DOI:
10.1039/C2RA01162J
(Paper)
RSC Adv., 2012,
2, 2055-2060
Rapid controllable high-concentration synthesis and mutual attachment of silver nanowires†
Received
22nd November 2011
, Accepted 14th December 2011
First published on 12th January 2012
Abstract
A new well-designed two-step injection solution-based method has been proposed to rapidly prepare uniform silver nanowires on a gram-scale. Uniform silver nanowires with different and controllable aspect ratios can be synthesized in high yields. Due to a high chemical reaction rate arising from high AgNO3 concentration, the whole synthetic process of the silver nanowires is rapid and can be controlled within 20 min. The influences of different experimental parameters on the morphologies of silver nanoproducts in such a high-concentration synthesis were investigated. It was found that the addition of an appropriate amount of Cl− ions had significant impact on the formation and the aspect ratios of the silver nanowires. Solution volume effect was also studied. It was shown that our synthetic method strongly favours the synthesis of silver nanowires in large solution volumes. A reasonable formation mechanism of the silver nanowires was put forward based on novel experimental phenomena. The mutual attachment of silver nanowires was observed and was found to play an important role in such high-concentration synthesis of silver nanowires. This facile scalable preparation method of silver nanowires can decrease the synthetic cost greatly and broaden the research and application areas of silver nanowires.
Introduction
With the fast development of nanotechnology, the application and commercialization of nanomaterials have been some of the most important issues that materials scientists and engineers who major in nanoscience and nanotechnology have to face,1 and to decrease the preparation cost while improving the quality of nanoproducts is an inevitable road to achieve this aim.2,3 As well-known, sol–gel,4 molten salt,5 and combustion methods6 and other solid-state top–down methods, such as explosion, physical grinding, high energy ball milling,7,8 have taken the lead in realizing the industrial production of nanomaterials, but apparently these methods are not very suitable for the preparation of those nanomaterials with uniform size, specified morphology and good solution or matrix dispersion. Solution-based methods, as some of the most effective routes to obtain high-quality nanomaterials at a low temperature, provide a good candidate approach for the large-scale acquisition of specified nanomaterials.9,10 However, in the past decade, the liquid-phase synthesis of nanomaterials has remained on the small lab scale with low solution concentrations and volumes. Due to the strong influence of solution concentration and volume effects, simple enlargement of solution volume or increase of precursor concentration based on lab-scale synthetic methods reported in the literature usually result in the generation of nanomaterials with quite different morphologies and sizes. Therefore, it is necessary to explore the preparation and formation mechanism of nanomaterials in large solution volumes or with high precursor concentrations.
As we know, the synthesis of nanomaterials in large solution volumes can obtain massive nanoproducts in one experiment. As a consequence, it can not only save a lot of time, energy and manpower, but it can also avoid excessive loss of nanoproducts in post-processing. Compared to large-volume synthesis, high-concentration synthesis is more economical as a result of a lower cost of raw materials, energy, time and manpower. As the name suggests, high-concentration synthesis is a kind of solution-based chemical synthetic method to obtain nanomaterials in solutions of high precursor concentration. Owing to the characteristic feature of high precursor concentration, in high-concentration solutions there are very different inside interactions from those in lower precursor concentrations, such as larger electrostatic forces of solute ions, stronger interaction of nanoproducts, different intermolecular interactions and surface tension, etc. All of these make a distinct chemical reaction environment. In the synthesis of inorganic nanomaterials, according to the equation of chemical reaction dynamics, higher reagent concentrations usually lead to faster chemical reaction rates, and simultaneously induce the formation of more inorganic particles. In order to decrease surface energy as much as possible, large spherical particles with lower surface energy will be the favourite products. In addition, due to stronger interactions of preformed particles arising from shorter adjacent distances in solutions of high product concentration, as-formed solid particles tend to break the surfactant effect and aggregate to further decrease surface energy. Naturally, in such a synthesis, high precursor concentration is always paired with bad product quality. Therefore, high-concentration solution-based synthesis of high-quality nanomaterials with specified morphology has had to encounter a lot of challenges in both theory and practice. However, obvious advantages in decreasing the preparation cost of nanomaterials make this kind of synthetic method significantly promising. In this paper, we will investigate the high-concentration and large-volume synthesis of silver nanowires so as to explore the positive significance of high-concentration and large-volume synthesis of nanomaterials and expect colleagues to carry out further studies in this field.
Silver
nanowires, as one of the noble metallic nanowires, have a wide application domain owing to their unique mechanical, electrical, thermal, plasmonic and chemical properties. Recently, they have been in dramatically increasing demand attributed to their exhibiting good potential application in conductive composite materials, plasmonic enhancement, flexible electronics, sterilization, catalysis, etc.11–17 Conventional low-mass preparation has struggled to meet the growing requirements for silver nanowires. Therefore, there is an urgent need to find some better ways to obtain massive high-quality silver nanowires.
However, although the synthesis of silver nanowires has been studied for a dozen years and a variety of silver nanowires have been synthesized by different methods,18–23 high-concentration gram-scale synthesis of high-quality silver nanowires has rarely been reported.24 Herein, we will present a new well-designed two-step injection method for the high-concentration synthesis of silver nanowires. In this method, gram-scale well-defined silver nanowires with controllable size and aspect ratios can be obtained in high yield. Based on novel experimental phenomena in our high-concentration synthesis, a developed formation mechanism of silver nanowires has been proposed. It was found that the mutual attachment of silver nanowires plays an important role in such high-concentration synthesis.
Experimental section
Materials
Ethylene glycol (EG) and NaCl were purchased from Sigma-Aldrich, and AgNO3 and polyvinylpyrrolidone (PVP) (Mw ≈ 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) were purchased from the Shanghai Chemical Reagent Company. All chemicals were used as received without further purification.
000) were purchased from the Shanghai Chemical Reagent Company. All chemicals were used as received without further purification.
Synthetic procedures
In a typical synthesis of silver nanowires, 2.005 g PVP (Mw ≈ 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was dissolved in 34 ml EG with moderate stirring and heated to 160 °C until the temperature was stable. Then 40 μl of 0.2 M NaCl was added into the flask. After one minute, 6 ml EG solution containing 2.0384 g of silver nitrate (AgNO3, CAgNO3 = 2 M) was added to the flask at the rate 100 μl/10 s by pipette. Once the reaction solution began to become cloudy from transparent, all residual precursor solution was added into the flask immediately. Then the flask was sealed until the solution become glistening, indicating the formation of silver nanowires. The products were collected by centrifugation.
000) was dissolved in 34 ml EG with moderate stirring and heated to 160 °C until the temperature was stable. Then 40 μl of 0.2 M NaCl was added into the flask. After one minute, 6 ml EG solution containing 2.0384 g of silver nitrate (AgNO3, CAgNO3 = 2 M) was added to the flask at the rate 100 μl/10 s by pipette. Once the reaction solution began to become cloudy from transparent, all residual precursor solution was added into the flask immediately. Then the flask was sealed until the solution become glistening, indicating the formation of silver nanowires. The products were collected by centrifugation.
Characterization
X-Ray diffraction (XRD) patterns were recorded on a Philips X'pert diffractometer with Cu-Kα radiation (λ = 1.54178 Å). The morphology and structure of the samples were investigated by field emission scanning electron microscopy (FE-SEM, JEOL JSM-6335F) and transmission electron microscopy (Philips FEG CM20) with an accelerating voltage of 200 kV. High-resolution transmission electron microscopy (HRTEM) was performed on a JEOL-2010 transmission electron microscope. The sample was dried at 60 °C for 24 h before characterization.
Results and discussion
Polyol synthesis is a well-established method used to prepare nanomaterials over the past decade.25,26 In a typical polyol synthesis of silver nanostructures through reducing AgNO3, metallic silver can be obtained through the following chemical reactions:27,28| | | 2HOCH2CH2OH → 2CH3CHO + 2H2O | (1) |
| | | 2Ag+ + 2CH3CHO → CH3COCOCH3 + 2Ag + 2H+ | (2) |
| | | 4HNO3 + 3Ag → 3AgNO3 + NO + 2H2O | (3) |
At high temperatures, ethylene glycol can be oxidized to aldehyde to reduce silver ions to metallic silver. At the same time, nitric acid was produced in situ due to the generation of protons and could dissolve silver solids into silver ions at high temperature again. Therefore, in such a synthesis, the total generation rate of silver solids was determined by both the reduction rate of silver ions and the oxidation rate of metallic silver. With the decomposition and escape of nitric acid at high temperature, the reaction (2) will dominate the reaction system gradually. In order to achieve better control of the morphology of silver nanostructures in high-concentration synthesis, it is necessary to decrease the generation rate of silver solids induced by high precursor concentration. Apparently, to increase the rate of reaction (3) is an effective route to achieve this purpose. As we know, aqua regia composed of hydrochloric acid and nitric acid has a stronger corrosive nature and thus a stronger etching ability on metal than nitric acid alone. Therefore, in our synthesis, NaCl was used to introduce chloride ions so as to enhance the etching ability of the reaction solution on metallic silver and decrease the total generation rate of silver solids.
Two-step injection solution-based synthesis of silver nanowires
In this work, a two-step injection method was adopted to obtain silver nanowires for the first time. In the preparation, polyvinylpyrrolidone (PVP) and a small amount of NaCl were firstly dissolved in ethylene glycol (EG) and heated to 160 °C. Then high-concentration AgNO3 solution was injected at a slow and constant rate until the solution become cloudy. Then a fast solution injection was executed immediately and all residual precursor solution was added quickly. This is a well-designed method to prepare silver nanowires. The first slow injection step can guarantee the formation of a large amount of small silver particles due to low precursor concentration and strong etching of Cl−/NO3− on crystal boundaries of as-generated large irregular polycrystalline silver solids. And the second fast injection step can increase the concentration of silver ions dramatically to induce the generation of a lot of pentagonal silver particles.29 Then the preformed small silver particles can be fed back to pentagonal particles to provide silver sources for the growth of silver nanowires.30 This method conformed well to the growth mechanism of silver nanowires and thus can ensure the high-yield formation of silver nanowires in theory. A lot of experiments have confirmed that the fast injection step can increase the yield ratio of silver nanowires significantly (Fig. S2†). In the injection process, we chose the time that the solution started to become cloudy as the start time that the fast injection was executed. We think that the emergence of cloudy solution is a sign that small silver particles become saturated and aggregated, and thus it should be the best critical time to go to the next step so as to guarantee the quality of silver nanowires. Generally, our two-step injection synthetic method is experimental phenomenon-dependent, and it can greatly avoid the interference from uncertain factors, such as humidity, impurities, etc., and guarantee high experimental reproducibility.
Fig. 1 shows SEM images of as-prepared typical silver nanowires and an HRTEM image of a selected single silver nanowire. As can be seen from the SEM images, high-yields of silver nanowires with good size uniformity and aspect ratios can be obtained. A TEM image of a typical silver nanowire is shown in Fig. 1e. A multiply twinned crystalline structure and obvious twin planes can be observed clearly. The HRTEM image in Fig. 1f shows lattice spacing of 1.45 Å perpendicular to the growth direction, corresponding to the interplanar distance of (220) faces, which indicates that the growth direction of the twinned crystalline silver nanowires is <220>. Parallel to the growth direction, the interplanar spacing is 2.37 Å, corresponding to the interplanar distance of the (111) faces, which means that a lot of (111) crystalline faces extended along the growth direction.
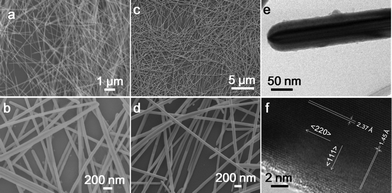 |
| | Fig. 1
SEM, TEM and HRTEM images of typical silver nanowires synthesized in our experiments with different precursor concentrations and magnifications. (a, b) CAgNO3 = 0.1 M; (c, d) CAgNO3 = 0.15 M. (e) TEM image of a typical silver nanowire synthesized when CAgNO3 = 0.1 M. (f) HRTEM image in the fringe of Fig. 1e. T = 160 °C, VEG = 40 ml. | |
The effects of the AgNO3 concentration on the morphology of silver products
The influence of the AgNO3 concentration on the morphology of the silver products was firstly investigated. In our experiments, the concentration of AgNO3 is generally several to ten times higher than the concentration commonly reported in the literature. Fig. 2 shows SEM images of silver nanowires formed using different AgNO3 concentrations. Obviously, high-yield silver nanowires can be formed in a wide concentration range. Few silver solids with other morphologies can be found in the products. On increasing the concentration of AgNO3, the average diameter of the silver nanowires increased slightly but the one-dimensional structure is maintained, which indicated that, in our synthesis, the concentration was no longer the main morphology-determining factor. It was thought that the two-step injection synthetic method played a critical role in reducing the influence of the concentration on the morphology of the products, because in such synthesis, silver particles were formed in polyol solution with a high molar ratio of PVP to silver ions, which was conducive to the control of the size, and grew in a lower definite ratio, which was beneficial for the formation of specified nanostructures. This feature is especially important for high-concentration synthesis. In our synthesis, even if the concentration of AgNO3 achieved 0.6 M (1 g AgNO3/10 ml EG), one-dimensional silver structures can still be obtained. In addition, a high AgNO3 concentration will bring more nitrate ions into the reaction system to increase the concentration of in situ generated nitric acid and enhance the etching ability of the reaction solution on large irregular silver particles, thus providing more small silver particles for the growth of silver nanowires.
 |
| | Fig. 2
SEM images of silver nanowires synthesized in ethylene glycol with different concentrations of AgNO3 precursors, (a) 0.1 M; (b) 0.15 M; (c) 0.3 M. CCl− = 0.4 mM, T = 160 °C, VEG = 40 ml. | |
The effects of the chloride ion concentration on the morphology of silver products
Subsequently, the effects of chloride ions were investigated. It was found that, in high-concentration synthesis, chloride ions played an especially important role, attributed to their enhanced corrosive nature as a result of the synergetic effects of hydrochloric acid and nitric acid (aqua regia). Recently, chloride ions have been widely used to control the morphology of metal nanostructures.28,31,32 In the case of silver nanowire synthesis through reducing AgNO3, the addition of chloride ions can greatly enhance the etching ability of in situ generated nitric acid on silver solids to facilitate the generation of small single crystalline or twinned crystalline silver particles that can play as silver sources of silver nanowires. Fig. 3 shows SEM images of silver nanoproducts formed using different concentrations of chloride ions. We can see from Fig. 3a, without the addition of NaCl, irregular silver nanoparticles were dominant. The addition of a small amount of chloride ions can change the morphology of the products markedly and help to obtain silver nanowires. Such a great change in the morphology of the silver products reflected the importance of chloride ions in controlling this reaction. However, too high a chloride ion concentration will bring about some bad effects due to the introduction of a lot of AgCl solids. We can see that, when the concentration of chloride ions reached 2 mM, the silver products began to become markedly non-uniform, and a lot of short and thick silver rods appeared (Fig. 3f). In our synthesis, in a large chloride ion concentration range, silver nanowires can be well fabricated. Moreover, the aspect ratio of silver nanowires can be well adjusted by controlling the concentration of chloride ions. We found that silver nanowires have a higher aspect ratio when CCl− = 0.4 mM than when CCl− = 0.2 mM and CCl− = 0.1 mM (Fig. 3b, c, d). It might be because chloride ions can decrease the reaction rate. In the experiments, we can obviously observe that the generation of cloudy solution can be postponed by the addition of chloride ions. On increasing the content of chloride ions, the time at which the reaction solution became cloudy increased. This should be ascribed to an enhanced etching ability of Cl−/NO3− pairs thus decreasing the generation rate of silver solids and a strong interaction between silver ions and chloride ions to reduce the concentration of free silver ions. In consequence, an increased reaction time in the first injection step can result in the generation of more small silver particles to provide more silver sources for the growth of silver nanowires derived from pentagonal twinned crystals and thus in obtaining longer silver nanowires.
 |
| | Fig. 3
SEM images of as-prepared silver nanowires with different aspect ratios under different concentrations of chloride ions, (a) without the addition of chloride ions; (b) CCl− = 0.1 mM; (c) CCl− = 0.2 mM; (d) CCl− = 0.4 mM; (e) CCl− = 0.8 mM; (f) CCl− = 2 mM. T = 160 °C, CAgNO3 = 0.15 M, VEG = 40 ml. | |
Solution volume effects
In order to expand this method for real large-scale production of silver nanowires, we also studied the solution volume effect on the formation of silver nanowires. Fig. 4 shows SEM images of as-synthesized silver nanowires formed in different solution volumes. It can be seen that, in small solution volumes, the products are non-uniform, and there exist a lot of thick and short silver rods. On increasing the solution volume, the quality of the silver nanowires can be improved obviously, which means the solution volume has significant impact on the morphology of silver nanowires and our method is strongly in favour of the large-scale synthesis of silver nanowires in large solution volumes. We think that solutions with larger volume have a wider molecular motion range, a smaller gas–liquid interface area per unit mass, a possible greater internal pressure and a better thermal stability, and every molecule or particle will be affected by more other molecules or particles through electrostatic effects and other interactions. All of those make a different reaction environment for the synthesis of nanomaterials. Herein, larger volumes can decrease the decomposition rate of nitric acid to guarantee their etching effect on metallic silver by decreasing the escape of gas products due to greater internal pressure and a smaller gas–liquid interface area thus improving the quality of silver nanowires. In our experiments, we can obviously observe that, for large-volume solutions, several minutes after the fast injection, when the glass stopper of the three-necked flask was uncovered, the reaction solution became reddish brown immediately, which meant a lot of nitrogen dioxide was generated due to the contact of preformed nitrogen monoxide in the solution with oxygen, and for small-volume solutions, the phenomenon is not obvious. This indicated that large solution volumes can hold gas products better so as to decrease the decomposition rate of nitric acid. In addition, better thermal stability should also be beneficial for the improvement of the quality of silver products.
 |
| | Fig. 4
SEM images of silver nanowires with different volumes of ethylene glycol, (a) 20 ml; (b) 40 ml; (c) 80 ml. CAgNO3 = 0.3 M, CCl− = 0.4 mM, T = 160 °C. | |
The influence of other factors on the morphology of silver products
As we know, the temperature is always an important factor affecting the chemical reaction rate and the shift of reaction equilibrium. For an endothermic reaction, the temperature can increase the reaction rate, and improve the conversion rate of reagents thus boosting the yield ratio of nanoproducts. In our synthesis, it was found that the influence of the temperature on the morphology of silver nanoproducts is not very obvious. It might be because the total generation rate of silver solids did not change a lot due to co-enhancement of generation and dissolution rate of silver solids with increasing temperature. In a broad temperature range, as investigated by us, from 140 °C to 180 °C, the morphology of the silver nanowires did not change a lot (Fig. S3†).
In order to guarantee high conversion rate from silver ions to metallic silver, we selected 160 °C as the reaction temperature. In addition, we also contrasted the quality of silver products obtained by one-step injection methods to that of those obtained by the two-step method. When only the slow injection was introduced, the silver nanowires had a much lower yield ratio (Fig. S2†); and when only the fast injection was introduced, the morphology of the silver products was affected significantly by the molar ratio of PVP to silver nitrate. It was found that there is a high yield ratio of silver nanowires when the molar ratio of PVP to silver nitrate is 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in such synthesis. However, single-step fast injection will result in an obvious increase of the diameter of silver nanowires (Fig. S4†). Therefore, we think that our two-step injection synthetic method also has advantages in improving the yield ratio of metallic silver due to good high-temperature operability and controlling the diameters of silver nanowires due to the superfast formation of silver nanowires after the fast injection.
1 in such synthesis. However, single-step fast injection will result in an obvious increase of the diameter of silver nanowires (Fig. S4†). Therefore, we think that our two-step injection synthetic method also has advantages in improving the yield ratio of metallic silver due to good high-temperature operability and controlling the diameters of silver nanowires due to the superfast formation of silver nanowires after the fast injection.
Growth mechanism of silver nanowires in high-concentration synthesis
As mentioned above, we have obtained high-quality silver nanowiresvia a facile rapid high-concentration polyol synthesis. In our two-step injection method, the experiments were simple and highly reproducible. Based on the intrinsic uniqueness of high-concentration synthesis, we investigated the growth mechanism of the silver nanowires. Fig. 5 shows the morphology evolution of silver nanoproducts at different reaction steps. Fig. 5a shows a TEM image of silver products at the fifth minute after injection. Small silver nanoparticles are the main products. With reaction time increasing to seven minutes, after the fast injection, larger twinned crystalline silver particles appeared and some short silver rods began to form (Fig. 5b). After another one-minute of reaction, silver nanowires emerged in multitude (Fig. 5c). When the reaction continued to the tenth minute, grown silver nanowires were the dominant products (Fig. 5d). From magnified TEM images, we can see that a lot of small silver particles were absorbed on the surfaces of the silver nanowires, which indicated that small silver nanoparticles were generated in considerable amounts during the reaction process and provided silver sources for the growth of silver nanowires. Basically, the experimental phenomena conformed to our original design thought. It is that, by strong etching of Cl−/NO3− pairs, to acquire a lot of small enough silver particles, and then through sudden fast injection of the precursor solution to induce the generation of pentagonal silver particles, and finally to obtain high-yield silver nanowiresvia back feed of preformed small silver particles onto pentagonal silver particles. The whole reaction process to obtain silver nanowires is very rapid. Basically, the reaction time can be controlled within 20 min.
 |
| | Fig. 5
TEM images of silver nanoproducts obtained directly from reaction solution diluted by ethanol at different reaction times: (a) 5 min; (b) 7 min; (c) 8 min; (d) 10 min; (c1, d1) magnified images of c and d. | |
In the TEM images, a lot of curved silver nanowires can be observed, and the junctions can be seen clearly as indicated by dashed circles in Fig. 6. Some active ends that absorbed a lot of small particles are also exhibited in dashed rectangles in Fig. 6. From Fig. 6a, we can see the left silver nanowire was composed of three-section silver rods with different diameters. We tend to think it was formed through mutual attachment of different silver rods but cannot completely exclude the influence of corrosion, although it is difficult to corrode a long multiply twinned crystalline silver rod homogeneously so as to change the diameter. In Fig. 6b, we can clearly see two silver nanowires connected by a lot of silver nanoparticles. These small particles can butt joint, slanting joint or side joint two nanowires. We think that curved nanowires should be from slanting-joint and side-jointed nanowires. Many curved silver nanowires can also be observed from SEM images (Fig. S5†). Joint traces with joint neck are clearly exhibited. The inset of Fig. 6a shows a special curved five-section silver nanowire. It might be formed by the mutual attachment of curved silver nanowires. Generally, it was thought that silver nanowires can attach to each other through two ways: the first one is the reconnection of the same nanowire after strong corrosion by recovering the corrosive junction; the second one is the mutual attachment of different nanowires at the active ends owing to larger attachment probability in high-concentration solution of silver nanowires. These two ways both play positive roles in elongating silver nanowires. Considering the mutual attachment of silver nanowires, we proposed a novel growth mechanism of silver nanowires in high-concentration synthesis and show it in Fig. 7. In this mechanism, we think the ends of silver nanowires can be activated by absorbing small silver particles and then attaching other silver nanowires to prolong themselves. Curved multisection silver nanowires can also be formed during this process.
 |
| | Fig. 6 Active ends of silver nanowires indicated by dashed rectangles and mutual attachment of silver nanowires marked by dashed circles. Inset of a, SEM image of a special five-section silver nanowire, scale bar is 200 nm. | |
 |
| | Fig. 7 The formation mechanism of silver nanowires. | |
In conclusion, we have found a facile rapid two-step injection synthetic method to prepare high-quality silver nanowires with controllable aspect ratios on a large scale. The preparation cost of silver nanowires was reduced greatly. Such a high-efficiency synthesis of silver nanowires can bring more opportunities for related studies and applications of silver nanowires, especially in the fields that require massive silver nanowires. In addition, curved multisection silver nanowires can be formed in our high-concentration synthesis. Based on novel experimental phenomena, we proposed a reasonable formation mechanism of silver nanowires in the high-concentration synthesis. Mutual attachment of silver nanowires was found and can provide some references for the synthesis of other one-dimensional metal nanomaterials.
References
- M. N. Helmus, Nat. Nanotechnol., 2006, 1, 157–158 CrossRef CAS
 .
.
- T. M. Osman, D. E. Rardon, L. B. Friedman and L. F. Vega, JOM, 2006, 58, 21–24 CrossRef
 .
.
- P. Shapira and J. Wang, Asian Business Management, 2009, 8, 461–489 CrossRef
 .
.
- J. D. Mackenzie and E. P. Bescher, Acc. Chem. Res., 2007, 40, 810–818 CrossRef CAS
 .
.
- Y. B. Mao, T. J. Park and S. S. Wong, Chem. Commun., 2005, 5721–5735 RSC
 .
.
- K. C. Patil, S. T. Aruna and T. Mimani, Curr. Opin. Solid State Mater. Sci., 2002, 6, 507–512 CrossRef CAS
 .
.
- E. Gaffet, M. Abdellaoui and N. Malhouroux-Gaffet, Mater. Trans. JIM, 1995, 36, 198–209 CAS
 .
.
- C. C. Koch, Rev. Adv. Mater. Sci., 2003, 5, 91–99 CAS
 .
.
- Y. N. Xia, P. D. Yang, Y. G. Sun, Y. Y. Wu, B. Mayers, B. Gates, Y. D. Yin, F. Kim and Y. Q. Yan, Adv. Mater., 2003, 15, 353–389 CrossRef CAS
 .
.
- X. Wang, J. Zhuang, Q. Peng and Y. D. Li, Nature, 2005, 437, 121–124 CrossRef CAS
 .
.
- W. H. Xu and S. H. Yu, Small, 2009, 5, 460–465 CrossRef CAS
 .
.
- X. H. Hu and C. T. Chan, Appl. Phys. Lett., 2004, 85, 5472 CrossRef CAS
 .
.
- H. Ditlbacher, A. Hohenau, D. Wagner, U. Kreibig, M. Rogers, F. Hofer, F. R. Aussenegg and J. R. Krenn, Phys. Rev. Lett., 2005, 95, 257403 CrossRef
 .
.
- L. B. Hu, H. S. Kim, J.-Y. Lee, P. Peumans and Y. Cui, ACS Nano, 2010, 4, 2955–2963 CrossRef CAS
 .
.
- R. F. Aroca, P. J. G. Goulet, D. S. dos Santos, R. A. Alvarez-Puebla and O. N. Oliveira, Anal. Chem., 2005, 77, 378–382 CrossRef CAS
 .
.
- R. J. Chimentao, F. Medina, J. L. G. Fierro, J. E. Sueiras, Y. Cesteros and P. Salagre, J. Mol. Catal. A: Chem., 2006, 258(1–2), 346–354 CrossRef CAS
 .
.
- D. T. Schoen, A. P. Schoen, L. B. Hu, H. Sun. Kim, S. C. Heilshorn and Y. Cui, Nano Lett., 2010, 10, 3628–3632 CrossRef CAS
 .
.
- Y. G. Sun and Y. N. Xia, Adv. Mater., 2002, 14, 833–837 CrossRef CAS
 .
.
- L. B. Hu, J. W. Choi, Y. Yang and Y. Cui, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 21490–21494 CrossRef CAS
 .
.
- X. L. Tang, M. Tsuji, P. Jiang and M. Nishio, Colloids Surf., A, 2009, 338, 33–39 CrossRef CAS
 .
.
- I. Park, E. U. Kim, K. J. Baeg, Y. Y. Noh, D. Y. Kim, T. Lee and G. Y. Jung, Nanotechnology, 2009, 20, 355302 CrossRef
 .
.
- C. L. Chen, H. Furusho and H. Mori, Nanotechnology, 2009, 20 Search PubMed
 .
.
- Z. H. Wang, J. W. Liu, X. Y. Chen, J. X. Wan and Y. T. Qian, Chem.–Eur. J., 2004, 11, 160 CrossRef CAS
 .
.
- W. J. Zhang, P. Chen, Q. S. Gao, Y. H. Zhang and Y. Tang, Chem. Mater., 2008, 20, 1699–1704 CrossRef CAS
 .
.
- F. Fievet, J. P. Lagier and M. Figlarz, MRS Bull., 1989, 14, 29 CAS
 .
.
- Y. N. Xia, Y. J. Xiong, B. Lim and S. E. Skrabalak, Angew. Chem., Int. Ed., 2009, 48, 60–103 CrossRef CAS
 .
.
- F. Fievet, J. P. Lagier, B. Blin, B. Beaudoin and M. Figlarz, Solid State Ionics, 1989, 32–33, 198 CrossRef
 .
.
- S. H. Im, Y. T. Lee, B. Wiley and Y. N. Xia, Angew. Chem., Int. Ed., 2005, 44, 2154 CrossRef CAS
 .
.
- M. Tsuji, K. Matsumoto, P. Jiang, R. Matsuo, X. L. Tang and K. S. N. Karnarudin, Colloids Surf., A, 2008, 316, 266–277 CrossRef CAS
 .
.
- Y. G. Sun, B. Mayers, T. Herricks and Y. N. Xia, Nano Lett., 2003, 3, 955–960 CrossRef CAS
 .
.
- B. Wiley, T. Herricks, Y. G. Sun and Y. N. Xia, Nano Lett., 2004, 4, 2057 CrossRef CAS
 .
.
- K. E. Korte, S. E. Skrabalak and Y. N. Xia, J. Mater. Chem., 2008, 18, 437–441 RSC
 .
.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary figures. See DOI: 10.1039/c2ra01162j |
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) were purchased from the Shanghai Chemical Reagent Company. All chemicals were used as received without further purification.
000) were purchased from the Shanghai Chemical Reagent Company. All chemicals were used as received without further purification.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was dissolved in 34 ml EG with moderate stirring and heated to 160 °C until the temperature was stable. Then 40 μl of 0.2 M NaCl was added into the flask. After one minute, 6 ml EG solution containing 2.0384 g of silver nitrate (AgNO3, CAgNO3 = 2 M) was added to the flask at the rate 100 μl/10 s by pipette. Once the reaction solution began to become cloudy from transparent, all residual precursor solution was added into the flask immediately. Then the flask was sealed until the solution become glistening, indicating the formation of silver nanowires. The products were collected by centrifugation.
000) was dissolved in 34 ml EG with moderate stirring and heated to 160 °C until the temperature was stable. Then 40 μl of 0.2 M NaCl was added into the flask. After one minute, 6 ml EG solution containing 2.0384 g of silver nitrate (AgNO3, CAgNO3 = 2 M) was added to the flask at the rate 100 μl/10 s by pipette. Once the reaction solution began to become cloudy from transparent, all residual precursor solution was added into the flask immediately. Then the flask was sealed until the solution become glistening, indicating the formation of silver nanowires. The products were collected by centrifugation.




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in such synthesis. However, single-step fast injection will result in an obvious increase of the diameter of silver nanowires (Fig. S4†). Therefore, we think that our two-step injection synthetic method also has advantages in improving the yield ratio of metallic silver due to good high-temperature operability and controlling the diameters of silver nanowires due to the superfast formation of silver nanowires after the fast injection.
1 in such synthesis. However, single-step fast injection will result in an obvious increase of the diameter of silver nanowires (Fig. S4†). Therefore, we think that our two-step injection synthetic method also has advantages in improving the yield ratio of metallic silver due to good high-temperature operability and controlling the diameters of silver nanowires due to the superfast formation of silver nanowires after the fast injection.


.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
