Self assembly of bivalent glycolipids on single walled carbon nanotubes and their specific molecular recognition properties†
Bandaru Narasimha
Murthy
a,
Susanne
Zeile
a,
Monessha
Nambiar
a,
Matthew R.
Nussio
a,
Christopher T.
Gibson
a,
Joseph G.
Shapter
a,
Narayanaswamy
Jayaraman
b and
Nicolas H.
Voelcker‡
*a
aFlinders University, School of Chemical and Physical Sciences, Sturt Road, Bedford Park, SA 5042, Australia. E-mail: nico.voelcker@unisa.edu.au; Fax: +61 88302 5613; Tel: +61 88302 5508
bDepartment of Organic Chemistry, Indian Institute of Science, Bangalore 560 012, India
First published on 18th January 2012
Abstract
Solubilization of single walled carbon nanotubes (SWNTs) in aqueous milieu by self assembly of bivalent glycolipids is described. Thorough analysis of the resulting composites involving Vis/near-IR spectroscopy, surface plasmon resonance, confocal Raman and atomic force microscopy reveals that glycolipid-coated SWNTs possess specific molecular recognition properties towards lectins.
Single walled carbon nanotubes (SWNTs) are a unique class of pseudo-1D nanostructures with considerable potential for nanomedicine including their use in drug delivery, bioimaging, cell transfection and as biosensors.1–3 However, pristine SWNTs are hydrophobic and insoluble in aqueous media.4 Moreover, SWNTs tend to form bundles, which are heavily entangled, giving rise to a complex and poorly described ensemble of aggregate structures.5 Improving the dispersion of SWNTs and establishing a well-defined interface between carbon nanotubes (CNTs) and biological systems is challenging yet key to accessing the immense potential of these materials for biological applications. This challenge has spawned intensive research efforts resulting in a broad palette of solubilization strategies.1,6 These strategies fall within two categories, namely covalent and non-covalent approaches.4,7 Glycoligand display on CNTs has been pursued using covalent attachment of amino sugars8,9 and β-aminophthaloyl appended sugars10via amidation, and non-covalent attachment (wrapping) of β-1,3-glucans,11–13starch,14alginic acid,15mucin mimics,16,17 glycodendrimers18 and sugars conjugated to pyrene.19 Such multivalent ligand display is relevant since molecular level clustering of glycoligands has been shown to result in enhancements of otherwise weak binding affinities to biological receptors.20–24 These affinity enhancements are attributed to kinetic and thermodynamic factors and are relevant to the design of optimized ligand–receptor interactions.
We recently described the synthesis of a series of mono- and bivalent α-D-mannopyranoside containing glycolipids and characterized their aggregation properties.25Glycolipid micelles were used as analytes in surface plasmon resonance (SPR) studies involving surfaces derivatized with the lectin Canavalia ensiformis agglutinin (Concanavalin A, Con A).25,26Glycolipid micelle–Con A interactions were found to be consistent with a bivalent analyte model, in which the micelle binds to two receptor sites independently.
Here, we show for the first time that bivalent synthetic glycolipids (GL-1 and GL-2, differing in the length of their ethylene glycol spacer) as well as monovalent glycolipid GL-3 can coat nanotubes in a non-covalent yet stable fashion by self assembly and can endow the resulting glycolipid-coated nanotubes (GL-SWNTs) with molecular recognition properties towards Con A (Fig. 1).
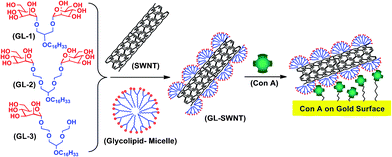 | ||
| Fig. 1 Schematic showing the three glycolipids used in this study (GL-1, GL-2 and GL-3), their self assembly on SWNTs (GL-SWNTs) and molecular recognition of tetrameric Con A presented on a gold surface. | ||
Sonication of both as-prepared and acid-treated (cut to a length of 400–1000 nm)27 SWNTs in water in the presence of any of the three glycolipids at a concentration above their critical micelle concentration (CMC) resulted in immediate and long-term solubilization of the SWNTs in the aqueous phase (Fig. 3A and Fig. S1†). The aqueous solutions of solubilized SWNTs were characterized by Vis/near-IR spectroscopy. Fig. 2 shows Vis/near-IR spectra of SWNTs in glycolipid solutions, in which D2O was used in place of H2O, as water absorbs in the near-IR region. The characteristic absorption bands in the Vis/near-IR region, attributed to the inter band transition between the mirror image spikes in the density of states (DOS) of the SWNTs,28 are clearly visible and their shapes are virtually identical to those for the individually dispersed SWNTs in the micelles of surfactants.29–31 This indicates that the aqueous glycolipid micelles used here have the ability to individually disperse the SWNTs.
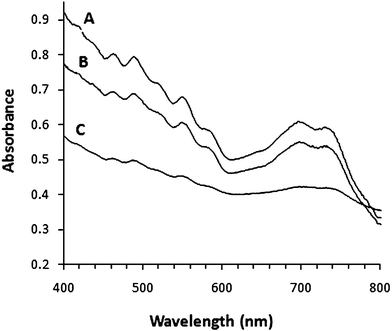 | ||
| Fig. 2 Vis/near-IR absorption spectra of SWNTs solubilized using glycolipid GL-2 (A), GL-1 (B) and GL-3 (C) solutions. | ||
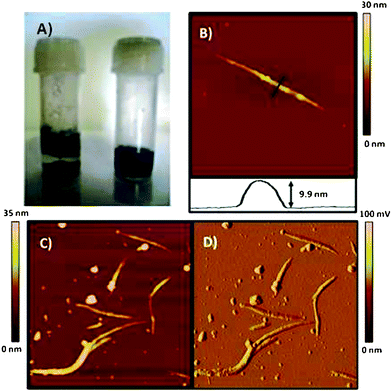 | ||
| Fig. 3 (A) Photograph of vials containing a suspension of SWNTs (left) and GL-1 coated SWNTs (right) in water taken one day after sonication. (B) Tapping mode AFM height image and cross section of GL-1 coated SWNTs, image scale 0.83 μm × 0.83 μm. (C) Height image of GL-1 coated SWNTs, image scale 2 μm × 2 μm. (D) Amplitude image of same region as Fig. 3C. Images of SWNTs were acquired on freshly cleaved mica surfaces. | ||
The topographical differences of SWNTs and GL-coated SWNTs were studied by means of atomic force microscopy (AFM). Uncut (as-prepared) SWNTs deposited from DMF onto mica ostensibly appear as bundles and heights of individual carbon nanotubes ranged from 1.2 to 2.5 nm (Fig. S2†). The inherent aggregation (bundling) behaviour of SWNTs was absent when nanotubes were suspended in an aqueous GL-1 solution, further confirming the successful coating of SWNTs by amphiphilic glycolipids and effective dispersion of nanotube bundles. AFM images shown in Fig. 3B–D provide evidence of a coating of cut SWNTs with GL-1. Nanotube coating was also successful with the other glycolipids and was found to be independent of the valency or spacer length of the glycolipids employed (Fig. S3†).
The glycolipids in the aqueous phase readily formed micelles with diameters of 6–8 nm.25 We hypothesize that during the solubilization process, individual SWNTs with diameters of 1–2 nm thread through micelles due to hydrophobic effects, resulting in a coating of the SWNTs with a glycolipid layer. This process is akin to the so-called “micellar solubilization” using surfactants.31 The amorphous material in Fig. 3C–D is likely to correspond to carbonaceous material formed during the manufacturing process of the SWNTs as shown in our earlier work.27
AFM height images in Fig. 3B–C show a striped topology of the SWNTs after GL coating which is in stark contrast with the rather smooth surface of uncoated SWNTs. The amplitude imaging mode is very sensitive to edges and topography. Indeed, the amplitude image in Fig. 3D confirms the striped topology of the coated nanotubes. These findings are consistent with those of the group of Shinkai11,12 for carbon nanotubes coated with helix-forming β-1,3-glycans. On the other hand, these results are in contrast to previous work using glycodendrimers which showed incomplete but homogeneous wrapping.18
The AFM height analysis of both uncoated and GL-SWNTs revealed an average 5–7 nm increase in nanotube height after the coating (Fig. 3B). The length of a GL-1 molecule is approximately 3.1 nm (Fig. S4†). The height analysis and striped patterns suggest that the glycolipids are assembled on the SWNT surface either with hemimicellar32 orientation or with a tilt angle of 50–60° from the surface normal. The striped topography may be a result of changes in the tilt angle of the glycolipids as expected for micelles.
Vibrational spectroscopy results further bolster our claim of successful glycolipid coating. Raman spectra of GL-SWNTs show a conspicuous change in the structure of the G band at 1590 cm−1 and a shift in the peak maximum in comparison to the bare SWNTs (Fig. 4). The narrowing of the G band can be ascribed for the electronic changes in SWNTs on glycolipid wrapping. Similar trends were observed in the literature for DNA-wrapped SWNTs.33 Furthermore, IR spectra of GL-coated SWNTs showed C–H valence vibrations of the alkyl chains, which are absent in the SWNT samples.
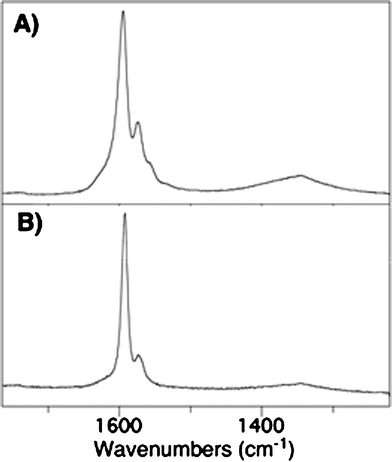 | ||
| Fig. 4 Raman spectra of as-prepared SWNTs (A) and GL-1 coated SWNTs (B). | ||
An assessment of the specific interaction of the GL-1 coated SWNTs with the lectin Con A immobilized on a carboxymethyl dextran-functionalized sensor chip surface (CM5) was conducted by means of surface plasmon resonance (SPR).34 Wheat germ agglutinin (WGA) and a bare CM5 sensor chip surface were used as controls to determine the level of non-specific binding and sample bulk refractive contributions.35 The sensorgrams demonstrate binding of GL-SWNTs to the immobilized Con A surface (Fig. 5) to the level of ∼6000 RU. WGA, on the other hand, gave a response that was comparable to the non-specific interaction on the bare CM5 sensor chip. The non-specific interaction observed for WGA could result from the hydrophobic interactions between partially coated GL-SWNTs and the lectin surface.
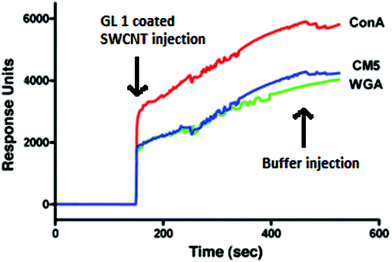 | ||
| Fig. 5 SPR sensorgrams obtained for the interaction of Con A, WGA, and the bare sensor chip (CM5) with GL-1 coated SWNTs. | ||
The specific recognition between GL-SWNTs and Con A follows the aggregate mechanism, in which the multivalent GL-SWNTs (glycoside clusters) could cross-link with the tetrameric lectin in a cooperative binding, thereby facilitating strong interactions between Con A and GL-SWNTs.
To further investigate the lectin recognition by GL-SWNTs, Con A and WGA were covalently immobilized on separate gold-coated glass slides and incubated with GL-SWNTs and bare SWNTs. These surfaces were then subjected to confocal Raman microscopy. The images in Fig. 6 demonstrate that only GL-1 coated nanotubes adsorbed on a glass slide onto which Con A had been immobilized (Fig. 6B). In contrast, unmodified SWNTs did not adsorb to Con A, nor did GL-SWNTs adsorb to a slide decorated with WGA instead of Con A. The non-specific interactions between GL-SWNTs and WGA that are observed in ‘real-time’ SPR were not seen in confocal Raman imaging due to detection limits and different sampling methods. The confocal Raman results provide ample evidence of specific molecular recognition of lectin Con A by mannose appended GL-SWNTs.
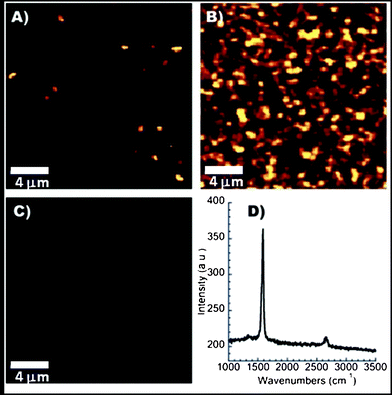 | ||
| Fig. 6 Confocal Raman images of Con A modified surfaces incubated with (A) unmodified SWNTs, or (B) GL-1 coated SWNTs. (C) WGA modified surface incubated with GL-1 coated SWNTs. Images are maps of the intensity at 1595 cm−1 and (D) Raman spectrum obtained from a point in image B. | ||
Laser scanning confocal microscopy of GL-SWNTs incubated with fluorescein isothiocyanate (FITC)-labelled Con A showed a network of clustered proteins, further corroborating the molecular lectin recognition by GL-SWNTs (Fig. S5†). No such clustering was observed for FITC-labelled Con A only.
Finally, AFM images of GL-SWNTs after incubation with Con A feature small spheres decorating and, in some cases, clustering around the nanotubes (Fig. S6†). The height of these spheres (around 4 nm) matches the expected size of the Con A proteins. These features were observed both on uncut and cut GL-SWNTs. Note that we have not investigated differences in the affinity for Con A between the three different glycolipids varying in valency and tether length. The study of multivalency effects will be the subject of a future study.
In conclusion, we have reported on a novel approach of displaying glycoligands on SWNTs using the self assembly of glycolipids. GL-SWNTs show specific molecular recognition properties in aqueous solution. This non-covalent coating approach is robust and versatile and can be readily adapted to other types of glycolipids with different recognition properties or valencies. Our strategy has several key advantages over the current methods of glycoligand display on carbon nanotubes. For example, the valency of the building blocks and the flexibility of the pendant glycoligand can be tuned. Furthermore, several building blocks can be mixed to either display multiple glycoligands on the nanotubes or to adjust the density of the glycoligands in order to optimize binding. CNTs functionalized by self assembly of glycolipids are poised to advance efforts in applying bio-functionalized nanotubes in drug delivery, bioimaging or biosensing. At the same time, the described functionalization approach may also improve the toxicological profile of carbon nanotubes.
Experimental
Vis/near-IR studies
About 0.5 mg of uncut-SWNTs was added to a D2O solution (5 ml) of a glycolipid (10 mM), and the mixture was sonicated by using an ultrasonic cleaner (Digitech) that operates at 100 W power for 1 h, followed by centrifugation (Sigma 3-18 K centrifuge) at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 2 h. The centrifugation was used to remove the metal catalysts and SWNT bundles. The upper 70–80% of the supernatant was then carefully decanted and then the supernatant was used for Vis/near-IR measurements. The spectra were recorded on a Cary 50 scan UV-Vis spectrophotometer (Varian).
000 g for 2 h. The centrifugation was used to remove the metal catalysts and SWNT bundles. The upper 70–80% of the supernatant was then carefully decanted and then the supernatant was used for Vis/near-IR measurements. The spectra were recorded on a Cary 50 scan UV-Vis spectrophotometer (Varian).
Atomic force microscopy
For AFM sample preparation, approximately 0.5 mg of SWNTs were added to 1 ml of DMF and sonicated for 2 h at room temperature. A small amount of the sample (∼5 μl) was placed onto a freshly cleaved mica surface and further dried in an oven at 50 °C for 6 h. In the case of the glycolipid coated SWNTs, 5 μl of the glycolipid coated SWNTs stock solution was transferred onto a freshly cleaved mica surface and further dried at room temperature for 12 h.Commercial Nanoscope IV and V AFMs (Veeco, USA) were used in tapping mode in air using silicon cantilevers (FESP, Veeco, USA) with a nominal spring constant of 2.8 N m−1 and operating at the cantilever resonance frequency (around 75 kHz). The diameters of the AFM tips were determined using the method described by Wang and Chen.36 All tip diameters measured were between 20 and 22 nm and were consistent with the tip diameter range quoted by the manufacturer. To obtain accurate height data of all nanotubes, AFM images were acquired with the scan rate, amplitude set-point and pixel number optimized according to the experimental details outlined by Poenitzsch and Musselman.37 It should still be noted that even with careful attention to AFM imaging parameters, radial compression of adsorbed single walled nanotubes can occur due to van der Waals forces. This can result in the nanotubes no longer being a perfect cylindrical system.38
Surface plasmon resonance
The surface plasmon resonance studies were performed on a Biacore 2000 (Pharmacia Biosensor AB, Sweden) operated using the software version 1.3. The research grade carboxymethylated dextran matrix (CM5) was used in the analysis and the chip contained four flow cells of dimensions (l × w × h) 2.4 × 0.5 × 0.05 mm3. After equilibration of the sensor surface with HEPES buffer (pH 7.4), the CM5 sensor chip was activated at a flow rate of 10 μl min−1 with a 6 min pulse of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of freshly prepared 0.1 M N-hydroxysuccinimide (NHS) and 0.1 M N-ethyl-N-(dimethylaminopropyl) carbodiimide. Con A was immobilized at a concentration of 100 μg ml−1 in 10 mM NaOAc buffer (pH 4.3) in one sensor channel. The remaining NHS esters were blocked by the addition of 1.0 M ethanolamine hydrochloride at pH 8.5 for 7 min. WGA was immobilized on a different sensor channel and this surface was used as a reference to the Con A functionalized surface. All binding experiments with GL-1 coated SWNTs were performed at a flow rate of 20 μl min−1.
1 mixture of freshly prepared 0.1 M N-hydroxysuccinimide (NHS) and 0.1 M N-ethyl-N-(dimethylaminopropyl) carbodiimide. Con A was immobilized at a concentration of 100 μg ml−1 in 10 mM NaOAc buffer (pH 4.3) in one sensor channel. The remaining NHS esters were blocked by the addition of 1.0 M ethanolamine hydrochloride at pH 8.5 for 7 min. WGA was immobilized on a different sensor channel and this surface was used as a reference to the Con A functionalized surface. All binding experiments with GL-1 coated SWNTs were performed at a flow rate of 20 μl min−1.
Raman spectroscopy
A commercial Raman microscope (Renishaw Ramascope System 1000) was used to collect Raman scattered light. The system consists of a single spectrograph fitted with holographic notch filters and a Peltier cooled CCD detector upon which the Raman spectrum is dispersed. The spectrograph is coupled to a Leica microscope (DMLM) with computer controlled sample stage, rigidly fixed to the spectrograph base plate. The spectrometer has a maximum lateral resolution of 2 μm and depth resolution of 2 mm; however, the effective field of view was increased to allow signal collection from a larger area. The spectrometer is equipped with three lasers for use in the standard normal incidence sampling geometry: 532 nm, 633 nm, and 785 nm. The two visible wavelengths deliver around 5 mW of power to the sample, whereas the 785 nm laser delivers around 100 mW. The 785 nm laser (Renishaw-badged compact diode laser) was used for the spectra reported in this work, and was focused onto the sample using a ×50 objective (Leica, NA = 0.75). The 785 nm laser produces a line focus at the sample, increasing the area analysed during acquisition. Calibration of the instrument was checked with the emission lines of a neon lamp.Confocal Raman microscopy
Gold coated glass slides were functionalized with a 20 mM solution of mercaptopropionic acid (MPA) in ethanol–water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent by incubating overnight at room temperature. The terminal carboxylic groups of MPA were then activated by immersion of the slide in a buffer (pH = 5.2) containing 0.002 M EDC and 0.005 M NHS for 1 h. Two proteins ConA (0.1 mg ml−1) and WGA (0.1 mg ml−1) were then immobilized on two separate MPA-treated and activated gold slides. The protein-immobilized gold surfaces were incubated with glycolipid-coated (GL-1) nanotubes for 1 h. As a control, a slide to which ConA had been immobilized was treated with plain cut SWNTs for 1 h. The three samples were imaged using a confocal Raman microscope Alpha 300RS from Witec (Germany) maintaining the same instrumental parameters.
1) solvent by incubating overnight at room temperature. The terminal carboxylic groups of MPA were then activated by immersion of the slide in a buffer (pH = 5.2) containing 0.002 M EDC and 0.005 M NHS for 1 h. Two proteins ConA (0.1 mg ml−1) and WGA (0.1 mg ml−1) were then immobilized on two separate MPA-treated and activated gold slides. The protein-immobilized gold surfaces were incubated with glycolipid-coated (GL-1) nanotubes for 1 h. As a control, a slide to which ConA had been immobilized was treated with plain cut SWNTs for 1 h. The three samples were imaged using a confocal Raman microscope Alpha 300RS from Witec (Germany) maintaining the same instrumental parameters.
Laser scanning confocal microscopy
Glycolipid coated SWNTs were incubated with FITC labelled Con A (Sigma, USA). 10 μl of the solution was applied to a glass slide, washed with Milli Q water and dried. 10 μl of prolong gold antifade reagent (Invitrogen, USA) was applied to a cover slip which was then lowered onto the glass slide. The corners of the cover slip were sealed with nail polish. The sample was then cured for 24 h in the dark before examination under the microscope. Confocal microscopy was performed on a Leica TCS SP5 laser scanning confocal microscope.Acknowledgements
This work was supported by funding from the Australian Research Council's Discovery Scheme.References
- F. S. Lu, L. R. Gu, M. J. Meziani, X. Wang, P. G. Luo, L. M. Veca, L. Cao and Y. P. Sun, Adv. Mater., 2009, 21, 139–152 CrossRef CAS.
- K. T. Constantopoulos, C. J. Shearer, A. V. Ellis, N. H. Voelcker and J. G. Shapter, Adv. Mater., 2010, 22, 557–571 CrossRef CAS.
- K. Kostarelos, A. Bianco and M. Prato, Nat. Nanotechnol., 2009, 4, 627–633 CrossRef CAS.
- D. Tasis, N. Tagmatarchis, A. Bianco and M. Prato, Chem. Rev., 2006, 106, 1105–1136 CrossRef CAS.
- B. Kim, Y. H. Lee, J. H. Ryu and K. D. Suh, Colloids Surf., A, 2006, 273, 161–164 CrossRef CAS.
- V. Georgakilas, N. Tagmatarchis, D. Pantarotto, A. Bianco, J. P. Briand and M. Prato, Chem. Commun., 2002, 3050–3051 RSC.
- C. A. Dyke and J. M. Tour, Chem.–Eur. J., 2004, 10, 812–817 CrossRef CAS.
- L. R. Gu, T. Elkin, X. P. Jiang, H. P. Li, Y. Lin, L. W. Qu, T. R. J. Tzeng, R. Joseph and Y. P. Sun, Chem. Commun., 2005, 874–876 RSC.
- H. F. Wang, L. R. Gu, Y. Lin, F. S. Lu, M. J. Meziani, P. G. J. Luo, W. Wang, L. Cao and Y. P. Sun, J. Am. Chem. Soc., 2006, 128, 13364–13365 CrossRef CAS.
- L. R. Gu, Y. Lin, L. W. Qu and Y. P. Sun, Biomacromolecules, 2006, 7, 400–402 CrossRef CAS.
- T. Hasegawa, T. Fujisawa, M. Numata, M. Umeda, T. Matsumoto, T. Kimura, S. Okumura, K. Sakurai and S. Shinkai, Chem. Commun., 2004, 2150–2151 RSC.
- M. Numata, M. Asai, K. Kaneko, A. H. Bae, T. Hasegawa, K. Sakurai and S. Shinkai, J. Am. Chem. Soc., 2005, 127, 5875–5884 CrossRef CAS.
- M. Numata, K. Sugikawa, K. Kaneko and S. Shinkai, Chem.–Eur. J., 2008, 14, 2398–2400 CrossRef CAS.
- A. Star, D. W. Steuerman, J. R. Heath and J. F. Stoddart, Angew. Chem., Int. Ed., 2002, 41, 2508–2512 CrossRef CAS.
- Y. Liu, P. Liang, H. Y. Zhang and D. S. Guo, Small, 2006, 2, 874–878 CrossRef CAS.
- X. Chen, G. S. Lee, A. Zettl and C. R. Bertozzi, Angew. Chem., Int. Ed., 2004, 43, 6111–6116 CrossRef CAS.
- X. Chen, U. C. Tam, J. L. Czlapinski, G. S. Lee, D. Rabuka, A. Zettl and C. R. Bertozzi, J. Am. Chem. Soc., 2006, 128, 6292–6293 CrossRef CAS.
- P. Wu, X. Chen, N. Hu, U. C. Tam, O. Blixt, A. Zettl and C. R. Bertozzi, Angew. Chem., Int. Ed., 2008, 47, 5022–5025 CrossRef CAS.
- M. Assali, M. P. Leal, I. Fernández, R. Baati, C. Mioskowski and N. Khiar, Soft Matter, 2009, 5, 948–950 RSC.
- Y. C. Lee and R. T. Lee, Acc. Chem. Res., 1995, 28, 321–327 CrossRef CAS.
- J. J. Lundquist and E. J. Toone, Chem. Rev., 2002, 102, 555–578 CrossRef CAS.
- O. Srinivas, N. Mitra, A. Surolia and N. Jayaraman, Glycobiology, 2005, 15, 861–873 CrossRef CAS.
- G. Thoma, M. B. Streiff, A. G. Katopodis, R. O. Duthaler, N. H. Voelcker, C. Ehrhardt and C. Masson, Chem.–Eur. J., 2006, 12, 99–117 CrossRef CAS.
- G. Thoma, A. G. Katopodis, N. Voelcker, R. O. Duthaler and M. B. Streiff, Angew. Chem., Int. Ed., 2002, 41, 3195–3198 CrossRef CAS.
- B. N. Murthy, N. H. Voelcker and N. Jayaraman, Glycobiology, 2006, 16, 822–832 CrossRef CAS.
- K. V. Brinda, A. Surolia and S. Vishveshwara, Biochem. J., 2005, 391, 1–15 CrossRef CAS.
- M. W. Marshall, S. Popa-Nita and J. G. Shapter, Carbon, 2006, 44, 1137–1141 CrossRef CAS.
- P. Kim, T. W. Odom, J.-L. Haung and C. M. Lieber, Phys. Rev. Lett., 1999, 82, 1225 CrossRef CAS.
- V. C. Moore, M. S. Strano, E. H. Haroz, R. H. Hauge and R. E. Smalley, Nano Lett., 2003, 3, 1379–1382 CrossRef CAS.
- A. Ishibashi and N. Nakashima, Chem.–Eur. J., 2006, 12, 7595–7602 CrossRef CAS.
- M. J. O'Connell, S. M. Bachio, C. B. Huffman, V. C. Moore, M. S. Strano, E. H. Haroz, K. L. Rialon, P. J. Boul, W. H. Noon, C. Kittrell, J. Ma, R. H. Hauge, R.B. Weisman and R. E. Smalley, Science, 2002, 297, 593–596 CrossRef CAS.
- E. J. Wallace and M. S. P. Sansom, Nanotechnology, 2009, 20, 045101 CrossRef.
- H. Kawamoto, T. Uchida, K. Kojima and M. Tachibana, Chem. Phys. Lett., 2006, 432, 172–176 CrossRef CAS.
- M. R. Nussio, M. J. Sykes, J. O. Miners and J. G. Shapter, ChemMedChem, 2007, 2, 366–373 CrossRef CAS.
- B. Krotkiewska, M. Pasek and H. Krotkiewski, Acta Biochim. Pol., 2002, 49, 481–490 CAS.
- Y. Wang and X. Chen, Ultramicroscopy, 2007, 107, 293–298 CrossRef CAS.
- V. Z. Poenitzsch and I. H. Musselman, Microsc. Microanal., 2006, 12, 221–227 CrossRef CAS.
- T. Hertel, R. E Walkup and P. Avouris, Phys. Rev. B, 1998, 58, 13870–13873 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials and methods, AFM images of uncoated SWNTs, GL-coated SWNTs, Con A incubated GL-SWNTs and laser scanning confocal microscopy images of FITC-Con A incubated GL-SWNTs. See DOI: 10.1039/c2ra01192a |
| ‡ Current address: Mawson Institute, University of South Australia, Mawson Lakes Blvd., Mawson Lakes, SA 5095, Australia |
| This journal is © The Royal Society of Chemistry 2012 |
