ethyl methyl carbonate (EMC) in 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 volume ratio (Techno Semichem) and 1 M lithium bis(trifluoromethylsulfonyl)imide (LiTFSI, Aldrich)/1-methyl-1-propylpyrrolidinium bis(trifluoromethylsulfonyl)imide (Py13TFSI, Citri), denoted as LiTFSI/Py13TFSI henceforth, with lithium reference and counter electrodes using a multichannel galvanostatic cycler (Won-A Tech). The rate capability of the SiO1.3 electrode was examined by cycling at the variable charge rates of 1.7 C, 3 C, 6 C and 15 C, while fixing the discharge rate as the 1.7 C. Note that the 1 C rate refers to the current density of 1132 mA g−1 applied to the cell to reach to the charge voltage of 0.1 V vs.Li/Li+ in 1 h. Electrolyte preparation, cell assembly/disassembly, electrochemical testing and sample preparation for ex-situ analyses were carried out in an argon-filled glove box.
7 volume ratio (Techno Semichem) and 1 M lithium bis(trifluoromethylsulfonyl)imide (LiTFSI, Aldrich)/1-methyl-1-propylpyrrolidinium bis(trifluoromethylsulfonyl)imide (Py13TFSI, Citri), denoted as LiTFSI/Py13TFSI henceforth, with lithium reference and counter electrodes using a multichannel galvanostatic cycler (Won-A Tech). The rate capability of the SiO1.3 electrode was examined by cycling at the variable charge rates of 1.7 C, 3 C, 6 C and 15 C, while fixing the discharge rate as the 1.7 C. Note that the 1 C rate refers to the current density of 1132 mA g−1 applied to the cell to reach to the charge voltage of 0.1 V vs.Li/Li+ in 1 h. Electrolyte preparation, cell assembly/disassembly, electrochemical testing and sample preparation for ex-situ analyses were carried out in an argon-filled glove box.
Surface characterization for the electrodes cycled at the 1.7 C rate was performed using ex-situ attenuated total reflectance (ATR) FTIR spectroscopy using an IR spectrometer (Bruker optics IFS66V/S) equipped with an MCT detector. The cycled electrodes were separated from the cells and washed with dimethyl carbonate (DMC, Techno Semichem) for 60 s for the removal of residual electrolyte followed by drying in the glove box at room temperature. The cycled electrodes were directly mounted on the tightly closed ATR unit in the glove box to avoid atmospheric contamination.
Surface compositional analysis of the cycled electrode was conducted using ex-situXPS by transferring the sample from the glove box to the XPS chamber using a vacuum-sealed container without exposure to air. The changes with cycling in the surface morphology of the SiO1.3 electrode were examined using ex-situfield emission SEM (JEOL JSM-7000F) at 5 kV.
3. Results & discussion
3.1. Composition and structural characterization
The surface of the pristine SiO1.3 film appears smooth as shown in Fig. 1a. Composition was determined using the Si 2p XPS depth profile from the bulk (<5 nm depth) of the film, which was obtained by Ar sputtering for 20 s. The experimental peak shape of Si 2p3/2 was modeled by employing multiple-splitting patterns derived for Si0, Si+, Si2+, Si3+ and Si4+ at 99.2, 100.1, 101.2, 102.2 and 103.1 eV from the standard compounds of the Si, Si2O, SiO, Si2O3 and SiO2, respectively.28–31 The individual peak components show full width at half maximum (FWHM) values of 1.6–1.7 eV, in correspondence with the values reported in similar studies.30,31Fig. 1b exhibits the original spectrum of the film with the fitted one. Average valence of the Si is determined to be 2.6 and the composition of the silicon oxide is thus SiO1.3. Parts of the Si atoms from the target seem to react with oxygen atoms with either full oxidization to form SiO2 or partial oxidation to form Si suboxides during PLD. Fig. 1c and d show elemental mapping results for the SiO1.3 film. Homogeneous distribution of Si (red) and O (green) atoms indicates the homogeneity in composition. | ||
| Fig. 1 SEM surface image (a), XPS Si 2p spectral curve fitting (b), and electron mapping of the (c) Si (red) and (d) O (green) elements for the pristine SiO1.3 film. | ||
The Raman spectrum of the as-prepared SiO1.3 film is compared with an amorphous Si film as a reference in Fig. 2. General spectral features of the SiO1.3 film are alike to that of amorphous Si,13,14,26 where broad bands are originated from structural disorder due to different environments around the Si atom probably together with surface dangling bonds. The Si atoms in the SiO1.3 have a lower probability of having Si neighbors than in the Si film. Note that the SiO2 generally shows broad bands at 490, 810, 1065, and 1200 cm−1 assigned to the SiO2 random network structure, and sharp bands at ∼495 and ∼610 cm−1 attributed to fourfold and threefold ring defects formed in the SiO2 network.32,33 In Fig. 2, a somewhat sharp band at 602 cm−1, and broad bands at 810 and 1063 cm−1, which are not observed for the amorphous Si, are direct evidence for the presence of fourfold and/or threefold ring defects of the SiO2 and random network structure. A broad band near 466–481 cm−1 is ascribed to the Si–Si clusters from both amorphous Si and SiO2/SiO.34 The SiO1.3 film is thus determined to be a mixture of homogeneously distributed Si, SiO and SiO2, and/or non-stoichiometric suboxides, consistent with the multivalence of the Si atom observed from the XPS data.
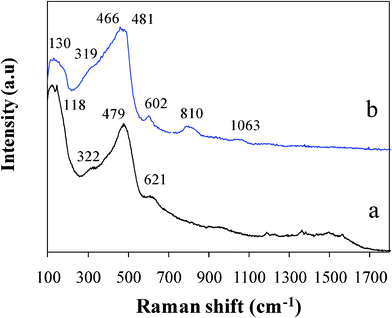 | ||
| Fig. 2 Raman spectra of (a) the Si film prepared by PLD as a reference and (b) the pristine SiO1.3 film. | ||
3.2. Cycling behavior
Voltage profiles of a lithium cell with the SiO1.3 film electrode in IL are shown in Fig. 3a. Initial charge and discharge capacities are 2010 and 1058 mA h g−1, respectively. The initial charge curve shows a negative potential overshoot, attributable to the nucleation and formation of a new phase (e.g., Li2O), as observed in SiO.35 In differential capacity (dQ/dV) plots, Fig. 3b, a shoulder at 0.5–0.3 V marked with an arrow is observed in the initial charge curve, but it disappears in the next cycles. This phenomenon is associated with electrolyte reduction that expends extra charge capacity while passivating the electrode surface and probably forming the SEI layer. According to the ab initio calculation by Howlett et al.,36TFSI is less stable than Py13 toward reduction and the TFSI reductive decomposition begins at a potential higher than ∼1 V vs.Li/Li+. On the SiO1.3 film electrode, the reductive decomposition of the TFSI and Py13 ions seems to occur most actively at 0.5–0.3 V, in Fig. 3b. A significant charge capacity peak below 0.3 V is due to the lithium reaction of the SiO1.3 forming new phases of lithium silicate and Li2O4–6 followed by the lithiation of Si forming LixSi. In the reverse process, a broad discharge peak at 0.4–1.0 V is due to delithiation from LixSi regenerating Si. This is followed by a low capacity discharge peak above 1.2 V by further delithiation toward the regeneration of SiO1.3. Irreversible initial capacity loss after charge is related to the presence of remaining lithium as LixSi and/or LixSiO1+y and initial electrolyte reduction during the lithiation process, resulting in a low initial Coulombic efficiency of ∼53%. After initial cycling, structural resolution of prominent peaks by lithiation and delithiation are quite consistent with 200 cycles. | ||
| Fig. 3 Voltage profiles (a) of lithium cells with the SiO1.3 electrode in 1 M LiTFSI/Py13TFSI between 0.1 and 1.5 V vs.Li/Li+, and (b) differential capacity (dQ/dV) plots at different cycle numbers. | ||
The cycling ability of the SiO1.3 electrode in IL is displayed in Fig. 4a and b, and compared with that in EL. In the IL, capacity retention at the 200th cycle is 88% (Fig. 4a), delivering 1058–930 mA h g−1 with maintained Coulombic efficiencies as 97% (Fig. 4b). In contrast, in the EL capacity rapidly fades with inferior efficiencies (Fig. 4b). The IL appears to play a role in preserving the electrode structure, via effective passivation of the electrode surface, and enhancing the cycling performance, as observed in Fig. 3b.
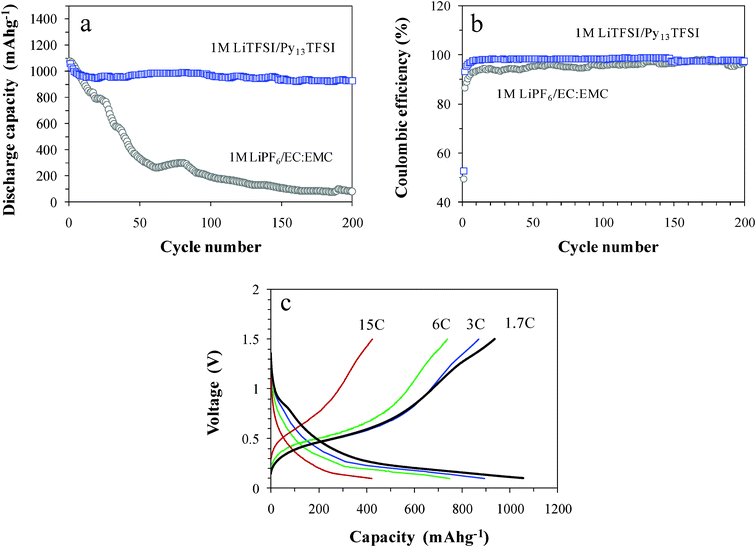 | ||
| Fig. 4 Plots of (a) discharge capacity and (b) Coulombic efficiency of lithium cells with the SiO1.3 electrodes in 1 M LiTFSI/Py13TFSI and 1 M LiPF6/EC:EMC as a function of cycle number, and (c) rate capability in 1 M LiTFSI/Py13TFSI. | ||
The rate capability of the SiO1.3 electrode, which is taken from the second cycle at each C-rate, is shown in Fig. 4c. The discharge capacity decreases with increasing the charge rate as 870, 737 and 424 mA h g−1 at the rates of 3 C, 6 C and 15 C, respectively. These correspond to 85, 71 and 39% of the discharge capacity at the 1.7 C rate, respectively. The discharge voltage plateau near 0.45 V, corresponding to the delithiation process, is maintained against high rates. Excellent rate performance is ascribed to the preservation of electrode structure by effective surface passivation and strong adherence of the electrode to the current collector substrate.
3.3. Ex-situ surface chemistry studies
Fig. 5 shows the IR spectra for the surface of the pristine SiO1.3 electrode and the electrodes cycled in IL and EL. Note that when the electrode was rinsed in DMC, soluble surface species could be washed off and just stable and insoluble surface precipitates remain. | ||
| Fig. 5 IR spectra for the surface of (a) the pristine SiO1.3 electrode, (b) cycled electrode in 1 M LiPF6/EC:EMC, (c) IL only of 1 M LiTFSI/Py13TFSI as a reference, and (d) cycled electrode in 1 M LiTFSI/Py13TFSI. | ||
For the surface of the pristine film, Fig. 5a, a broad feature ranging from 1250–950 cm−1 is due to the ν(Si–O) of the SiO1.3.31,37 Peak broadening is associated with the structurally disordered amorphous characteristics and the presence of different types of Si–O bonds by the multivalence of the Si atom27,37,38 as observed from the XPS and Raman spectra. A low absorbance peak at 825 cm−1 is attributed to the ν(Si–O) of the Si–OH silanol group.11,37 The surface of the pristine SiO1.3 film consists of native silicon oxides and silanols.
For the cycled electrode in the EL, Fig. 5b reveals broad and multiple low absorbance peaks at 1300–850 cm−1, mostly due to inorganic compounds. Peaks at 1262, 1020 and below 900 cm−1 are assigned to ν(P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), ν(P–O–C)asym and ν(P–O–C)sym, respectively, and peaks below 932 cm−1 to ν(P–F) probably in different molecular bonding modes.11–13,37 Those functionalities are ascribed to the LiPF6-derived decomposition products. This indicates that the resistive LiF also forms on the electrode surface by the following equilibrium and reaction with a trace amount of water; LiPF6 ⇆ LiF + PF5, PF5 + H2O → PF3O + 2HF.16 The strong Lewis acids PF5 and PF3O as well as the HF can attack the surface of the SiO1.3 electrode forming the PF- and/or F-containing surface species, consistent with our IR data. Tiny peaks at 2960–2850 cm−1 together with fingerprints at 1466 cm−1 are characteristic of CH3– methyl and –CH2– methylene groups for alkyl functionality.11–13,37 The data suggest that organic phosphorus fluorine compounds (–O
O), ν(P–O–C)asym and ν(P–O–C)sym, respectively, and peaks below 932 cm−1 to ν(P–F) probably in different molecular bonding modes.11–13,37 Those functionalities are ascribed to the LiPF6-derived decomposition products. This indicates that the resistive LiF also forms on the electrode surface by the following equilibrium and reaction with a trace amount of water; LiPF6 ⇆ LiF + PF5, PF5 + H2O → PF3O + 2HF.16 The strong Lewis acids PF5 and PF3O as well as the HF can attack the surface of the SiO1.3 electrode forming the PF- and/or F-containing surface species, consistent with our IR data. Tiny peaks at 2960–2850 cm−1 together with fingerprints at 1466 cm−1 are characteristic of CH3– methyl and –CH2– methylene groups for alkyl functionality.11–13,37 The data suggest that organic phosphorus fluorine compounds (–O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PF–OR, R = alkyl) are major surface species, similar to the Si.11–13 The absence of the peak for the ν(Si–O) from the pristine SiO1.3 indicates the full surface coverage of the electrode by new surface species.
PF–OR, R = alkyl) are major surface species, similar to the Si.11–13 The absence of the peak for the ν(Si–O) from the pristine SiO1.3 indicates the full surface coverage of the electrode by new surface species.
The cycled electrode in the IL in Fig. 5d however exhibits the remaining features of ν(Si–O) at 1179 cm−1 from the pristine SiO1.3. The surface of the cycled SiO1.3 must be partially covered by the SEI components and/or the surface layer is thinner than that in the EL. Peaks at 1581 and 1501 cm−1 together with fingerprints at 1446 and 1354 cm−1 and those at 2960–2850 cm−1 are due to the RCO2−Mn+ (M = Li/Si) carboxylate metal salt.11–13,27,37 This organic compound is the decomposition product of the Py13 cation, probably coupled with oxygen from surface oxygen of the electrode and/or the TFSI anion. Peaks from the Li2CO3 are overlapped at 1501 and 881 cm−1.37
Spectral comparison with the IL only (Fig. 5c) reveals that dominant multiple peaks at 1180–1050 cm−1 and tiny peaks at 1354–1333 cm−1 in Fig. 5d are the –SO2– and –CF3 functionalities from the TFSI anion, probably together with its decomposed fragments.22,27,37 Peaks at 1059 and 791 cm−1 attributed to ν(SNS) support this assignment. A shoulder below 1059 cm−1 may include the peaks due to the sulfinate salt –SO2−Mn+, sulfate salt M2(SO42−)n, and/or sulfite salt M2(SO32−)n.27,37 They might form by the combination of radicals that are produced on the electrochemical reduction of the TFSI anion.
IR analysis results show that the SEI layer formed in the IL is thin and/or partially covers the SiO1.3 electrode surface. The SEI layer is composed of relatively higher concentration levels of organic compounds than that in the EL, which originate from the Py13. The surface also includes high concentration levels of TFSI-derived species. The presence of original TFSI anion undecomposed indicates that the presence of oxygen in the SiO1.3 suppresses electron transfer-driven reduction of the TFSI anion, in contrast to the Si that exhibited all decomposition products of the Py13 cation and TFSI anion.20 The interfacial compatibility between IL and the SiO1.3, and thin but efficient passivation of the electrode surface with a stable SEI layer are reasons for the excellent cycling performance.
Fig. 6 shows XPS spectra for the top surface of the pristine SiO1.3, together with the cycled electrodes in IL and EL. The spectrum of the pristine SiO1.3 exhibits the presence of surface SiO2, i.e., oxygen-abundant surface. Comparison of the Si 2p spectra before and after cycling, regardless of electrolyte type, reveals that charge–discharge cycling tends to result in a red-shift of the peak by the presence of the LixSi, LixSiO1+y and/or the SEI compounds bonded to the surface Si. The appearance of a new C peak and enhancement of O and C peaks, in particular, on the cycled electrode in IL, indicates that the electrode surface is covered by new SEI components.
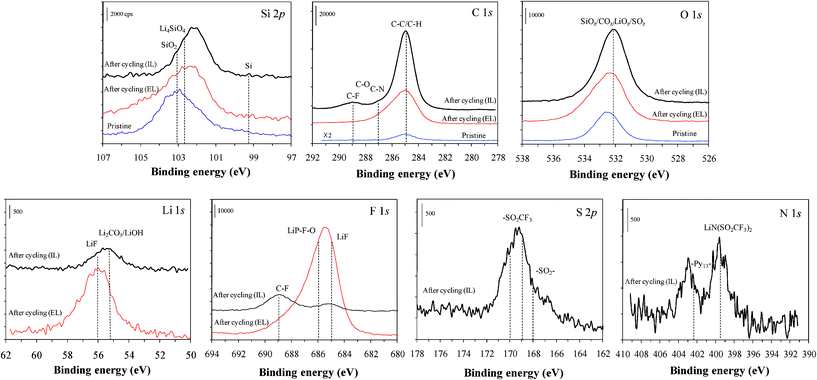 | ||
| Fig. 6 XPS high resolution spectra of the Si, C and O atoms for the top surface of the pristine SiO1.3 electrode (blue bottom lines), and cycled electrodes in 1 M LiPF6/EC:EMC (EL, red middle lines) and 1 M LiTFSI/Py13TFSI (IL, black uppermost lines), and the spectra of the Li, F, S and N atoms for the cycled electrodes. | ||
For the C 1s spectrum, several peaks around 283–290 eV are attributable to C–C/C–H, C–N, and C–O groups from C-containing compounds.27–29 The clear appearance of the new peak at 289 eV on the cycled electrode in IL is ascribed to the C–F whose source is the TFSI anion. In the F 1s spectrum, a broad peak at 688–691 eV, which is associated with the CF-containing compounds27,28 supports the presence of the C–F group.
In the Li 1s spectrum of the cycled electrodes in IL, a broad peak near 55–56 eV is attributable to the Li–O type bond of the Li2CO3/LiOH and O-containing organic compounds such as the carboxylate lithium salt or lithium sulfate/sulfite as observed from the IR data.27–29 A part of the peak is also attributed to the LiF28,29 as confirmed in the F 1s spectrum, and to the LixSi and/or LixSiOy. However, the cycled electrode in EL exhibits mainly an intense peak at 56 eV due to the Li–F type compound. A significant peak at 684–687 eV for the LiF, Li–P–F bond in the F 1s spectrum, probably together with the LixSi–F group, confirms the assignment.28,29 This is consistent with the IR data and the P 2p spectrum (not shown). This is the evidence of the active participation of LiPF6 in the interfacial reaction and the formation of plenty of the insulating compounds (e.g., LiF). On the contrary, the F 1s spectrum for the IL exhibits a tiny peak at 685 eV. The TFSI decomposition produces a limited amount of Li- and F-containing species.
In the S 2p spectrum, the peak at 167–172 eV is assigned to the S–O bonding27–29 from the TFSI anion and/or the fragments of TFSI. The SO-containing surface species and Li–O, C–O and Si–O containing species contribute to the broad peak around 532 eV in the O 1s spectra.
In the N 1s spectrum, a peak at 399.5 eV is attributable to the Li/LixSi–TFSI, and another peak at 403 eV to pyrrolidinium-containing species.27–29 The peak at 403 eV, which is at a higher binding energy than 402.6 eV for the reference Py13, reflects the presence of stronger bonding with the N atom (e.g., LixSi–Py13). The main framework of some of the pyrrolidinium ring and TFSI seem to remain undecomposed, consistent with the IR data.
The XPS analysis results suggest that the SEI layer formed on the cycled SiO1.3 electrode in IL consists of the pyrrolidinium ring and TFSI ions and their decomposition products. The surface of the cycled electrode in EL however includes mostly insulating LiF and PF-containing species, alike to the Si.
3.4. Morphology changes with cycling
Fig. 7 shows the changes in the surface morphology of the SiO1.3 electrode with cycling. The pristine film in Fig. 1a was dense and had a smooth surface. After cycling in IL, in Fig. 7a, the film surface remains as dense and flat, despite a rougher surface than the pristine one. Round shaped particles with an average diameter of 50 nm are observed. They however appear well adhered to the substrate. By contrast, after cycling in EL, in Fig. 7b, the film surface became remarkably rough and porous with aggregated particles that are a few hundreds of nanometre in size. Effective surface passivation of the SiO1.3 electrode in IL also contributes to suppress the morphology change. This should be related to the enhanced cycling performance of the SiO1.3 electrode as observed in Fig. 3 and 4. | ||
| Fig. 7 SEM surface images for the SiO1.3 electrodes after cycling in (a) 1 M LiTFSI/Py13TFSI and (b) 1 M LiPF6/EC:EMC. | ||
4. Conclusions
Enhanced cycling performance of the SiO1.3 electrode in the non-flammable ionic liquid electrolyte of 1 M LiTFSI/Py13TFSI and the impact of interfacial behavior on the cycling ability are demonstrated. ATR FTIR and X-ray photoelectron spectroscopic analysis results provide the evidence that the SEI layer formed in the ionic liquid electrolyte is composed of the pyrrolidinium and TFSI ions and their decomposition products. The effective passivation of the electrode surface with a stable SEI layer leads to suppressed change in particle morphology and enhanced cycling performance. This is in contrast to a rapid performance fade in the commercial electrolyte due to the interfacial instability and the accumulation of insulating LiF and Li–PF-containing surface species. Detailed mechanism studies for the reductive decomposition of the ionic liquid electrolyte are in progress. Control of interfacial compatibility is believed to be one of the essential technologies for having a stable cycling performance of silicon oxide anodes and battery safety.Acknowledgements
This work was supported partly by the National Research Foundation (No. 2010-0027665) and partly by the Converging Research Center Program (2011K000687) through the MEST of Korea.References
- M. N. Obrovac and L. J. Krause, J. Electrochem. Soc., 2007, 152, A103 CrossRef.
- J. R. Szczech and S. Jin, Energy Environ. Sci., 2011, 4, 56 CAS.
- T. Tabuchi, H. Yasuda and M. Yamachi, J. Power Sources, 2005, 146, 507 CrossRef CAS.
- M. Miyachi, H. Yamamoto, H. Kawai, T. Ohta and M. Shirakata, J. Electrochem. Soc., 2005, 152, A2089 CrossRef CAS.
- M. Miyachi, H. Yamamoto and H. Kawai, J. Electrochem. Soc., 2007, 154, A376 CrossRef CAS.
- T. Kim, S. Park and S. M. Oh, J. Electrochem. Soc., 2007, 154, A1112 CrossRef CAS.
- C.-H. Doh, C.-W. Park, H.-M. Shin, D.-H. Kim, Y.-D. Chung, S.-I. Moon, B.-S. Jin, H.-S. Kim and A. Veluchamy, J. Power Sources, 2008, 179, 367 CrossRef CAS.
- H. Fukui, H. Ohsuka, T. Hino and K. Kanamura, ACS Appl. Mater. Interfaces, 2010, 2, 998 CAS.
- Q. Sun, B. Zhang and Z.-W. Fu, Appl. Surf. Sci., 2008, 254, 3774 CrossRef CAS.
- K. A. Striebel, C. Z. Deng, S. J. Wen and E. J. Cairns, J. Electrochem. Soc., 1996, 143, 1821 CrossRef CAS.
- S.-W. Song, R. P. Reade, E. J. Cairns, J. T. Vaughey, M. M. Thackeray and K. A. Striebel, J. Electrochem. Soc., 2004, 151, A1012 CrossRef CAS.
- S.-W. Song and S.-W. Baek, Electrochem. Solid-State Lett., 2009, 12, A23 CrossRef CAS.
- C. C. Nguyen and S.-W. Song, Electrochim. Acta, 2010, 55, 3026 CrossRef CAS.
- H. Choi, C. C. Nguyen and S.-W. Song, Bull. Korean Chem. Soc., 2010, 31, 2519 CrossRef CAS.
- D. Liu and G. Cao, Energy Environ. Sci., 2010, 3, 1218 CAS.
- K. Xu, Chem. Rev., 2004, 104, 4303 CrossRef CAS.
- M. Galiński, A. Lewandowski and I. Stępniak, Electrochim. Acta, 2006, 51, 5567 CrossRef.
- A. Lewandowski and A. Swiderska-Mocek, J. Power Sources, 2009, 194, 601 CrossRef CAS.
- P. C. Howlett, D. R. MacFarlane and A. F. Hollenkamp, Electrochem. Solid-State Lett., 2004, 7, A97 CrossRef CAS.
- A. Fernicola, F. Croce, B. Scrosati, T. Watanabe and H. Ohno, J. Power Sources, 2007, 174, 342 CrossRef CAS.
- M. Ishikawa, T. Sugimoto, M. Kikuta, E. Ishiko and M. Kono, J. Power Sources, 2006, 162, 658 CrossRef CAS.
- E. Markevich, R. Sharabi, V. Borgel, H. Gottlieb, G. Salitra, D. Aurbach, G. Semrau and M. A. Schmidt, Electrochim. Acta, 2010, 55, 2687 CrossRef CAS.
- P. Reale, A. Fernicola and B. Scrosati, J. Power Sources, 2009, 194, 182 CrossRef CAS.
- J. Hassoun, A. Fernicola, M. A. Navarra, S. Panero and B. Scrosati, J. Power Sources, 2010, 195, 574 CrossRef CAS.
- K.-H. Lee and S.-W. Song, ACS Appl. Mater. Interfaces, 2011, 3, 3697 CAS.
- V. Baranchugov, E. Markevich, E. Pollak, G. Salitra and D. Aurbach, Electrochem. Commun., 2007, 9, 796 CrossRef CAS.
- C. C. Nguyen and S.-W. Song, Electrochem. Commun., 2010, 12, 1593 CrossRef CAS.
- C. D. Wagner, W. M. Riggs, L. E. Davis and J. F. Moulder, in Handbook of X-Ray Photoelectron Spectroscopy, ed. G. E. Muilenberg, Perkin-Elmer Corp, Minnesota, 1979 Search PubMed.
- http://srdata.nist.gov/xps/ .
- F. G. Bell and L. Ley, Phys. Rev. B, 1988, 37, 8383 CrossRef CAS.
- J. J. van Hapert, A. M. Vredenberg, E. E. van Faassen, N. Tomozeiu, W. M. Arnoldbik and F. H. P. M. Habraken, Phys. Rev. B, 2004, 69, 245202 CrossRef.
- M. Yoshikawa, K. Iwagami, N. Morita, T. Matsunobe and H. Ishida, Thin Solid Films, 1997, 310, 167 CrossRef CAS.
- F. L. Galeener, R. A. Barrio, E. Martinez and R. J. Eliott, Phys. Rev. Lett., 1984, 17, 2429 CrossRef.
- T. P. Nguyen and S. Lefrant, Solid State Commun., 1986, 57, 235 CrossRef CAS.
- A. Netz, R. A. Huggins and W. Weppner, J. Power Sources, 2003, 119–121, 95 CrossRef CAS.
- P. C. Howlett, E. I. Izgorodina, M. Forsyth and D. R. MacFarlane, Z. Phys. Chem., 2006, 220, 1483 CrossRef CAS.
- G. Socrates, in Infrared Characteristic Group Frequencies, Tables and Charts, John Wiley & Sons, New York, 1994 Search PubMed.
- P. G. Pai, S. S. Chao, Y. Takagi and G. Lucovsky, J. Vac. Sci. Technol., A, 1986, 4, 689 CAS.
| This journal is © The Royal Society of Chemistry 2012 |
