An octanuclear helical ‘molecular wheel’ from hierarchical assembly of four dinuclear Cu2 units in a mixed-ligand array†
Adel M.
Najar
,
Ian S.
Tidmarsh
and
Michael D.
Ward
*
Department of Chemistry, University of Sheffield, Dainton Building, Sheffield, S3 7HF, UK. E-mail: m.d.ward@sheffield.ac.uk
First published on 3rd January 2012
Abstract
Reaction of Cu(II) salts with a combination of two different types of ligand affords an unusual ‘molecular wheel’, based on a cyclic array of four pyrazolate-bridged dinuclear Cu(II) units which are interconnected by bis-bidentate bridging ligands, in a hierarchical self-assembly process.
The continuing appeal of self-assembly is the remarkable array of elaborate and elegant structures that can form from deceptively simple components. In coordination chemistry these components are labile metal ions and polydentate bridging ligands, and the ways in which the geometric preferences of metal cations (as expressed in stereoelectronic effects and coordination number preferences) and bridging ligands (as expressed in the number and disposition of binding sites) can combine in mutually agreeable ways continues to be remarkable.1 The resulting assemblies are of appeal for a combination of their intrinsic beauty, the understanding they provide on how to understand and control self-assembly, and in some cases the useful functional behaviour of the assemblies as molecular hosts, catalysts, and materials with useful opto-electronic or magnetic properties.
Amongst the wide variety of structural types that have been formed from self-assembly methods, two dimensional cyclic arrays (‘molecular wheels’) are notable.2,3 These have been of particular interest for their magnetic properties, particularly when the metal ions are connected by single-atom bridging ligands;2 and for their recognition and guest-binding properties, if the central cavity of the cyclic array can accommodate a guest species which may act as a template for the assembly.3
Here we report the synthesis and structural characterisation of a new example of a ‘molecular wheel’ which is unusual in two respects. Firstly the assembly consists, not of isolated metal cations, but of a set of four dinuclear subunits which are based on a Cu2(μ–L)2 core [L = anion of 3-(2-pyridyl)-pyrazole, which provides a 1,2-pyrazolate bridge between two Cu(II) centres], which are further connected into a cyclic array using a different bridging ligand Lbiph (see Scheme 1). The second unusual feature follows from this, which is that the complex illustrates an example of a two-stage self-assembly process in which there is a clear hierarchy: small components assemble to give (in this case) the Cu2(μ–L)2 core, and interconnection of these with the bis-bidentate ligand Lbiph affords the octanuclear array. A conceptually related example was described by us recently in which octahedral mononuclear complexes of Co(II) and Ni(II), with pendant nitrile binding sites, reacted with Ag(I) ions in a separate step to form coordination networks in which the Co(II) or Ni(II) species became crosslinked via CN⋯Ag interactions.4 This type of hierarchical self-assembly relying on discrete stages has been termed ‘subcomponent self-assembly’ by Nitschke and used as the basis of some remarkable self-assembled polynuclear complexes from simple components.5
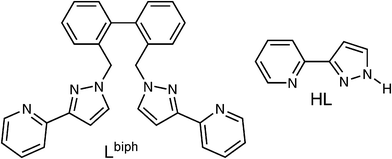 | ||
| Scheme 1 Structural formulae of the ligands used in this work. | ||
Reaction of Cu(ClO4)2, HL and Lbiph (see Scheme 1)‡ in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in MeOH/CH2Cl2 at room temperature for 24 h followed by partial removal of solvents under reduced pressure afforded a microcrystalline green solid in 78% yield;§ X-ray quality crystals were grown by diffusion of acetone vapour into a solution of the complex in dmf. The most significant peak in the ES mass spectrum occurred at m/z 984, with a spacing between components of the isotope cluster of 0.25 Daltons, and is ascribed to {Cu8(μ–L)8(Lbiph)4(ClO4)4}4+ in agreement with the crystal structure which is shown in Fig. 1–3,¶6,7 implying that the assembly observed in the solid state (described below) is retained in solution.
1 ratio in MeOH/CH2Cl2 at room temperature for 24 h followed by partial removal of solvents under reduced pressure afforded a microcrystalline green solid in 78% yield;§ X-ray quality crystals were grown by diffusion of acetone vapour into a solution of the complex in dmf. The most significant peak in the ES mass spectrum occurred at m/z 984, with a spacing between components of the isotope cluster of 0.25 Daltons, and is ascribed to {Cu8(μ–L)8(Lbiph)4(ClO4)4}4+ in agreement with the crystal structure which is shown in Fig. 1–3,¶6,7 implying that the assembly observed in the solid state (described below) is retained in solution.
![A view of one of the two independent complex cations in [Cu8(μ–L)8(Lbiph)4] (ClO4)8•2(dmf)•4(acetone) (the other is essentially identical). It lies on a twofold axis running vertically through the centre in this image such that opposite ligands (e.g. blue and green) are crystallographically equivalent. In the centre is a highly disordered perchlorate anion. For typical Cu–N bond distances, see main text.](/image/article/2012/RA/c2ra01177h/c2ra01177h-f1.gif) | ||
| Fig. 1 A view of one of the two independent complex cations in [Cu8(μ–L)8(Lbiph)4] (ClO4)8•2(dmf)•4(acetone) (the other is essentially identical). It lies on a twofold axis running vertically through the centre in this image such that opposite ligands (e.g. blue and green) are crystallographically equivalent. In the centre is a highly disordered perchlorate anion. For typical Cu–N bond distances, see main text. | ||
![Two partial views of a complex cation in [Cu8(μ–L)8(Lbiph)4] (ClO4)8•2(dmf)•4(acetone), showing how (a) two near-planar Cu2(μ–L)2 units (shown in grey, with Cu atoms in orange) are connected by a twisted bridging ligand Lbiph; and (b) how a Cu2(μ–L)2 unit is connected to the termini of two different bridging ligands Lbiph (in red and green). In this latter view it can be seen how the phenyl ring of Lbiph lies stacked with one of the coordinated L ligands due to the twisted conformation of Lbiph.](/image/article/2012/RA/c2ra01177h/c2ra01177h-f2.gif) | ||
| Fig. 2 Two partial views of a complex cation in [Cu8(μ–L)8(Lbiph)4] (ClO4)8•2(dmf)•4(acetone), showing how (a) two near-planar Cu2(μ–L)2 units (shown in grey, with Cu atoms in orange) are connected by a twisted bridging ligand Lbiph; and (b) how a Cu2(μ–L)2 unit is connected to the termini of two different bridging ligands Lbiph (in red and green). In this latter view it can be seen how the phenyl ring of Lbiph lies stacked with one of the coordinated L ligands due to the twisted conformation of Lbiph. | ||
![A space-filling view of the complex cation of [Cu8(μ–L)8(Lbiph)4] (ClO4)8•2(dmf)•4(acetone) viewed ‘edge-on’ (perpendicular to the view in Fig. 1). The Cu2(μ–L)2 units are again shown in grey with the pyridyl ligands removed for clarity such that only the Cu2(μ–pz)2 units are visible. The helical ‘over and under’ arrangement of the bridging ligands around the periphery is clear.](/image/article/2012/RA/c2ra01177h/c2ra01177h-f3.gif) | ||
| Fig. 3 A space-filling view of the complex cation of [Cu8(μ–L)8(Lbiph)4] (ClO4)8•2(dmf)•4(acetone) viewed ‘edge-on’ (perpendicular to the view in Fig. 1). The Cu2(μ–L)2 units are again shown in grey with the pyridyl ligands removed for clarity such that only the Cu2(μ–pz)2 units are visible. The helical ‘over and under’ arrangement of the bridging ligands around the periphery is clear. | ||
The formulation of the complex is [Cu8(μ–L)8(Lbiph)4](ClO4)8•2(dmf)•4(acetone). The octanuclear complex cation lies on a twofold axis such that half of it is unique; there are two crystallographically independent (but very similar) half-molecules in the asymmetric unit. The complex cation consists of four essentially planar Cu2(μ–L)2 units that are shown in Fig. 2a. In these fragments the deprotonated ligand L acts as a tridentate donor, being bidentate chelating to one Cu(II) ion and monodentate to the other, providing a pyrazolate bridge such that there is Cu2(μ–pyrazolate)2 core with each Cu(II) ion having three donor atoms from this pair of bridging ligands.
All of these Cu–N distances are short with the Cu–N(pyrazolate) distances in the range 1.95–1.99 Å, and the Cu–N(pyridyl) distances in the range 2.06–2.07 Å. This Cu2(μ–L)2 unit is a well-known feature of Cu(II) complexes based on pyridyl-pyrazole chelates.8 The remaining two coordination sites around each (five-coordinate) Cu(II) centres are then occupied by a neutral bidentate fragment from a bridging ligand Lbiph which is the basis of the assembly of four Cu2(μ–L)2 units into a cyclic array.
Each adjacent pair of Cu2(μ–L)2 units is spanned by one bridging Lbiph which adopts a highly twisted conformation to allow this to happen [Fig. 2(b) and 3], with the dihedral angles between the two phenyl rings of the central biphenyl units being 81° and 83° in the two crystallographically independent ligands. Each phenyl ring of a biphenyl unit ends up lying approximately parallel to, and stacked between, a pair of pyrazolyl-pyridine units: one from a ligand L and one from a ligand Lbiph, such that there are eight of these three-layer stacks around the periphery of the complex. Each phenyl ring is additionally involved in CH⋯π interactions with another coordinated ligand L to which it is approximately perpendicular, such that every phenyl ring from a bridging ligand is involved in three non-covalent interactions (two π–stacks and a CH⋯π interaction) with other ligands. Each bridging ligand Lbiph is twisted in the same sense, with the result that the complex overall has a cyclic helical structure as emphasised in the space-filling view in Fig. 3. As is often the case with other cyclic helicates, the central cavity contains a (disordered) anion.
Formation of this structure is clearly driven in part by the preference of Cu(II) ions for a basically square-pyramidal coordination environment arising from the non-symmetrical dz22/d(x2-y2)1 configuration (Fig. 2). In keeping with this the axial Cu–N distances, involving the pyrazolyl units of Lbiph, are all significantly longer than the basal Cu–N distances at ca. 2.3 Å. The highly conserved Cu2(μ–L)2 unit, which is a regular feature of Cu(II) complexes with this type of pyridyl-pyrazole ligand,8 leaves two coordination sites free around each (five coordinate) Cu(II) ion. Each Cu(II) ion can therefore accommodate one additional bidentate ligand, i.e. there are two per Cu2(μ–L)2 unit. Consequently bis-bidentate bridging ligands of the type Lbiph—and many others of the same family—are ideal for connecting these Cu2(μ–L)2 units into linear or cyclic arrays, allowing self-assembly to be based on use of a dinuclear fragment as a main component part (effectively a two-connecting node) rather than on single metal ions as a component part.
This is reminiscent of the way that kinetically inert dimetal units have been extensively used as subcomponents for self-assembly by Cotton and co-workers.9 The difference here is that the Cu2(μ–L)2 unit is labile and itself assembles in the reaction, such that there is a clear two-step hierarchy in the self-assembly process with formation of Cu2(μ–L)2 units as one step and connection of these into a cyclic helical array by Lbiph units as the other, cf. the ‘subcomponent self-assembly’ process of Nitschke et al.5 Importantly, the self-assembly of [Cu8(μ–L)8(Lbiph)4](ClO4)8 selects for the mixture of ligands: when Cu(ClO4)2, HL and Lbiph are combined in the correct proportions this is the only compound isolated, and in good yield; homoleptic complexes such as [Cu4L6]2+, for example,6e are not detected. This principle will allow us to extend the wide range of self-assembled systems that we have studied with bridging ligands of the Lbiph class to a new family based on dinuclear Cu2 nodes.
We thank the University of Garyounis in Libya for a PhD studentship to Dr Adel Najar.
References
- (a) D. Fiedler, D. H. Leung, R. G. Bergman and K. N. Raymond, Acc. Chem. Res., 2005, 38, 349 CrossRef CAS; (b) M. Fujita, M. Tominaga, A. Hori and B. Therrien, Acc. Chem. Res., 2005, 38, 369 CrossRef CAS; (c) S. R. Seidel and P. J. Stang, Acc. Chem. Res., 2002, 35, 972 CrossRef CAS; (d) M. D. Ward, Chem. Commun., 2009, 4487 RSC; (e) J. J. Perry, J. A. Perman and M. J. Zaworotko, Chem. Soc. Rev., 2009, 38, 1400 RSC; (f) S. Alvarez, Dalton Trans., 2006, 2209 Search PubMed; (g) B. Breiner, J. K. Clegg and J. R. Nitschke, Chem. Sci., 2011, 2, 51 RSC; (h) D. Philp and J. F. Stoddart, Angew. Chem., Int. Ed. Engl., 1996, 35, 1155 CrossRef CAS.
- (a) K. J. Taft, C. D. Delfs, G. C. Papaefthymiou, S. Foner, D. Gatteschi and S. H. Lippard, J. Am. Chem. Soc., 1994, 116, 823 CrossRef CAS; (b) M. Manoli, A. Precsimone, A. Mishra, S. Parsons, G. Christou and E. K. Brechin, Dalton Trans., 2007, 532 RSC; (c) J. Dreiser, O. Waldmann, G. Carver, C. Dobe, H.-U. Güdel, H. Weihe and A.-L. Barra, Inorg. Chem., 2010, 49, 8729 CrossRef CAS; (d) S. J. Shah, C. M. Ramsey, K. J. Heroux, A. G. DiPasquale, N. S. Dalal, A. L. Rheingold, E. del Barco and D. N. Hendrickson, Inorg. Chem., 2008, 47, 9569 CrossRef CAS; (e) Z.-H. Ni, L.-F. Zhang, V. Tangoulis, W. Wernsdorfer, A.-L. Cui, O. Sato and H.-Z. Kou, Inorg. Chem., 2007, 46, 6029 CrossRef CAS; (f) O. Waldmann, Coord. Chem. Rev., 2005, 249, 2550 CrossRef CAS; (g) E. J. L. McInnes, S. Piligkos, G. A. Timco and R. E. P. Winpenny, Coord. Chem. Rev., 2005, 249, 2577 CrossRef CAS; (h) M. L. Baker, S. Piligkos, A. Bianchi, S. Carretta, D. Collison, J. J. W. McDouall, E. J. L. McInnes, H. Mutka, G. A. Timco, F. Tuna, P. Vadivelu, H. Weihe, H. U. Güdel and R. E. P. Winpenny, Dalton Trans., 2011, 40, 8533 RSC.
- (a) P. L. Jones, K. J. Byrom, J. C. Jeffery, J. A. McCleverty and M. D. Ward, Chem. Commun., 1997, 1361 RSC; (b) B. Hasenknopf, J.-M. Lehn, B. O. Kneisel, G. Baum and D. Fenske, Angew. Chem., Int. Ed. Engl., 1996, 35, 1838 CrossRef CAS; (c) G. Baum, E. C. Constable, D. Fenske, C. E. Housecroft and T. Kulke, Chem. Commun., 1995, 195 Search PubMed; (d) F. Tuna, J. Hamblin, A. Jackson, G. Clarkson, N. W. Alcock and M. J. Hannon, Dalton Trans., 2003, 2141 RSC; (e) O. Mamula, A. von Zelewsky, P. Brodard, C. W. Schlapfer, G. Bernardinelli and H. Stoeckli-Evans, Chem.–Eur. J., 2005, 11, 3049 CrossRef CAS; (f) S. P. Argent, H. Adams, T. Riis-Johannessen, J. C. Jeffery, L. P. Harding, O. Mamula and M. D. Ward, Inorg. Chem., 2006, 45, 3905 CrossRef CAS; (g) N. K. Al-Rasbi, H. Adams, L. P. Harding and M. D. Ward, Eur. J. Inorg. Chem., 2007, 4770 CrossRef CAS; (h) C. S. Campos-Fernandez, B. L. Schottel, H. T. Chifotides, J. K. Bera, J. Bacsa, J. M. Koomen, D. H. Russell and K. R. Dunbar, J. Am. Chem. Soc., 2005, 127(37), 12909 CrossRef CAS.
- H. Fenton, I. S. Tidmarsh and M. D. Ward, Dalton Trans., 2010, 39, 3805 RSC.
- (a) V. E. Campbell and J. R. Nitschke, Synlett, 2008, 3077 CAS; (b) R. A. Bilbeisi, J. K. Clegg, N. Elgrishi, X. De Hatten, M. Devillard, B. Breiner, P. Mal and J. R. Nitschke, J. Am. Chem. Soc. DOI:10.1021/ja2092272; (c) J. R. Nitschke, Acc. Chem. Res., 2007, 40, 103 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr. Sect. A, 2008, 64, 112 CrossRef.
- G. M. Sheldrick, SADABS: A program for absorption correction with the Siemens SMART system, University of Göttingen, Germany, 1996 Search PubMed.
- (a) K. Singh, J. R. Long and P. Stavropoulos, Inorg. Chem., 1998, 37, 1073 CrossRef CAS; (b) J. Pons, F. J. Sanchez, J. Casabó, A. Alvarez-Larena, J. F. Piniella and J. Ros, Inorg. Chem. Commun., 2003, 6, 833 CrossRef CAS; (c) J. Mukherjee and R. Mukherjee, Dalton Trans., 2006, 1611 RSC; (d) V. Mishra, F. Lloret and R. Mukherjee, Eur. J. Inorg. Chem., 2007, 2161 CrossRef CAS; (e) K. L. V. Mann, E. Psillakis, J. C. Jeffery, L. H. Rees, N. M. Harden, J. A. McCleverty, M. D. Ward, D. Gatteschi, F. Totti, F. E. Mabbs, E. J. L. McInnes, P. C. Riedi and G. M. Smith, J. Chem. Soc., Dalton Trans., 1999, 339 RSC; (f) M. Munakata, L. P. Wu, M. Yamamoto, T. Kuroda-Sowa, M. Maekawa, S. Kawata and S. Kitagawa, J. Chem. Soc., Dalton Trans., 1995, 4099 RSC.
- (a) F. A. Cotton, C. Y. Liu, C. A. Murillo and X. Wang, Inorg. Chem., 2006, 45, 2619 CrossRef CAS; (b) F. A. Cotton, C. A. Murillo and R. Yu, Dalton Trans., 2006, 3900 RSC; (c) F. A. Cotton, C. A. Murillo, S.-E. Stiriba, X. Wang and R. Yu, Inorg. Chem., 2005, 44, 8223 CrossRef CAS; (d) F. A. Cotton, C. Lin and C. A. Murillo, Acc. Chem. Res., 2001, 34, 759 CrossRef CAS.
Footnotes |
| † CCDC reference numbers 855588. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra01177h |
| ‡ The ligand LBiph was prepared from 2,2’-bis(bromomethyl)biphenyl and HL under basic conditions, exactly according to the method used for numerous other ligands in this series (ref. 1d), and has satisfactory routine analytical data. Full details will be published in a later full paper. |
| § A solution of Cu(ClO4)2·6H2O (0.050 g, 135 μmol) in MeOH (7 cm3) was added to a mixture of LBiph (0.031 g, 67 μmol) and HL (0.019 g, 135 μmol) in CH2Cl2 (7 cm3). The mixture was stirred at RT for 24 h; the solvent was then removed in vacuo to afford a pale green powder (0.032 g, 78%). X-ray quality crystals were grown by slow diffusion of acetone into a dmf solution of the complex. ESMS: m/z 984, {Cu8(μ–L)8(Lbiph)4(ClO4)4}4+. Found: C, 49.9; H, 4.1; N, 15.7%. Required for [Cu8(μ–L)8(Lbiph)4](ClO4)8·5H2O•2dmf: C, 50.0; H, 3.7; N, 15.3%. |
¶ Crystal data for [Cu8(μ–L)8(Lbiph)4](ClO4)8•2(dmf)•4(acetone) (C202H182N50O38Cl8Cu8): monoclinic, space groupP2/c, Mr = 4709.9, a = 37.5021(11), b = 15.4876(4), c = 42.4530(12), β = 114.5940 (10), V = 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 420.5(11), Z = 4, Dc = 1.395 g cm−3, μ(Mo-Kα) = 0.919 mm−1. A crystal of size 0.28 × 0.25 × 0.20 mm3 was mounted on a Bruker APEX-2 diffractometer under a stream of cold N2 and intensity data were collected at 100 K. 202 420.5(11), Z = 4, Dc = 1.395 g cm−3, μ(Mo-Kα) = 0.919 mm−1. A crystal of size 0.28 × 0.25 × 0.20 mm3 was mounted on a Bruker APEX-2 diffractometer under a stream of cold N2 and intensity data were collected at 100 K. 202![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 537 reflections were collected with 2θmax = 53° which after merging afforded 45897 independent data with Rint = 0.057. Refinement of 2516 parameters with 1407 restraints converged at R1 [selected data with I > 2σ(I)] = 0.110; wR2 (all data) = 0.361. Software used for structure solution and refinement was SHELXS-97 and SHELX-97;6 the absorption correction was applied with SADABS.7 The crystal contains two independent complex cations, each of which lies on a twofold axis such that one half of each lies in the asymmetric unit. There is extensive disorder of perchlorate anions which in many cases have been refined over two or more sites with fractional occupancies; one of the acetone molecules is disordered over two sites. All anion and solvent molecules were refined with isotropic displacement parameters, and extensive use was made of geometric restraints on the disordered components in the final refinement. The SQUEEZE function in PLATON was used to eliminate regions of diffuse electron density that could not be satisfactorily modelled (see cif for details). 537 reflections were collected with 2θmax = 53° which after merging afforded 45897 independent data with Rint = 0.057. Refinement of 2516 parameters with 1407 restraints converged at R1 [selected data with I > 2σ(I)] = 0.110; wR2 (all data) = 0.361. Software used for structure solution and refinement was SHELXS-97 and SHELX-97;6 the absorption correction was applied with SADABS.7 The crystal contains two independent complex cations, each of which lies on a twofold axis such that one half of each lies in the asymmetric unit. There is extensive disorder of perchlorate anions which in many cases have been refined over two or more sites with fractional occupancies; one of the acetone molecules is disordered over two sites. All anion and solvent molecules were refined with isotropic displacement parameters, and extensive use was made of geometric restraints on the disordered components in the final refinement. The SQUEEZE function in PLATON was used to eliminate regions of diffuse electron density that could not be satisfactorily modelled (see cif for details). |
| This journal is © The Royal Society of Chemistry 2012 |
