Preparation of proton exchange membranes with high performance by a pulsed plasma enhanced chemical vapor deposition technique (PPECVD)†
Zhongqing
Jiang
*a and
Zhong-jie
Jiang
*b
aDepartment of Chemical Engineering, Ningbo University of Technology, Ningbo, 315016, Zhejiang, China. E-mail: zhongqingjiang@hotmail.com; Tel: +86-574-88477381
bDepartment of Nature and Sciences, University of California, Merced, 95343, USA. E-mail: zhongjiejiang1978@hotmail.com
First published on 28th February 2012
Abstract
A pulsed plasma enhanced chemical vapor deposition technique has been employed to prepare sulfonated plasma polymerized membranes. The technique adopting such a pulse plasma discharge can avoid the further dissociation of active species and polymers formed by the plasma discharge during their polymerization and deposition and produces membranes with a higher content of proton exchange groups. The obtained membranes have higher water uptake capability and IECs, low methanol permeability and activation energy for proton conduction, and therefore show great promise for application in direct alcohol fuel cells.
The ever-increasing pressure from increased oil prices and environmental issues has stimulated a world-wide effort towards the development of high energy density power sources.1,2 In line with this effort, direct alcohol fuel cells (DAFCs), which use naturally regenerative liquid alcohols as fuels, are receiving a great deal of attention due to their high power density, low operating temperature, low pollutant emission, easy handling and processing of liquid fuels, and are therefore considered as an alternative energy source with great potential to alleviate the pressure or even solve the problems from the energy crisis.3–5 However, the practical situation is not as one might expect. It has been noticed that the real applications of DAFCs have been greatly hampered by some fundamental problems, such as high cost, low operation temperature and high fuel permeability of membranes, and low activity and poor durability of electrocatalysts, although significant progress has been made during the past decades.6–9 At the heart of these problems are polymer proton exchange membranes (PEMs), which have been considered as the major source of failure.10–13 As the key component of a DAFC, a PEM serves a bifunction of separating reactants and conducting protons, and therefore largely determines the performance of DAFCs.14,15 A highly qualified PEM is required to be able to efficiently transfer the protons, but block the fuel diffusion through the membrane, which can improve the electrochemical performance of DAFCs and protect the cathode from poisoning by the products of the undesired reactions of permeated fuels.16 Up to now, great efforts, ranging from modification of Nafion (a current commercial PEM) to the exploitation of new fabrication methods, have been paid to prepare PEMs of low cost and desired properties, such as high operation temperature and low fuel permeability of membranes, and high proton conductivity.7,8 Among various approaches reported for the membrane fabrication and modification, a technique which utilizes a plasma discharge to initiate the polymerization of monomers for the fabrication of PEMs has attracted significant attention.17,18 In a process called plasma polymerization, the dissociation of monomers caused by the plasma discharge and the polymer formation due to the recombination of active species generated by the monomer dissociation occurs simultaneously, allowing for the fabrication of PEMs with a highly cross-linked structure.13 The fabricated plasma membranes therefore exhibit significantly low fuel permeability and can protect the electrode catalysts from poisoning, which make them particularly attractive as PEMs for DAFCs, because their highly cross-linked structure formed during the plasma polymerization can effectively block fuels from diffusion through the membranes.13,19 However, the conventional plasma polymerization techniques usually produce PEMs with a low content of proton exchange groups.19 The dissociation of functional groups during the plasma discharge makes the introduction of ion-exchange moieties into the polymer membranes rather difficult.19,20
To address this problem, we recently introduced a new plasma polymerization technique which uses a low-frequency, after-glow capacitively coupled plasma (AGCCP) discharge method to synthesize PEMs.21,22 The obtained membranes are reported to have a high content of proton exchange groups, high proton conductivity and low methanol permeability. However, one problem is that these membranes exhibit a significantly higher activation energy for proton conduction, because their highly cross-linked structure requires more energy for the proton migration from one free-site to another,21,22 which is disadvantageous for their use in DAFCs. The low activation energy for proton conduction favors the proton migration in the membranes especially at low temperatures, and is thus helpful to reduce energy losses and improves the utilization of energy during the electrochemical process. In this sense, the preparation of PEMs, not only with high proton conductivity but with low fuel permeability and activation energy for proton conduction, is of great significance. In this work we utilize a pulsed plasma enhanced chemical vapor deposition (PPECVD) technique which involves alternating the plasma on and off to prepare PEMs, in which styrene and trifluoromethane sulfonic acid are used as starting monomers. Using such a technique, the polymerization of the monomers used for the PEMs is initially initiated by the short period of plasma discharge, which is followed by a longer period of plasma off allowing for the recombination of the active species generated due to the dissociation of monomers by the plasma discharge and the deposition of the obtained polymers. In this way, the degree of monomer dissociation can be well controlled and the further dissociation of the formed polymers by the plasma discharge during the polymer deposition can be avoided, which are therefore expected to produce PEMs of relatively less cross-linked structures with high contents of proton exchange groups. The results show that the plasma membranes synthesized by the PPECVD technique have a high content of proton exchange groups, higher ion-exchange capacities and proton conductivity and low methanol permeability, which indicate that they are quite suitable as PEMs for DAFC applications.
The PEMs synthesized by the PPECVD technique exhibit a dense structure with no cracks detectable at the membrane surfaces, which is demonstrated in Fig. 1 showing a typical SEM image of the obtained PEMs. The inset of Fig. 1 indicates that these membranes are relatively uniform with thicknesses tunable by changing the duration of the fabrication times (see ESI† for the experimental details). At the surface of the membranes are some small particles, which are considered to be formed from the growth of the activated species generated during the plasma discharge and the subsequent deposition due to gravitational force. This is a typical phenomenon associated with the plasma fabrication of PEMs.23–25
 | ||
| Fig. 1 SEM image of the obtained PEM. The inset is an SEM image of the cross section of PEM and its corresponding thickness is indicated. | ||
Up to now, although much work has been devoted to the plasma fabrication of membranes, their formation mechanism is still a subject of debate due to the complexity of plasma reactions. A generally accepted mechanism for the membrane formation is that the plasma discharge generates energetic particles, which collide with monomer molecules to produce the active species, such as excited molecules, free radicals and ions, due to the dissociation of monomer molecules. The formation of membranes is a result of the recombination of these active species. In the conventional plasma polymerization with a continuous wave plasma discharge, the monomer molecules are prone to dissociation due to their random collisions with the energetic plasma particles and the nonuniformity of energy distribution of these energetic particles. Thus, the fabricated membranes are reported to have a lack of proton exchange groups due to the dissociation of proton exchange groups even in the process of the polymer deposition. The technique adopting the PPECVD can, however, protect proton exchange groups from dissociation during the plasma off for the deposition of polymer, and is expected to produce PEMs with higher contents of proton exchange groups. Fig. 2 shows typical Fourier transform infrared (FTIR) spectra of the obtained PEMs. It shows that the PEMs fabricated by the PPECVD technique contain molecular segments similar to Nafion® 117, which can be evidenced from their strong absorptions at 1200–1230 cm−1 and 1030 cm−1, assigned to the respective feature vibrations of the CFx and the sulfonic acid group (–SO3H). The relatively higher peak intensity at 1030 cm−1 indicates that the plasma fabricated PEMs contain a higher content of –SO3H. The existence of other absorption peaks, such as those at 1490, 1450, 880, 830 and 760 cm−1, etc., indicates that the plasma PEMs consist of more complicated structures than Nafion® 117. The results of X-ray photoelectron spectroscopy are in good agreement with those obtained from the FTIR spectra shown above. The existence of sulfonic acid groups can be demonstrated from the S2p spectra shown in Fig. 3. A spectra deconvolution demonstrates that the S2p spectra of the membranes comprise four components. The first two components at 170 and 169 eV can be assigned to sulfonic acid groups and RSO2(OR′), while the other two components with sulfur at lower oxidation states 166 and 164.5 eV are attributed to RSO2R′ or RSOR′. A rough estimate done by the quantitative analysis of the XPS results shows that the membranes obtained by the PPECVD technique have a significantly higher content of sulfonic acid groups in comparison to Nafion® 117 (as shown in Table 1), which is an important feature of the obtained plasma PEMs for their special use in DAFCs because the higher content of sulfonic acid group provides a great possibility to improve the proton conductivity of the membranes. These results demonstrate that the method using the PPECVD technique for the fabrication of PEMs can effectively protect sulfonic acid groups from the dissociation during the membrane fabrication, and produces membranes with a higher content of proton exchange groups as expected.
 | ||
| Fig. 2 FTIR spectra of (a) the obtained plasma PEM and (b) Nafion® 117. | ||
 | ||
| Fig. 3 XPS spectra of S2p for (a) the obtained plasma membranes and (b) the Nafion® 117. | ||
| Membrane | Pulsed plasma membrane | Continuous wave plasma membranec | Nafion®117 |
|---|---|---|---|
| a The content of –SO3H is measured by a rough quantitative analysis of the XPS results. The value obtained for Nafion®117 is very close to that reported in the literature.13,19 b The data for σ presented in the Table 1 are obtained at 20 °C. c Continuous wave plasma membrane is synthesized by an after-glow capacitively coupled plasma discharge technique. | |||
| Content of –SO3H(%)a | 4.83 ± 0.13 | 4.84 ± 0.12 | 1.23 ± 0.04 |
| Water uptake (%) | 75.4 ± 2.3 | 64.4 ± 1.9 | 34.6 ± 1.1 |
| IEC (meq g−1) | 1.77 ± 0.04 | 1.40 ± 0.03 | 0.93 ± 0.01 |
| σ (mS cm−1)b | 194.4 ± 6.6 | 181.0 ± 5.3 | 155.3 ± 3.8 |
| E a (kJ mol−1) | 11.13 ± 0.33 | 27.29 ± 4.03 | 10.20 ± 0.22 |
| P (m2 s−1) | 1.51 ± 0.002 × 10−11 | 6.64 ± 0.005 × 10−12 | 2.06 ± 0.005 × 10−10 |
For desirable PEMs, they are generally expected to have a high water uptake and ion exchange capacity (IEC) except for a high content of proton exchange groups. As is well known, the performance of a PEM is dependent upon proton conductivity, which indeed greatly relies on the water uptake capability since water assists the migration of protons through the membrane. In this sense, a membrane with a high level of water uptake would be beneficial for improving the electrochemical performance of DAFCs. The measurement of the water uptake of a membrane is done using a gravimetric technique (see ESI† for details). Table 1 compares the water uptake of the obtained plasma membranes with that of Nafion® 117. Clearly, the plasma membranes obtained by the PPECVD technique exhibit significantly higher water uptake capability. The higher water uptake capability of the plasma PEMs might be attributed from their higher content of sulfonic acid groups, whose strong hydrophilicity promotes the adsorption of more water in the membranes. The IEC, which measures the relative concentration of acid groups within PEMs that can be used as proton exchange groups participating in the electrochemical processes, is therefore an important parameter reflecting the electrochemical properties of PEMs. The data shown in Table 1 demonstrate that the obtained plasma membranes have a higher degree of IEC, which is in good agreement with the results presented above that the obtained plasma membranes have a higher content of proton exchange groups.
The measurements of the proton conductivity of the membranes are carried out by an AC impedance method. The proton conductivity σ of the membranes can be deduced from the impedance diagram using eqn (1):
 | (1) |
 | ||
| Fig. 4 (a) Nyquist impedance plot of a plasma polymerized membrane synthesized by the PPECVD technique. The inset is the Nyquist impedance plot of the Nafion® 117. (b) Arrhenius plot showing temperature dependence of membranes proton conductivity for the plasma PEMs and Nafion® 117. The dots are the experimental data. The straight lines are the Arrhenius-type fitting of the experimental data. | ||
The ionic conduction in a solid electrolyte membrane is a thermally activated process. Therefore, the proton conductivity of a membrane follows a simple Arrhenius-type law as a function of temperature. Fig. 4b shows the temperature dependence of the proton conductivity of the plasma membranes and the Nafion® 117, from which the activation energy Ea (kJ mol−1) for proton conduction can be derived by a linear least square fit of the temperature dependence of conductivity using eqn (2).

| (2) |
The occurrence of proton migration through a polymer PEM is mainly via two ways, i.e. proton hopping-Grothuss (or “jump”) mechanism and vehicle mechanism. The Grothuss mechanism suggests that the migration of the protons through membranes is along the chain of water molecules which is accompanied by the successive formation and deformation of H3O+, while in the vehicle mechanism, the migration of protons is believed to occur via their combination with water molecules to diffusionally transport through the membrane in the form of a complex like H+(H2O)n. For the proton migration in perflourinated Nafion® 117, it is generally accepted that these two mechanisms coexist, whereas the vehicle mechanism which requires less energy for the proton migration is considered to play a big contribution because the low cross-linked structure of Nafion® 117 allows the migration of the hydrated protons through the membrane. Due to the similarity of the plasma PEMs with Nafion® 117 in the proton exchange groups and the chemical composition (see ESI† for the elemental analysis, Table S1), we believe that the proton migration through these PEMs is also a synergistic process of these two mechanisms, and the vehicle mechanism is also considered to play the main role in the proton migration due to the comparative activation energies with Nafion® 117. This might be different from the proton migration in the plasma membrane synthesized by the continuous wave after-glow capacitively coupled plasma discharge technique where the proton migration occurs mainly via the Grothuss mechanism because the highly cross-linked structures is unfavorable for the migration of protons via the vehicle mechanism.
The results presented above show that the plasma membranes synthesized by the PPECVD technique are greatly suitable for the application in the DAFCs because these membranes are reported to have thin and tunable thicknesses, higher contents of proton exchange groups, higher water uptake capability and IEC, and low activation energy. However, for practical uses, these membranes are also required to have low alcohol diffusion permeability. The alcohol crossover through the membrane would result in the decrease of the cathode's performance and the waste of fuel, which is therefore a severe problem associated with DAFCs using Nafion as the PEM. Although the plasma membranes synthesized by the continuous wave after-glow capacitively coupled plasma discharge technique exhibit higher water uptake, IECs and proton conductivity and low alcohol permeability (as shown in Table 1), their significantly higher activation energy for the proton conduction makes the proton migration relatively difficult, which is disadvantageous for their use in DAFCs. The results presented in this work, however, demonstrate that the technique using a pulsed plasma discharge to initiate the polymerization of monomers can produce PEMs not only with all the advantages of the plasma membranes but also with low alcohol diffusion permeability. As shown in Fig. 5, the diffusion of methanol through the plasma membranes is measured (see ESI† for details). Using eqn (3), the methanol permeability through the membranes can be obtained and is presented in Table 1.
![Plots of [(CB(t)VBLm)/(CAS)] vs. diffusion time for the plasma PEMs and Nafion® 117 (in the inset). The straight lines in the figures are the corresponding fits of the experimental data, and the slops correspond to their methanol permeability.](/image/article/2012/RA/c2ra01244h/c2ra01244h-f5.gif) | ||
| Fig. 5 Plots of [(CB(t)VBLm)/(CAS)] vs. diffusion time for the plasma PEMs and Nafion® 117 (in the inset). The straight lines in the figures are the corresponding fits of the experimental data, and the slops correspond to their methanol permeability. | ||
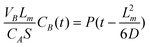
| (3) |
In conclusion, a pulse plasma discharge technique has been used for the preparation of PEMs for DAFC applications. The technique using the pulse plasma discharge can reduce the degree of monomer dissociation and avoid the further dissociation of polymers formed by the plasma discharge during their deposition and produces membranes with higher contents of proton exchange groups. The obtained membranes are reported to have a higher water uptake capability and IEC, low activation energy for the proton conduction and methanol permeability, and are therefore quite suitable for applications in DAFCs.
Acknowledgements
This work was financially supported by the Chinese National Natural Science Foundation (No. 11105078), the Ningbo Natural Science Foundation (No. 2011A610209), and the start-up foundation of Ningbo University of Technology (No. 0080011540018). The author gratefully acknowledges the support of K.C. Wong Education Foundation.References
- A. D'Epifanio, M. A. Navarra, F. C. Weise, B. Mecheri, J. Farrington, S. Licoccia and S. Greenbaum, Chem. Mater., 2010, 22, 813–821 CrossRef CAS.
- S. Yun, H. Im, Y. Heo and J. Kim, J. Membr. Sci., 2011, 380, 208–215 CrossRef CAS.
- B. P. Ladewig, R. B. Knott, A. J. Hill, J. D. Riches, J. W. White, D. J. Martin, J. da Costa and G. Q. Lu, Chem. Mater., 2007, 19, 2372–2381 CrossRef CAS.
- Z. C. Zhang, E. Chalkova, M. Fedkin, C. M. Wang, S. N. Lvov, S. Komarneni and T. Chung, Macromolecules, 2008, 41, 9130–9139 CrossRef CAS.
- Y. Gao, G. P. Robertson, M. D. Guiver, S. D. Mikhailenko, X. Li and S. Kaliaguine, Macromolecules, 2004, 37, 6748–6754 CrossRef CAS.
- B. C. Lin, S. Cheng, L. H. Qiu, F. Yan, S. M. Shang and J. M. Lu, Chem. Mater., 2010, 22, 1807–1813 CrossRef CAS.
- L. Wang, B. L. Yi, H. M. Zhang and D. M. Xing, J. Phys. Chem. B, 2008, 112, 4270–4275 CrossRef CAS.
- J. L. Lu, S. F. Lu and S. P. Jiang, Chem. Commun., 2011, 47, 3216–3218 RSC.
- D. Truffier-Boutry, A. De Geyer, L. Guetaz, O. Diat and G. Gebel, Macromolecules, 2007, 40, 8259–8264 CrossRef CAS.
- K. Xu, C. Chanthad, M. R. Gadinski, M. A. Hickner and Q. Wang, ACS Appl. Mater. Interfaces, 2009, 1, 2573–2579 CAS.
- S. Matsumura, A. R. Hlil, C. Lepiller, J. Gaudet, D. Guay, Z. Q. Shi, S. Holdcroft and A. S. Hay, Macromolecules, 2008, 41, 281–284 CrossRef CAS.
- K. Matsumoto, T. Higashihara and M. Ueda, Macromolecules, 2009, 42, 1161–1166 CrossRef CAS.
- S. Roualdes, I. Topala, H. Mahdjoub, V. Rouessac, P. Sistat and J. Durand, J. Power Sources, 2006, 158, 1270–1281 CrossRef CAS.
- P. V. Komarov, I. N. Veselov, P. P. Chu and P. G. Khalatur, Soft Matter, 2010, 6, 3939–3956 RSC.
- J. Liu, H. T. Wang, S. A. Cheng and K. Y. Chan, J. Membr. Sci., 2005, 246, 95–101 CrossRef CAS.
- C. Felice, S. Ye and D. Y. Qu, Ind. Eng. Chem. Res., 2010, 49, 1514–1519 CrossRef CAS.
- A. Ennajdaoui, S. Roualdes, P. Brault and J. Durand, J. Power Sources, 2010, 195, 232–238 CrossRef CAS.
- S. Roualdes, M. Schieda, L. Durivault, I. Guesmi, E. Gerardin and J. Durand, Chem. Vap. Deposition, 2007, 13, 361–369 CrossRef CAS.
- H. Mahdjoub, S. Roualdes, P. Sistat, N. Pradeilles, J. Durand and G. Pourcelly, Fuel Cells, 2005, 5, 277–286 CrossRef CAS.
- F. Finsterwalder and G. Hambitzer, J. Membr. Sci., 2001, 185, 105–124 CrossRef CAS.
- Z. Q. Jiang, Z. J. Jiang and Y. D. Meng, J. Membr. Sci., 2011, 372, 303–313 CrossRef CAS.
- Z. Q. Jiang, Z. J. Jiang, X. Y. Yu and Y. D. Meng, Plasma Processes Polym., 2010, 7, 382–389 CrossRef CAS.
- Z. Q. Jiang, Y. D. Meng, Z. J. Jiang and Y. C. Shi, Surf. Rev. Lett., 2007, 14, 1165 CrossRef CAS.
- Z. Q. Jiang, Y. D. Meng and Y. C. Shi, Jpn. J. Appl. Phys., 2008, 47, 6891 CrossRef CAS.
- Z. Q. Jiang, Y. D. Meng, Z. J. Jiang and Y. C. Shi, Surf. Rev. Lett., 2009, 16, 297 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental section. See DOI: 10.1039/c2ra01244h/ |
| This journal is © The Royal Society of Chemistry 2012 |
