Ultrafast microwave-assisted synthesis of MCNCs with high saturation magnetization and sustained aqueous stability†
Shuai
Xu
ab,
Zhimin
Luo
b,
Yujie
Han
b,
Jia
Guo
a and
Changchun
Wang
*ab
aState Key Laboratory of Molecular Engineering of Polymers, Department of Macromolecular Science, Fudan University, Shanghai, 200433, P. R. China. E-mail: ccwang@fudan.edu.cn
bLaboratory of Advanced Materials, Fudan University, Shanghai, 200438, P. R. China
First published on 27th February 2012
Abstract
A facile and green microwave route was developed to ultra-quickly synthesize magnetite colloidal nanocrystal clusters (MCNCs) by reducing iron(III) chloride with ethylene glycol within 10 min at 150 °C. The obtained uniform MCNCs exhibited excellent crystallinity, saturation magnetization and sustained aqueous stability upon addition of stabilizers.
Magnetic nanoparticles (MNPs), which are a significant class of materials with unique functionality, have attracted considerable attention due to their promising applications in fundamental science and sophisticated technology, such as ferrofluids, photonic crystals, drug delivery systems, and magnetic resonance imaging (MRI).1,2 To fulfil specific requirements, the physiochemical properties of magnetic nanomaterials must be rationally tailored and optimized.3 MNPs with high saturation magnetization and suitable size are critically important because it allows a rapid response to an external magnetic field for enhancement of enrichment and separation efficiency in bio-medical applications.4
Recently, an one-pot polyol-mediated solvothermal method was developed to fabricate magnetite colloidal nanocrystal clusters (MCNCs) that possess greatly improved magnetic responsiveness, tunable granular sizes, and well-defined structures.5 This leads to a remarkable progress in the synthetic technology of magnetic nanomaterials. However, the synthesis of MCNCs has often been subjected to severe reaction conditions including an elevated temperature (up to 200 °C), a long reaction time (more than 10 h), and a sealed autoclave.4 Hence, developing a simple and green way to obtain MCNCs with uniform size and high saturation magnetization is highly demanded. Microwave irradiation has been proved to be an efficient and green method to accelerate chemical reactions through rapid volumetric heating.6 Compared with the conventional solvothermal method, microwave irradiation offers remarkable merits, including dramatically shortened reaction time, lower thermal gradients, reduced energy consumption, and higher yields.7 Based on this, the microwave-assisted synthesis strategy has been widely used for synthesis of versatile nanomaterials, such as polymer microspheres,8 metallic nanoparticles,9 metal oxides,10 and quantum dots (QDs).11 Recently, magnetite nanoparticles were also synthesized using microwave irradiation.12 However, the resulting water-dispersible MNPs usually suffered from a wide size distribution,12a–c while the hydrophobic MNPs exhibited the weak magnetic responsiveness,12d,e which was responsible for limiting their potential applications in bio-related fields.
Herein, uniform MCNCs with high magnetic susceptibility were prepared via a rapid and green microwave method with iron chloride hexahydrate (FeCl3·6H2O) as an iron precursor, ammonia acetate (NH4OAc) as an alkaline source, and ethylene glycol (EG) as reducing agent and microwave-absorbing solvent (Fig. S1, ESI†). In a typical synthesis, the homogeneous dispersion of precursors in EG was transferred into a 35 ml vessel with a crimp cap, heated by a single-mode microwave irradiation of 2.45 GHz (CEM Discovery, CEM Inc. USA), and the other reaction parameters were rationally modulated (Experimental details and Fig. S2, ESI†). In sharp comparison to the known solvothermal process occurring in an autoclave, the microwave-assisted reaction was dramatically speeded up and completed within minutes. As displayed in Fig. 1a, the representative TEM image demonstrated the formation of spherical MCNCs with a diameter of approximate 273 nm. Also, the SEM image revealed that the clusters were formed in the fashion of aggregation of many small primary nanocrystals (Fig. 1b). The selected area electron diffraction (SAED) pattern (inset of Fig. 1c), recorded by focusing an electron beam on the boxed part, exhibited a single-crystalline diffraction pattern of the cluster.13 Additionally, it was estimated that the periodic fringe spacing of the crystallographic planes was about 0.48 nm, agreeing well with the interplanar spacing between the (111) lattice planes of the Fe3O4 crystal (Fig. 1d). To our knowledge, this is the first example for microwave-assisted preparation of MCNCs with well-defined structures and uniform sizes in just a few minutes.
 | ||
| Fig. 1 (a) TEM and (b) SEM image of MCNCs (inset is an enlarged one), (c) Enlarged TEM image of a part of a single cluster, inset is the selected area electron diffraction (SAED) pattern, and (d) HRTEM image of the boxed region in image c. | ||
Fig. 2 showed the TEM images of products synthesized at different temperatures within 10 min. At 120 °C, due to the limited energy supply, there were no MCNCs formed during the microwave irradiation (Fig. 2a). At elevated temperatures, it was observable that many small magnetite nanocrystals could be aggregated into clusters (Fig. 2b–c). For example, at 150 °C, almost all primary nanocrystals gathered into uniform and intact MCNCs (Fig. 2d). When the temperature was further increased from 160 °C to 200 °C, the resulting MCNCs could afford similar morphologies (Fig. 2e–f). Based on the above results, one can conclude that temperature plays a vital role in the formation of MCNCs.
 | ||
| Fig. 2 TEM images of MCNCs synthesized at (a) 120 °C, (b) 130 °C, (c) 140 °C, (d) 150 °C, (e) 160 °C, and (f) 200 °C for 10 mins. All scale bars are 200 nm. | ||
Fig. 3a showed the powder XRD patterns of the products; all peaks could be indexed well to the magnetic cubic structure of magnetite (JCPDS No.75-1610). X-ray photoelectron spectroscopy (XPS) of the products synthesized at 150 °C for 10 min exhibited peaks at 711 and 724 eV (Fig. 3b), which are the characteristic peaks of Fe 2p3/2 and Fe 2p1/2 oxidation states, respectively.4b Together with XRD results, it was clear that the magnetite phase had been synthesized by this facile and simple microwave pathway. As for the products prepared at 120 °C, only one weak and broad peak was found around 24°, which could be ascribed to the amorphous nanomaterials (Fig. 3a(i)). With increasing temperature, the peak at 24° disappeared, and the characteristic peaks of magnetite became more and more distinct, indicating the enhanced crystallization of the magnetite nanoparticles (Fig. 3a(ii–vi)). The magnetization curves (Fig. 3c) revealed that when a higher temperature was adopted in the microwave irradiation, a better saturation magnetization of the products was obtained. The MCNCs prepared below 100 °C were found to have no magnetism. As the reaction temperature was increased from 120 °C to 150 °C to 200 °C, the magnetization values of the products were dramatically enhanced from 4.5 to 71.5 to 79.1 emu g−1. Moreover, it is worthwhile to notice that the magnetic hysteresis loops (Hc < 25 Oe) could be negligible for all the samples, indicating the superparamagnetic property of the MCNCs (300 K). Such an excellent magnetic property is beneficial to the applications of MCNCs in biomolecule separation, and magnetically-guided drug delivery.
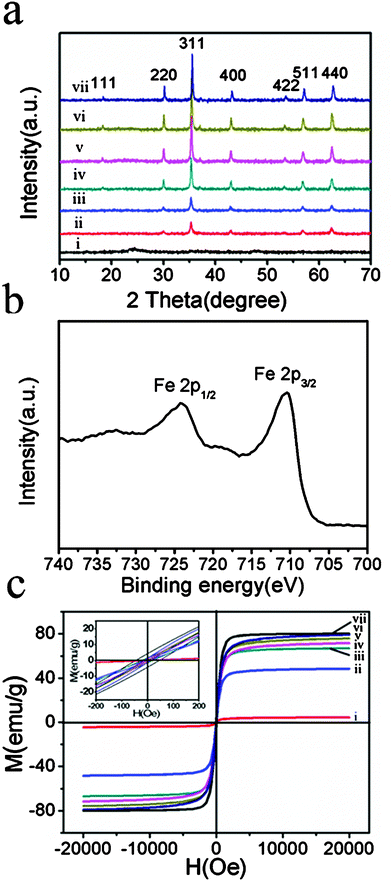 | ||
| Fig. 3 (a) Power XRD patterns, (b) XPS spectrum of MCNCs synthesized at 150 °C for 10 min, and (c) magnetization curves (T = 300 K) of MCNCs synthesized at (i) 120 °C, (ii) 130 °C, (iii) 140 °C, (iv) 150 °C, (v) 160 °C, and (vi) 200 °C for 10 mins and (vii) Fe3O4 CNCs obtained from solvothermal process at 200 °C for 15 h. | ||
In addition, we investigated the effect of different reaction times on the evolution of MCNCs at 150 °C. The typical TEM images of the products synthesized with different times showed their similar morphologies (Fig. S3, ESI†). Also, we were aware that, after the reaction proceeded for 5 min, the morphology of the products didn't undergo dramatic changes, which implied that the microwave heating could take effect only using 5 min. The powder XRD patterns of products with various reaction times gave almost the same position and intensity of peaks, indicating the difference of crystallinity among these products was slight and negligible (Fig. S4, ESI†). In addition, the saturation magnetizations of the four samples, as expected, were very close with respect to each other (Fig. S5, ESI†). Apart from temperature and time, the microwave power was also investigated. If the power used was less than 150 W, inferior crystallinity and low magnetization were obtained due to the insufficient microwave energy supply.
To elucidate the influence of reaction temperature and time on the crystallite sizes and magnetization, the foregoing results were compiled, and displayed in Fig. 4. The crystallite sizes of the MCNCs were determined according to the Scherrer's equation.14 As shown in Fig. 4a, the crystallite sizes of the MCNCs increased gradually from 21.8 to 35.7 nm, as the temperature was elevated from 130 °C to 200 °C. Also, this increasing tendency was observed when the saturation magnetizations were plotted as a function of temperature. On the other hand, as the reaction time prolonged from 5 min to 60 min, the crystallite sizes of MCNCs were all in the range of 30 nm (Fig. 4b). Compared with the MCNCs (200 °C, 15 h) synthesized by the typical solvothermal method, the microwave-synthesized MCNCs (200 °C, 10 min) give a similar grain size (35.7 nm vs. 37.4 nm), comparable saturation magnetization (79.1 emu g−1vs. 80.0 emu g−1), and high crystalline degree; all of these disclose the powerful microwave effect in the reaction. However, the microwave irradiation pathway is proved to have a remarkably decreased reaction time, lower temperature, and reduced energy consumption, thereby leading to an overall reduction in energy consumption and high efficiency.
 | ||
| Fig. 4 Influence of the reaction (a) temperature at a fixed time of 10 min and (b) time at a fixed temperature of 150 °C over the crystallite size (nm) and magnetization values (emu g−1) of as-prepared MCNCs. | ||
In the typical synthesis conditions of MCNCs, we found that the pressure inside the vessel quickly reached about 140 psi, indicating that the solvent EG might be in the boiling state. Therefore, it can be rationally figured out that the polar solvent EG with high dielectric losses has excellent microwave absorbing capacity,9a and can create “hot spots” in the bulk solution. This will lead to acceleration of the mass transfer and crystal growth.15 Meanwhile, Fe(III) precursors are partially reduced to Fe(II) species by EG in alkaline conditions. Then the reaction system could reach a high temperature under the microwave irradiation, resulting in a spontaneous crystallographic fusion of nuclei into nanocystals. The freshly formed crystallites attach to each other, and tend to aggregate rapidly into clusters for a lower surface energy.
In addition, the in situ surface modification was conducted to enhance the colloidal stability for potential applications of MCNCs. Due to the strong affinity between –COOH and iron species, citrate acid (CA), poly(acrylate acid) (PAA) and poly(γ-glutamic acid) (PGA), were selected as stabilizers. As shown in Fig. 5, the resultant carboxyl-stabilized MCNCs were uniform in size and much smaller than non-modified ones (e.g. 192 nm for CA-stabilized MCNCs). As shown in Fig. 6, the transmittance of PBS dispersion (pH 7.4, 50 mM) for non-modified MCNCs increased rapidly in minutes, and finally reached 80% after 3 h of setting, while the transmittance of modified ones maintained at a low level of about 5% after the same time scale of 3 h. From the corresponding photos inside, it could be found that, compared to the transparency of non-modified MCNCs, the surface fictionalized ones still keep turbid without obvious precipitates after 3 h, reflecting the sustained aqueous stability of the particles. The reason responsible for the prolonged stability is the significant difference of surface charges. The MCNCs without stabilizer had slight positive charges of about +4 mV. In contrast, all the modified MCNCs were negatively charged, and showed surface charges of −39, −40 and −30 mV for CA, PAA and PGA, respectively. Thus, these MCNC dispersions could remain stable for several hours via the electrostatic repulsion interaction.
 | ||
| Fig. 5 TEM images of MCNCs stabilized by (a) CA, (b)PAA, (c)PGA, and (d) zeta potential values of the above MCNCs and bare MCNCs. All scale bars are 200 nm. | ||
 | ||
| Fig. 6 The plots of transmittance as a function of time measured for PBS dispersion (pH 7.4, 50 mM) of bare MCNCs and MCNCs stabilized by CA, PAA, and PGA, respectively. Insets are photographs of above samples after 3 h of setting. | ||
In conclusion, we have developed a simple, energy-saving and environmentally friendly microwave-assisted method to fabricate MCNCs. Both reaction temperature and microwave power acted as the key factors in directing the formation of the MCNCs. The obtained products were well-crystallized, uniform in size, and possessed a high magnetization. CA, PAA, PGA could serve as stabilizing agents to remarkably prolong the aqueous stability of the MCNCs, and such surface modification also can fulfil the further immobilization of biomolecular ligands. This facile, economical and green microwave pathway would undoubtedly offer a new avenue for the synthesis of well-defined magnetic nanostructures.
This work was supported by National Science Foundation of China (Grant No. 20974023, 21034003, 51073040 and 21128001), Shanghai Committee of Science and Technology, China (Grant No. 10XD1400500). We thank Prof. L. H. Wang for his kind help in the use of the microwave instrument.
References
- (a) S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. V. Elst and R.N. Muller, Chem. Rev., 2008, 108, 2064–2110 CrossRef CAS; (b) A. H. Lu, E. L. Salabas and F. Schuth, Angew. Chem., Int. Ed., 2007, 46, 1222–1244 CrossRef CAS.
- (a) D. L. Shi, N. M. Bedford and H. S. Cho, Small, 2011, 7, 2549–2567 CrossRef CAS; (b) J. H. Gao, H. W. Gu and B. Xu, Acc. Chem. Res., 2009, 42, 1097–1107 CrossRef CAS; (c) J. P. Ge and Y. D. Yin, Angew. Chem., Int. Ed., 2011, 50, 1492–1522 CrossRef CAS.
- (a) S. Palchoudhury, W. An, Y. L. Xu, Y. Qin, Z. T. Zhang, N. Chopra, R. A. Holler, C. H. Turner and Y. P. Bao, Nano Lett., 2011, 11, 1141–1146 CrossRef CAS; (b) S. Palchoudhury, Y. L. Xu, J. Goodwin and Y. P. Bao, J. Mater. Chem., 2011, 21, 3966–3970 RSC.
- (a) Y. H. Deng, D. W. Qi, C. H. Deng, X. M. Zhang and D. Y. Zhao, J. Am. Chem. Soc., 2008, 130, 28–29 CrossRef CAS; (b) B. Luo, S. Xu, A. Luo, W. R. Wang, S. L. Wang, J. Guo, Y. Lin, D. Y. Zhao and C. C. Wang, ACS Nano, 2011, 5, 1428–1435 CrossRef CAS.
- (a) H. Deng, X. L. Li, Q. Peng, X. Wang, J. P. Chen and Y. D. Li, Angew. Chem., Int. Ed., 2005, 44, 2782–2785 CrossRef CAS; (b) J. P. Ge, Y. X. Hu, M. Biasini, W. P. Beyermann and Y. D. Yin, Angew. Chem., Int. Ed., 2007, 46, 4342–4345 CrossRef CAS; (c) B. Luo, X. J. Song, F. Zhang, A. Xia, W. L. Yang, J. H. Hu and C. C. Wang, Langmuir, 2010, 26, 1674–1679 CrossRef CAS.
- (a) S. A. Galema, Chem. Soc. Rev., 1997, 26, 233–238 RSC; (b) J. M. Patete, X. H. Peng, C. Koenigsmann, Y. Xu, B. Karn and S. S. Wong, Green Chem., 2011, 13, 482–519 RSC; (c) J. A. Dahl, B. L. S. Maddux and J. E. Hutchison, Chem. Rev., 2007, 107, 2228–2269 CrossRef CAS.
- (a) J. A. Gerbec, D. Magana, A. Washington and G. F. Strouse, J. Am. Chem. Soc., 2005, 127, 15791–15800 CrossRef CAS; (b) X. L. Hu and J. C. Yu, Chem.–Asian J., 2006, 1, 605–610 CrossRef CAS.
- Z. S. An, W. Tang, C. J. Hawker and G. D. Stucky, J. Am. Chem. Soc., 2006, 128, 15054–15055 CrossRef CAS.
- (a) H. Kawasaki, Y. Kosaka, Y. Myoujn, T. Narushima, T. Yonezawa and R. Arakawa, Chem. Commun., 2011, 47, 7740–7742 RSC; (b) C. Vollmer, E. Redel, K. A. Shandi, R. Thomann, H. Manyar, C. Hardacre and C. Janiak, Chem.–Eur. J., 2010, 16, 3849–3858 CrossRef CAS.
- (a) K. L. Ding, Z. J. Miao, Z. M. Liu, Z. F. Zhang, B. X. Han, G. M. An, S. D. Miao and Y. Xie, J. Am. Chem. Soc., 2007, 129, 6362–6363 CrossRef CAS; (b) X. L. Hu, J. C. Yu, J. M. Gong, Q. Li and G. S. Li, Adv. Mater., 2007, 19, 2324–2329 CrossRef CAS.
- (a) Y. He, H. T. Lu, L. M. Sai, W. Y. Lai, Q. L. Fan, L. H. Wang and W. Huang, J. Phys. Chem. B, 2006, 110, 13352–13356 CrossRef CAS; (b) A. L. Washington II and G. F. Strouse, J. Am. Chem. Soc., 2008, 130, 8916–8922 CrossRef.
- (a) W. W. Wang, Y. J. Zhu and M. L. Ruan, J. Nanopart. Res., 2007, 9, 419–426 CrossRef CAS; (b) M. Edrissi and R. Norouzbeigi, J. Nanopart. Res., 2010, 12, 1231–1238 CrossRef CAS; (c) R. Y. Hong, T. T. Pan and H. Z. Li, J. Magn. Magn. Mater., 2006, 303, 60–68 CrossRef CAS; (d) I. Bilecka, I. Djerdj and M. Niederberger, Chem. Commun., 2008, 44, 886–888 RSC; (e) H. Y. Hu, H. Yang, P. Huang, D. X. Cui, Y. Q. Peng, J. C. Zhang, F. Y. Lu, J. Lian and D. L. Shi, Chem. Commun., 2010, 46, 3866–3868 RSC.
- A. Narayanaswamy, H. Xu, N. Pradhan and X. G. Peng, Angew. Chem., Int. Ed., 2006, 45, 5361–5364 CrossRef CAS.
- K. Tanaka, S. Nakashima, K. Fujita and K. Hirao, J. Phys.: Condens. Matter, 2003, 15, 469–474 CrossRef.
- J. C. Jia, J. C. Yu, Y. X. J. Wang and K. M. Chan, ACS Appl. Mater. Interfaces, 2010, 2, 2579–2584 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, characterization and physicochemical properties. See DOI: 10.1039/c2ra01169g |
| This journal is © The Royal Society of Chemistry 2012 |
