One-pot synthesis of monodisperse latex particles with single-cavity structure†
Zifu
Li
,
Xiaoling
Wei
and
To
Ngai
*
Department of Chemistry, The Chinese University of Hong Kong, Shatin N.T., Hong Kong. E-mail: tongai@cuhk.edu.hk; Fax: +(852) 2603 5057; Tel: +(852) 3943 1222
First published on 6th January 2012
Abstract
This paper describes a simple and flexible method for fabricating polymeric particles with single-cavity structure by a modified soap-free emulsion polymerization (SFEP). The key is to introduce a functional comonomer (methyl methacrylate, MMA) and a crosslinker (divinylbenzene, DVB) to a conventional three-component SFEP system, followed by polymerization. Anisotropic latex particles with good monodispersity in both size and shape are directly produced in one-pot synthesis with a high yield. The importance of the presence of MMA and DVB in the SFEP with regards to the anisotropic particles formation is discussed.
Polymeric latex particles with structural and composition anisotropies are of great interest for fundamental studies as well as for potential applications.1 Particle anisotropy can be defined either by the particle shape, such as faceted, branched, and patterned particles, or by the surface chemistry such as patchy and Janus particles.1–7 Special attention has been paid to identifying and exploiting anisotropic particles in order to tailor the optical properties of colloidal assemblies,1–8 control suspension rheology,9 and even engineer biomaterials.10 Various ingenious techniques, such as clusterization,11,12 electrical jetting,13–15 seeded emulsion polymerization,16–20 controlled nucleation and precipitation,21microcontact printing,22 liquid protrusion,23 and use of microfluidic devices,24–26 are being developed to synthesize or fabricate particles with novel anisotropic shapes. In spite of the success, it is worth pointing out that most of these methods cannot be easily applied to large-scale production because of multiple steps involved in a typical synthesis process. Moreover, several methods even require a sorting process to produce homogeneous samples. Thus, it is important to develop a convenient and direct means of producing uniform-sized anisotropic particles for technological applications.
Herein we report a simple and versatile procedure for generating monodisperse, anisotropic polymer particles by using a modified soap-free emulsion polymerization (SFEP) process. SFEP is an important industrial process for the manufacture of spherical polymer nanoparticles with excellent water resistance and adhesion properties.27,28 Typically, the principal ingredients for a standard SFEP include only three components; namely water, hydrophobic monomer, such as styrene, and hydrophilic initiator. In the absence of surfactant, the stability of the resulting polymer particles is ensured by the covalently bound stabilizing groups that originate from the ionic initiator molecules. We simply modified the conventional SFEP of styrene by further incorporating a small amount of hydrophilic comonomer, methyl methacrylate (MMA), and a crosslinker, divinylbenzene (DVB) into the system. Surprisingly, uniform-sized latex particles with single dimples and a dimension on the order of a few hundred nanometres (Fig. 1) are directly produced after the one-pot polymerization. This synthesis especially features high yields of stable nonspherical particles.
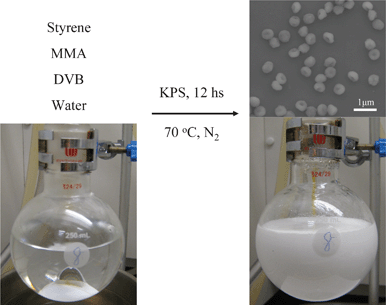 | ||
| Fig. 1 Preparation of anisotropic polymer particles by incorporation a functional monomer, MMA and a crosslinker, DVB to the conventional three-component SFEP system. | ||
We performed various series of SFEP of styrene in the presence of different amounts of MMA and DVB in order to have the best recipe for the manufacture of uniform anisotropic polymer particles and to understand the influence of MMA and DVB on the particle formation. All reactions were carried out at 70 °C under nitrogen using potassium persulfate (KPS, 0.14 g) as initiator. The total volume of the reaction mixture was 150 mL and the polymerizations lasted for 12 h. In our first series, we fixed the amounts of styrene (4.50 g) and MMA (0.14 g) and we varied the amounts of DVB, that is, 0, 0.07, 0.14, 0.28 and 0.42 g to influence the crosslinking density of the growing polymer particles. Emulsion polymerizations carried out in the absence or presence of a small amount of DVB (0.075 g) led to monodisperse spherical poly(styrene-co-methyl methacrylate) (PS-PMMA) particles. The Scanning electron microscopy (SEM, FEI Quanta 400 FEG, 10 kV) pictures showed that the diameter of the resulting polymer particles is in the range of around 380 nm (Fig. 2a and 2b), which agrees well with previous reports.29 Unprecedentedly, when the DVB amount was increased to 0.14 g, nonspherical shape polymer particles with single dimples were formed (Fig. 1 and Fig. 2c). Essentially the produced particles have good monodispersity not only in size but also in shape. The morphology of the polymer particles has changed completely at a higher content of DVB. Further increasing amounts of DVB (0.28 or 0.42 g) resulted in irregular shaped or even partially aggregated particles with a broad size distribution (Fig. 2d and Fig. S1, ESI†). It is worth mentioning that the DVB is scarcely added to the conventional SFEP. A few previous studies have shown that the introduction of crosslinker may easily lead to intensive coagulation of the particles and form filterable solids and scraps.27,28 However, in our system, the morphology of the resulting latex particles could be well controlled by the amount of DVB. We will come to this point later.
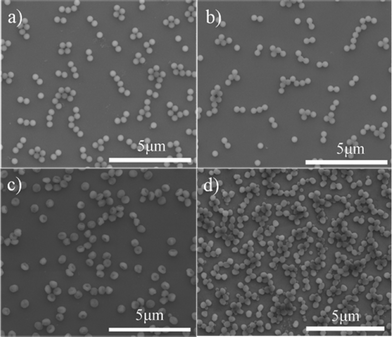 | ||
| Fig. 2 SEM images of the prepared PS-PMMA particles after the addition of different amount of crosslinking agent DVB, (a) 0 g, (b) 0.075 g, (c) 0.14 g, and (d) 0.28 g, to the SFEP of styrene. | ||
We also investigated the influence of added MMA on the structure of polymer particles. Fig. 3 shows the SEM images of the prepared polymer particles with varying amount of MMA, that is, 0.07, 0.14, 0.28, 0.42, 0.70 and 0.84 g, while the amounts of styrene and DVB constant is kept at 4.50 and 0.14 g, respectively. At a low MMA content (0.07 g), some anisotropic polymer particles coexisting with other smaller spherical polymer particles were produced (Fig. 3a). Increasing the amount of MMA to 0.14 g only resulted in monodisperse polymer particles with a single cavity (Fig. 3b), displaying a flat brimmed hat-like structure with a diameter about 520 nm. The particle has a small dimple, and the diameter to height ratio is about 1.5. Monodisperse anisotropic particles could still be obtained when the amount of MMA was increased to 0.28 g (Fig. 3c). The particles were smaller (430 nm), indicating that the increase of the functional monomer MMA can decrease the size of the resultant polymer particles. The diameter to height ratio, however, decreased to nearly 1.0. As the amount of MMA continuously increases, the polydispersity of the polymer particles increases (Fig. 3d–f). Also, the resulting particles were not highly uniform in shape. In particular, when the amount of MMA was higher than 0.70 g, no anisotropic polymer particles were found. We have thus well illustrated that the morphology of the polymer particles can be manipulated by simply adjusting the amount of DVB or MMA in a modified SFEP process.
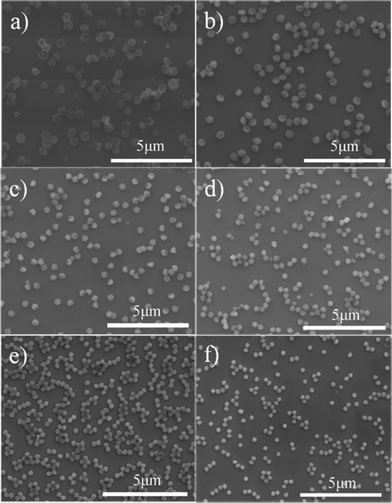 | ||
| Fig. 3 SEM images of the prepared PS-PMMA particles after the addition of different amount of functional monomer MMA, (a) 0.07 g, (b) 0.14 g, (c) 0.28 g, (d) 0.42 g, (e) 0.70 g and (f) 0.84 g, to the SFEP of styrene. Note that Fig. 4b is the same as Fig. 2c. | ||
To obtain a better understanding of the anisotropic particle formation mechanism in the presence of DVB and MMA, we further investigated the evolution of the PS-PMMA particles growth mechanism as a function of the reaction time (Fig. 4). Based on the aforementioned observation, the best recipe, that is, 4.50 g styrene, 0.14 g MMA, 0.14 g DVB, 0.14 g KPS, and 150 mL water has been proposed to manufacture the anisotropic polymer particles with the single cavity. The evolution of the particle growth at different time was thus performed by this recipe. Fig. 4a shows a SEM image of an intermediate product sampled 1 h after polymerization. It can be seen that the particle nuclei are spherical with a diameter of about 90 nm. These primary particles then grow in size not only due to swelling caused by the addition of monomers but also aggregation to form large colloidally stable polymer particles in the emulsion polymerization process (Fig. 4b). Interestingly, when the polymerization reaction was continued after 5 h, an obvious cavity in the particles was observed (Fig. 4c). Such dimples then deepen in time to reach their final morphology after 12 h polymerization (Fig. 4d). Note that latex particles with single or multi-cavity structures have recently been reported viaemulsion polymerization.30,31 In contrast, the cavity structure of the latex particles was controlled by modifying the feeding mode of the DVB during the emulsion polymerization. This different feeding mode of DVB affects the spatial distribution of the crosslinking density across the latex particles, which in turn leads to the formation of the latex particles with different cavity structures after the phase separation. The detail mechanism of the particle formation, however, has yet to be explored.
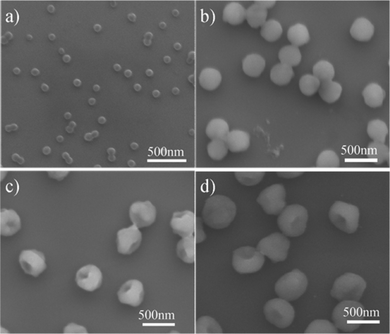 | ||
| Fig. 4 SEM images of the reaction intermediate of SFEP in the presence of MMA and DVB obtained at (a) 1 h, (b) 3 h, (c) 5 h, and (d) 12 h, respectively, after the initiation of the polymerization. | ||
Transmission electron microscopy (TEM, FEI CM120, 120 kV) was also used to examine the structure of the as-prepared anisotropic latex particles shown in Fig. 2c. Fig. 5a and 5b clearly show that monodisperse polymer particles with a single cavity are obtained. The particles have small dimples, and the diameter to height ratio is about 1.5. In order to have a clear picture of the spatial distribution of the PS and PMMA in the resulting particles, high resolution TEM (HRTEM, Tecnai F20, 200 kV) was also applied to view these particles. Fig. 5c shows the high resolution TEM image of the brim of a single polymer particle, and oxygen element map from this place was obtained and shown in Fig. 5d. The bright filed, which corresponds to abundant oxygen was clearly observed in the brim of the particle, indicating that the PMMA polymers are predominantly located in the flat brim because oxygen is only present in PMMA polymers. Similar results were obtained in other areas around the brim from the same polymer particle (Fig. S2, ESI†). We thereby conclude that for the prepared latex particles, the dimple is mainly composed of PS polymer, while the PMMA polymer is mostly distributed around the flat brims.
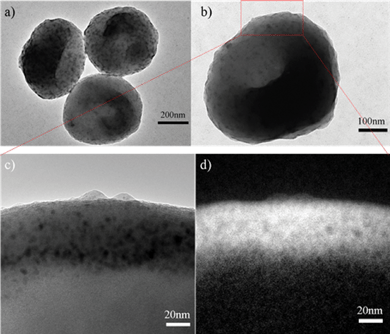 | ||
| Fig. 5 (a), (b) TEM images of the prepared PS-PMMA particles contain 4.50 g styrene, 0.14 g MMA, and 0.14 g DVB. (c) HRTEM image of the PS-PMMA particle; (d) Oxygen element map of the PS-PMMA particle from the same place as shown in (c). | ||
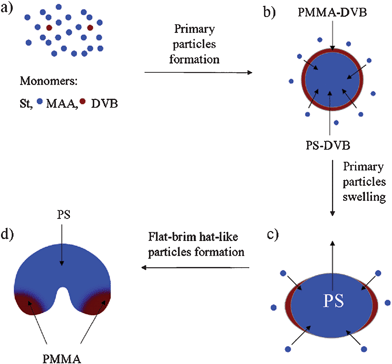 | ||
| Fig. 6 A schematic illustration showing the possible formation mechanism of the single-cavity latex particles via the modified SFEP of styrene. | ||
Based on our above results, the possible formation procedure of the anisotropic particles with single cavity structure in the bulk process was schematically shown in Fig. 6. In general, the conventional SFEP can be divided into two stages, e.g., the particle nucleation process and the particle growth. The resulting particle size and particle size distribution synthesized by using ionic initiator depend not only on the initial composition of the system but also on the dynamics of these two processes.27,28 At the polymerization temperature (70 °C), water-soluble initiator decomposes to produce free radicals. In the case of KPS, initiator decomposition leads to the formation of sulfate radicals that attack the free monomers (St, MMA, and DVB) in the water phase, followed by radical propagation and chain growth to from precursor particles (Fig. 4a). These primary particles are expected to have a core-shell structure with the crosslinked hydrophobic PS as the core surrounded by the more hydrophilic PMMA shell (Fig. 6b).31 The primary crosslinked particles swell by adsorbing unreacted monomer mixture (St, MMA and DVB), and the particle growth is then followed by monomer consumption (Fig. 4b), especially to the MMA and DVB monomers since they are only a small portion of all the monomers (both ratio of MMA/St and DVB/St are 3.1%, respectively). When all the MMA and DVB monomers are consumed, the unreacted St monomers continuously diffuse in the crosslinked PS core to swell the particles. Consequently, this swelling and polymerization reaction causes the protrusion to develop on the surrounded PMMA shell (Fig. 6c). As more and more unreacted St monomers are diffused in, the protrusive PS continues to grow and at the same time squeeze the PMMA shell to the edge of the particles (Fig. 6c). Finally, the phase separation between PS and PMMA results in the flat brimmed hat-like structure with most PMMA located in the flat brims (Fig. 6d). Based on this mechanism, there may be a wettability difference between the two sides of the resulting particles during the cavity formation process. The protrusive head side is more hydrophobic PS, while the flat brim side is more hydrophilic PMMA that has been confirmed by the TEM results (Fig. 5d).
In this proposed formation mechanism, the elasticity of the crosslinked primary particles plays an important role on the final particle morphology. The elasticity of the primary particles can be controlled by two parameters: namely, the amount of DVB crosslinker and the thickness of the surrounded hydrophilic PMMA shell. For the effect of DVB crosslinker, weakly crosslinked primary particles shrinks almost freely during the polymerization, leading to spherical particles with a slightly corrugated surface (Fig. 2a and 2b). However, for highly crosslinked primary particles, the particles can resist shrinkage and deformation, but large amount of DVB often results in aggregated particles due to secondary nucleation process (Fig. 2d and Fig. S1, ESI†). Particles with single cavity can only be formed when the primary particles contain the proper crosslinking density (Fig. 2c). This result is a little bit different from the previous results, where the increase of DVB can enlarge the cavity.30 The reason might be caused by the fact that surfactant is added in the previous case and that the particles formation mechanism is different.31 For the presence of MMA, insufficient MMA cannot form a strong crosslinked PMMA shell to withstand the extrusion of the PS core and results in a mixture of slightly deformed anisotropic particles and spherical particles (Fig. 3a). Also, anisotropic particles with single cavity can be obtained with proper MMA content, as shown in Fig. 3b and 3c. Moreover, the aspect ratio of these particles can be tuned by slightly increasing the MMA content from 0.14 to 0.28 g. Further increase of the MMA content may lead to a thick crosslinked PMMA shell in which unreacted St monomers are unlikely to diffuse into the core and cause the second nucleation process, eventually resulting in a broad size distribution of the particles as illustrated in Fig. 3d–f.
In conclusion, we have prepared uniform, monodisperse, and nonspherical latex particles with a single cavity using a modified soap-free emulsion polymerization. The shape of the resulting polymer particles can be easily controlled by adjusting the amount of MMA or DVB in each single batch polymerization. High resolution TEM images confirm that there is wettability difference between the two sides of the resulting particles, in which the protrusive head side is more hydrophobic PS, while the flat brim side is more hydrophilic PMMA. This approach is highly reproducible and also features high yields (3.2 wt% solid content) without any sorting process. The resulting anisotropic particles are suitable for studies on controlling colloidal self-assembly, complex dispersion properties, and as templates for advanced materials.
We would like to thank Dr T. Ming from the Department of Physics for the help of TEM characterization and Mr. Z. Chu for the help of high resolution TEM characterization. The financial support of this work by the Hong Kong Special Administration Region (HKSAR) General Research Fund (CUHK402809, 2160387) is gratefully acknowledged.
References
- S. Glotzer, Science, 2004, 306, 419–420 CrossRef CAS.
- A. van Blaaderen, Nature, 2006, 439, 545 CrossRef CAS.
- S. Glotzer and M. Solomon, Nature Mater., 2007, 6, 557–562 CrossRef.
- A. Hynninen, J. Thijssen, E. Vermolen, M. Dijkstra and A. van Blaaderen, Nature Mater., 2007, 6, 202–205 CrossRef CAS.
- S. Yang, S. Kim, J. Lim and G. Yi, J. Mater. Chem., 2008, 18, 2177–2190 RSC.
- A. Pawar and I. Kretzschmar, Macromol. Rapid Commun., 2010, 31, 150–168 CrossRef CAS.
- D. Nagao, M. Hashimot, K. Hayasaka and M. Konno, Macromol. Rapid Commun., 2008, 29, 1484–1488 CrossRef CAS.
- M. Mishchenko, J. Hovenier and L. Travis, Light Scattering by Nonspherical Particles: Theory, Measurements, and Applications; Academic Press, San Diego, 2000 Search PubMed.
- R. Larson, The Structure and Rheology of Complex Fluids; Oxford University Press: New York, 1998 Search PubMed.
- S. Mitragotri and J. Lahann, Nature Mater., 2009, 8, 15–23 CrossRef CAS.
- V. Manoharan, M. Elsesser and D. Pine, Science, 2003, 301, 483–487 CrossRef CAS.
- G. Yi, V. Manoharan, E. Michel, M. Elsesser, S. Yang and D. Pine, Adv. Mater., 2004, 16, 1204–1208 CrossRef CAS.
- K. Roh, D. Martin and J. Lahann, Nature Mater., 2005, 4, 759–763 CrossRef CAS.
- K. Roh, D. Martin and J. Lahann, J. Am. Chem. Soc., 2006, 128, 6796–6797 CrossRef CAS.
- S. Bhaskar, K. Pollock, M. Yoshida and J. Lahann, Small, 2010, 6, 404–411 CrossRef CAS.
- J. Kim, R. Larsen and D. Weitz, J. Am. Chem. Soc., 2006, 128, 14374–14377 CrossRef CAS.
- J. Kim, R. Larsen and D. Weitz, Adv. Mater., 2007, 19, 2005–2009 CrossRef CAS.
- A. Ohnuma, E. Cho, P. Camargo, L. Au, B. Ohtani and Y. Xia, J. Am. Chem. Soc., 2009, 131, 1352–1353 CrossRef CAS.
- J. Park, J. Forster and E. Dufresne, Langmuir, 2009, 25, 8903–8906 CrossRef CAS.
- J. Park, J. Forster and E. Dufresne, J. Am. Chem. Soc., 2010, 132, 5960–5961 CrossRef CAS.
- J. Ge, Y. Hu, T. Zhang and Y. Yin, J. Am. Chem. Soc., 2007, 129, 8974–8975 CrossRef CAS.
- O. Cayre, V. Paunov and O. Velev, J. Mater. Chem., 2003, 13, 2445–2450 RSC.
- D. Kraft, W. Vlug, C. van Kats, A. van Blaaderen, A. Imhof and W. Kegel, J. Am. Chem. Soc., 2009, 131, 1182–1186 CrossRef CAS.
- T. Nisisako, T. Torii, T. Takahashi and Y. Takizawa, Adv. Mater., 2006, 18, 1152 CrossRef CAS.
- Z. Nie, S. Xu, M. Seo, P. Lewis and E. Kumacheva, J. Am. Chem. Soc., 2005, 127, 8058–8063 CrossRef CAS.
- D. Dendukuri, D. Pregibon, J. Collins, T. Hatton and P. Doyle, Nature Mater., 2006, 5, 365–369 CrossRef CAS.
- K. Tauer, R. Deckwer, I. Kühn and C. Schellenberg, Colloid Polym. Sci., 1999, 277, 607–626 CAS.
- I. Kühn and K. Tauer, Macromolecules, 1995, 28, 8122–8128 CrossRef.
- S. Zhang, J. Chen and M. Taha, J. Appl. Polym. Sci., 2009, 114, 1598–1605 CrossRef CAS.
- L. Xu, H. Li, J. Wang, L. Li, Y. Song and L. Jiang, Macromol. Rapid Commun., 2010, 31, 1422–1426 CrossRef CAS.
- Y. Huang, J. Wang, J. Zhou, L. Xu, Z. Li, Y. Zhang, J. Wang, Y. Song and L. Jiang, Macromolecules, 2011, 44, 2404–2409 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details concerning polymerization recipes and particle characterization. See DOI: 10.1039/c2ra01209j |
| This journal is © The Royal Society of Chemistry 2012 |
