Label-free detection of target DNA sequence and single-base mismatch in hepatitis C virus corresponding to oligonucleotide by resonance light scattering technique
Zhanguang
Chen
*a,
Sihua
Qian
a,
Xi
Chen
b,
Junhui
Chen
*c,
Yuejuan
Lin
a and
Jinbin
Liu
d
aDepartment of Chemistry, Shantou University, Shantou 515063, China. E-mail: kqlu@stu.edu.cn; Fax: +86 75482902767; Tel: +86 75482903330
bGuangdong Pharmaceutical University, Guangzhou 510006, China
cInterventional Oncology & Minimally Invasive Therapies Department, Peking University Shenzhen Hospital, Shenzhen 518036, China. E-mail: chenjhpush@126.com
dDepartment of Chemistry, University of Texas at Dallas, Richardson, Texas 75080, USA
First published on 8th February 2012
Abstract
A highly sensitive assay to detect sequence-specific DNA by the resonance light scattering (RLS) technique has been developed based on the enhanced RLS intensities of the RLS spectra at 399.5 nm. It was found that Hoechst 33258, when bound to dsDNA and ssDNA in electrolyte solution, displayed different RLS signals. This encouraged us to perform a direct and unlabelled sequence-specific DNA assay. At optimal conditions, this RLS assay can detect complementary target DNA over a range of 4.0 × 10−9–3.8 × 10−7 mol L−1, and the limit of detection was around 1.7 × 10−9 mol L−1. Additionally, the results of experiments showed that different RLS signals reflected a different degree of mismatch between probe DNA and target DNA, and mismatched variants of target DNA with single-base difference can even be well discriminated. Herein, we moved the RLS technique one-step further toward sensitivity, rapidity, simplicity and non-toxicity. The RLS assay results were identified with a fluorescence method, fluorescence polarization assay and atomic force microscope measurement.
Introduction
The detection of specific DNA sequence provides the basis for detecting a wide variety of microbial and viral pathogens.1,2 The early and rapid detection of disease-associated sequence-specific DNA correlated to specific human genes, such as hepatitis C virus genotypes,3 has become a main topic in the medical field.The traditional DNA sequence detection methods were not applicable to a routine environment. This was due to the complex procedures, the use of radioisotopes, and the lengthy time to achieve results.4,5 The polymerase chain reaction, which was a major advancement in molecular biology, had been widely used to detect infectious viruses with easy handling and high sensitivity. However, this method also encountered the complex procedures, the lengthy time to achieve results and the difficulty in quantifying the targeted gene.6 The fluorescence method, based on the determination of fluorescent messages, was a more accurate DNA detection method.7,8 Nevertheless, it had to depend on the fluorescence of the tagged oligonucleotide probes,9,10 which can not avoid sophisticated preparation of oligonucleotide-functionalized probes. Surface plasmon resonance techniques were also used for DNA sensitive detection, but this assay had unavoidable drawbacks of being time consuming and labor intensive.11 Recently, sequence-selective biosensors for DNA detection have become popular.12 However, they had drawbacks of sophisticated modification and washing steps. Thus, simple, sensitive, and rapid assays for specific DNA sequence detection are highly desirable.
As a kind of elastic light scattering, RLS was produced when the incident beam was close to its molecular absorption band.13 Pasternack and coworkers developed the RLS technique to study biological macromolecules with a common fluorescence spectrometer.14 Subsequently, Huang et al. successfully employed the RLS technique for quantitative analysis of biomacromolecules.15 Recently, RLS studies have attracted great interest among researchers due to the sensitivity, rapidity and simplicity of this technique.16–22 The successful application of the RLS technique to directly screen DNA-targeted anticancer drugsin vitro has been demonstrated.23–25 The RLS technique has already been employed for the detection of DNA hybridization. Bao et al.26 applied RLS particles to detect DNA hybridization on cDNA microarrays. However, this assay involved sophisticated modification processes of array fabrication, RNA labeling and RLS particle binding. Zhang et al.27 designed a RLS detection method for DNA sequence-specificity with multiwalled carbon nanotubes (MWCNTs) as optical probes. Though without modification processes, their developed assay needed sophisticated oxidation of MWCNTs.28 Hence, in order to simplify the detection processes of specific DNA sequence, a RLS assay without modification processes of DNA and complicated procedures is desirable.
In this contribution, we have developed a novel label-free detection method for DNA sequence specificity using Hoechst 33258 (H33258) as an optical indicator based on the measurements of RLS signals. We carefully evaluated the roles that the concentration of DNA and H33258 played in determining the selectivity and sensitivity of the H33258 indicator. We also employed mitoxantrone to test its ability as an indicator for the detection of sequence-specific DNA. In order to support and visualize the conclusion on the interaction between H33258 and DNA, fluorescence method and fluorescence polarization assay were employed, and the atomic force microscope images were also obtained.
Experimental
Materials and reagents
Tris–HCl buffer (pH 7.4) containing 5.0 × 10−2 mol L−1NaCl was prepared by dissolving 0.3029 g Tris and 0.7305 g NaCl in 100 mL of doubly distilled water, adding an appropriate amount of 0.2 mol L−1HCl, and diluting to 250 mL with doubly distilled water.10 OD oligonucleotide sequences were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China), and they were used as received. Probe DNA (pDNA), 5′-GGA GGT CTC GTA GAC CGT GC-3′, this oligonucleotide, which corresponds to the sense strand of the hepatitis C virus 5′ untranslated region was used as the DNA probe; complementary target DNA (cDNA), 5′-GCA CGG TCT A![[C with combining low line]](https://www.rsc.org/images/entities/b_char_0043_0332.gif) G AGA CCT CC-3′, this oligonucleotide, which corresponds to the antisense strand of the hepatitis C virus 5′ untranslated region was used as the complementary target DNA; one-base-mismatched DNA (omDNA), 5′-GCA CGG TCT A
G AGA CCT CC-3′, this oligonucleotide, which corresponds to the antisense strand of the hepatitis C virus 5′ untranslated region was used as the complementary target DNA; one-base-mismatched DNA (omDNA), 5′-GCA CGG TCT A![[T with combining low line]](https://www.rsc.org/images/entities/b_char_0054_0332.gif) G AGA CCT CC-3′, this oligonucleotide was identical to the complementary DNA with a substitution (C/T) at position 11 shown with highlighted and underlined letters, leading to a single base mismatch with the probe; the non-complementary oligomer (ncDNA), 5′-CCA TGT GTA AGC CTA ATG GC-3′. 5.0 × 10−6 mol L−1 of these oligonucleotides was prepared by dissolving them in 26.5 mL, 25.0 mL, 24.5 mL and 25.5 mL of Tris–HCl buffer solution (pH 7.4), respectively.
G AGA CCT CC-3′, this oligonucleotide was identical to the complementary DNA with a substitution (C/T) at position 11 shown with highlighted and underlined letters, leading to a single base mismatch with the probe; the non-complementary oligomer (ncDNA), 5′-CCA TGT GTA AGC CTA ATG GC-3′. 5.0 × 10−6 mol L−1 of these oligonucleotides was prepared by dissolving them in 26.5 mL, 25.0 mL, 24.5 mL and 25.5 mL of Tris–HCl buffer solution (pH 7.4), respectively.
H33258 and Calf thymus DNA (ctDNA) were all purchased from the Sigma Chemicals Co., China. 0.025 g H33258 was dissolved in 50 mL of doubly distilled water to prepare a 1.0 × 10−3 mol L−1 stock solution. The subsequent concentrations of the needed working solutions were prepared by serial dilution of proper amounts of the stock solutions. The working solution of ctDNA was 40 mg L−1, prepared by dissolving 0.0040 g ctDNA in 100 mL of doubly distilled water.
All the above stocks need to be stored at 4 °C in the refrigerator. The Tris–HCl buffer solution was used to adjust the acidity of the solutions. The doubly distilled water was used throughout, and all chemicals used without other illumination were of analytical grade or the best grade commercially available.
Apparatus
The RLS spectra, fluorescence spectra, and fluorescence polarization spectra were all measured with an LS-55 spectrofluorometer (Perkin-Elmer, USA) equipped with a quartz cuvette (1.0 cm × 1.0 cm). The pH values of the solution were measured with an SA 720 instrument (Orion Research, USA). DF-101S collection hot-like magnetic force heating mixer (Gongyi City Yuhua Instrument Co., Ltd, Henan, China) was employed to control the temperature.Procedures of DNA hybridization
pDNA was hybridized with different sequences of oligonucleotides (volume ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Briefly, pDNA at a concentration of 5.0 × 10−6 mol L−1 and different complementary oligonucleotides with the same concentration were added into 10.0 mL calibrated flasks at 37 °C for 30 min. Then gradually cooled to room temperature, and incubated at 4 °C overnight.
1). Briefly, pDNA at a concentration of 5.0 × 10−6 mol L−1 and different complementary oligonucleotides with the same concentration were added into 10.0 mL calibrated flasks at 37 °C for 30 min. Then gradually cooled to room temperature, and incubated at 4 °C overnight.
Procedures of ctDNA denaturation
For the thermal denaturation experiment, ctDNA (dsDNA) was heated for 15 min in a boiling water bath followed by quick cooling in ice water. 15 min later, ssDNA was formed.RLS spectra assay
Into a 10.0 mL calibrated flask were added appropriate amounts of DNA and H33258. The mixture was diluted to the mark with Tris–HCl buffer (pH 7.4) solution and stirred thoroughly. All RLS spectra were obtained by simultaneously scanning the excitation and emission monochromators (λex = λem) from 250.0 to 700.0 nm with 10.0 nm wide excitation and emission slits. Based on the spectra, the RLS intensities were measured at 399.5 nm. The increase of the H33258 RLS intensity from adding DNA is represented as ΔIRLS = IRLS − I0RLS, where IRLS and I0RLS were the RLS intensities of H33258 with and without DNA, respectively.Fluorescence assay
Appropriate amounts of DNA and H33258 were added into a 10.0 mL calibrated flask, and then the mixture was diluted to the mark with Tris–HCl buffer (pH 7.4) solution and stirred thoroughly. All samples were illuminated at an emission wavelength of 479.0 nm, and the fluorescence excitation was scanned from 300.0 to 400.0 nm.Fluorescence polarization assay
The procedures were started by adding suitable volumes of H33258 and DNA in a 10.0 mL calibrated flask. The mixture was finally diluted to the mark with Tris–HCl buffer (pH 7.4) and stirred thoroughly. The fluorescence polarization spectra measurement was made by keeping the slit width for excitation and emission at 2.5 nm. The excitation and emission wavelengths were 343.0 nm and 479.0 nm, respectively.Atomic force microscope (AFM) measurements
The samples for AFM measurements were treated in accordance with the literature.29 All DNA concentrations were 1.5 × 10−9 mol L−1 and the H33258 concentration was 1.0 × 10−5 mol L−1. Then H33258–DNA solutions were deposited onto Mg2+-treated mica surfaces. All AFM images were obtained using a Nanoscope IIIa controller with a Multimode AFM (Digital Instruments, Santa Barbara, CA). The investigation was carried out in the contact mode using a DI cantilever of 0.16 N m−1 spring constant.Results and discussion
Features of RLS spectra
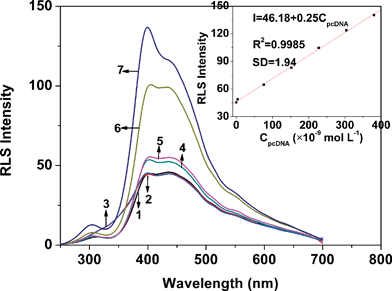 | ||
| Fig. 1 The RLS spectra of ssDNA (curve 1), dsDNA (curve 2), H33258 (curve 3) and H33258 in the presence of ssDNA (curve 4), pncDNA (curve 5), pomDNA (curve 6) and pcDNA (curve 7). All types of DNA were kept at 2.5 × 10−7 mol L−1; the concentration of H33258 was 1.0 × 10−5 mol L−1; pH 7.4. The inset shows the RLS intensities of H33258–pcDNA measured at 399.5 nm versus the pcDNA concentration. | ||
The protocol of our strategy is displayed in Scheme 1. The sensitivity and selectivity of our assay depended on the hybridization indicator which recognized dsDNA specifically. H33258, a DNA minor groove binder,30 was more selective to dsDNA due to its binding style. The minor groove existed only in the double-helix DNA. Therefore, H33258, when bound to dsDNA or ssDNA in an electrolyte solution, displays different RLS signals.
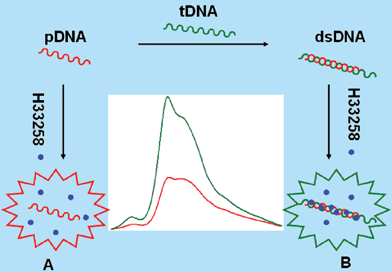 | ||
| Scheme 1 Schematic of RLS detection of sequence-specific DNA: (A) RLS intensity of H33258 without obvious change in the presence of ssDNA; (B) enhanced RLS intensity of H33258 in the presence of dsDNA. | ||
In this work, to demonstrate the different binding capabilities of ssDNA and dsDNA with H33258, ctDNA was employed. As Fig. 2 shows, the RLS signals of H33258, ctDNA and denatured ctDNA were weak while the RLS intensity of H33258–ctDNA was very strong. However, under the same conditions, the RLS intensity of H33258–ssDNA (denatured ctDNA) was weaker than that of H33258–ctDNA. Consequently, dsDNA has a binding capability with H33258 different to that of ssDNA. This high difference was employed here for the specific determination of DNA hybridization.
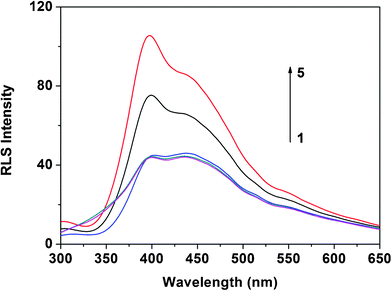 | ||
| Fig. 2 RLS spectra of ctDNA (curve 1), denatured ctDNA (curve 2), H33258 (curve 3) and H33258 in the presence of denatured ctDNA and ctDNA (curve 4 and curve 5). The concentrations of ctDNA and denatured ctDNA were 4.0 μg mL−1; the concentration of H33258 was 1.0 × 10−5 mol L−1; pH 7.4. | ||
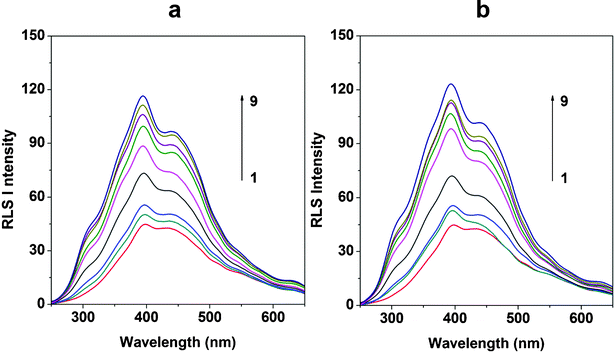 | ||
| Fig. 3 RLS spectra of MIT–dsDNA (a) and MIT–ssDNA (b). The concentration of MIT was 4.0 μg mL−1; the concentrations of DNA (μg mL−1) were: 1–9: 0, 0.2, 0.4, 0.8, 1.2, 1.6, 2.0, 2.4, 2.8; pH 7.4. | ||
Effects of DNA concentration and H33258 concentration
Taking pcDNA for example, it was shown that there was a good linear relationship between the RLS intensity of the H33258–pcDNA system and pcDNA concentration when the concentration ranged from 4.0 × 10−9 to 3.8 × 10−7 mol L−1. When pcDNA concentration reached 2.5 × 10−7 mol L−1, the RLS intensity of H33258 at 399.5 nm was increased more greatly by adding pcDNA than by adding pomDNA or pncDNA. Therefore, 2.5 × 10−7 mol L−1 was chosen for further experiments. The RLS intensities of dsDNA and ssDNA were both weak when their concentrations were in the range of 4.0 × 10−9–4.0 × 10−7 mol L−1.The effect of H33258 concentration on the RLS intensities was also examined. From our experiments, it can be clearly concluded that H33258 concentration had a great influence on the RLS intensities of the H33258–dsDNA systems. When H33258 concentration reached 1.0 × 10−5 mol L−1, the RLS intensity of the H33258–pcDNA system was increased more greatly than that of the other two H33258–dsDNA systems. Thus, 1.0 × 10−5 mol L−1 of H33258 solution was selected for the detection in this work. The results displayed that the RLS signal of H33258 was weak when its concentration was in the range of 1.0 × 10−6–1.0 × 10−4 mol L−1.
Verification of hoechst assay using other techniques
Fluorescence spectra
In order to prove this new assay, a fluorescence method was performed. Fig. 4 displays fluorescence excitation spectra of H33258 in the absence and presence of DNA. The Figure shows that H33258 had one excitation peak located at 343.0 nm. The addition of dsDNA induced a significantly increased fluorescence of H33258 as well as a red-shift in excitation from 343.0 nm to 351.0 nm. As can be seen from the Figure, the addition of pcDNA induced a stronger fluorescence intensity than that of pomDNA and pncDNA. However, in the case of the ssDNA being added, a smaller extent of fluorescence enhancement was observed in the fluorescence spectra of H33258. Therefore, the selectivity of H33258 to dsDNA was determined homogeneously by fluorescence spectroscopy. Herein, though the fluorescence method was more sensitive than the RLS assay, it had to depend on not only the selectivity of the probe to dsDNA, but also the fluorescence of the probe. However, our developed RLS assay only relied on the selectivity of the probe to dsDNA. Consequently, probes, as long as they have a different binding ability with dsDNA and ssDNA, can be employed to detect DNA hybridization by a RLS technique.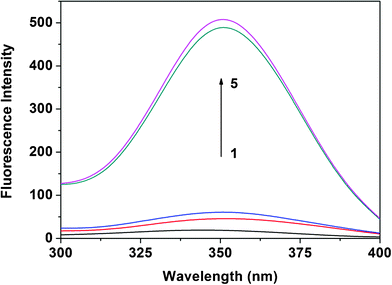 | ||
| Fig. 4 Fluorescence excitation spectra of H33258 in the absence and presence of ssDNA and dsDNA. Curve 1 represents the fluorescence excitation spectrum of H33258 at 1.0 × 10−6 mol L−1. Curves 2–5 represent fluorescence excitation spectra of H33258 in the presence of ssDNA, pncDNA, pomDNA, and pcDNA, respectively; the DNA concentration was 5.0 × 10−7 mol L−1; pH 7.4. | ||
Fluorescence polarization assay
Mere binding to the phosphate backbone or to the DNA grooves does not result in enhanced fluorescence polarization.31 H33258 is the archetypal DNA minor groove binder and is known to be A/T-selective. However, multiple binding modes were found by Loontiens et al.32 Apart from binding in the minor groove of A+T-rich DNA, H33258 also acts as a non-classical intercalator.33When H33258 intercalates into the DNA helix, its rotational motion should be restricted and, hence, fluorescence from the bound chromophore should be polarized.31Table 1 displays the polarization values of H33258 in the presence of different DNA. As is shown in the Table, fluorescence from the chromophore of H33258 was polarized (polarization value: 0.200) due to the rapid tumbling motion of the chromophore in aqueous media. When ssDNA was added into the H33258 solution, no increase in the measured polarization was observed over the polarization of free H33258 (polarization value: 0.207). After the mixing of H33258 and pcDNA, the fluorescence was more polarized (polarization value: 0.312). The increase in the polarization upon binding to dsDNA suggested intercalation of H33258 into the helix. Similarly, an increased polarization value was obtained in the presence of pomDNA (polarization value: 0.316). However, with the addition of pncDNA, the polarization value had no change (polarization value: 0.208). Poor intercalation of H33258 to ssDNA was consistent with the absence of polarized emission. The fluorescence polarization experiment revealed that it was unable to discriminate the perfectly complementary target and one-base-mismatched DNA, which demonstrated the high sensitivity and selectivity of our developed RLS assay.
| H33258 in the presence of DNA | Without DNA | ssDNA | pcDNA | pomDNA | pncDNA |
|---|---|---|---|---|---|
| Polarization value | 0.200 | 0.207 | 0.312 | 0.316 | 0.208 |
AFM image
AFM is a powerful tool for observing particles on the single molecule scale,29,34 because AFM can visualize particles in three dimensions with sub-nanometre height resolution and reflect near-physiological conditions in sample preparations.Within the present study, the size changes of H33258 after the addition of various DNA were examined by AFM imaging. Fig. 5a shows the native size of H33258 in the absence of DNA and the size of the largest particle in it was not more than 10−1 μm. Therefore, the RLS intensity of H33258 without DNA was weak. The AFM image in Fig. 5b displays the size of H33258 in the presence of ssDNA, which exhibited no larger particles appearing and thus no stronger RLS intensity of H33258–ssDNA than that of H33258 turning up. Those in Fig. 5c and 5d show the sizes of H33258 in the presence of pomDNA and pcDNA, in which larger particles (the sizes of most particles were more than 10−1 μm) were observed. Fig. 5 reveals that H33258 had different sizes after being bound to different types of DNA. According to the Rayleigh formula,13 the RLS intensity is related to the size of the formed particle in a certain range and directly proportional to the square of the molecular volume. The larger the size of the particle, the stronger the signal of the scattering light. The results from AFM confirmed the conjecture and gave more information in detail which explained the characteristics appearing in the RLS spectra.
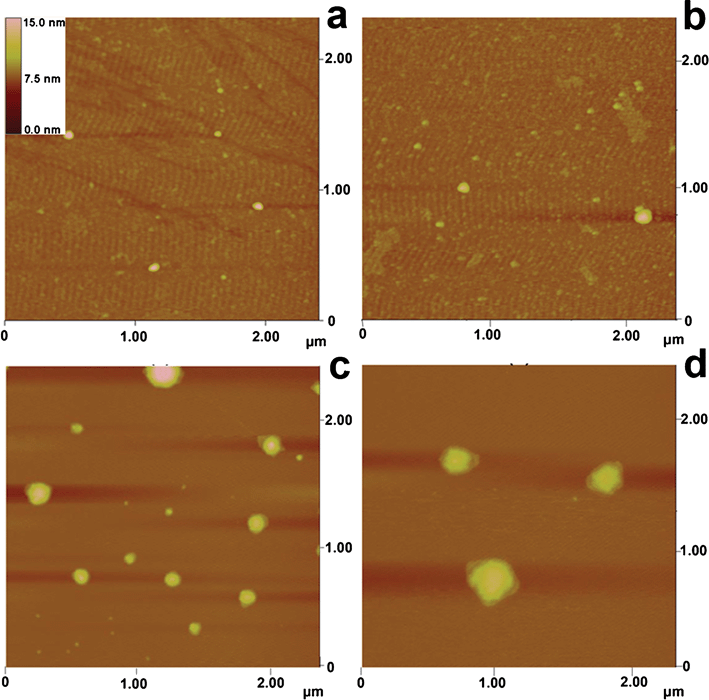 | ||
| Fig. 5 AFM images of: a) H33258; b) H33258–ssDNA; c) H33258–pomDNA; d) H33258–pcDNA. The concentration of H33258 was 1.0 × 10−5 mol L−1 and DNA was 1.5 × 10−9 mol L−1; pH 7.4. | ||
Conclusions
We developed a simple, sensitive, and selective detection method for sequence-specific DNA. H33258 was more selective to dsDNA than to ssDNA in an electrolyte solution, displaying different RLS signals that could be applied for the detection of sequence-specific DNA and the discrimination of complementary DNA from single-base mismatch and non-complementary oligonucleotides. Our RLS assay provided several advantages over conventional approaches for sequence-specific DNA detection methods. First, the RLS assay was simple and was only performed on a common spectrofluorometer. Second, there was no time-dependent monitoring required and this furnished the developed strategy with improved technical robustness and operational convenience. In addition, the developed approach avoided sophisticated modification and washing steps required in the popular electrochemical biosensor methods. In view of these advantages, this new assay holds great promise as a sensitive, selective, simple, and robust platform for quantitative assay of sequence-specific DNA. Moreover, the present assay can be an automatic process for sequence-specific DNA detection when combined with other techniques such as a flow injection analysis system.35,36Acknowledgements
The authors gratefully acknowledge the financial support of the Natural Science Foundation of Guangdong Province (9151022501000019).References
- K. L. Nathanson, Biochem. Pharmacol., 2010, 80, 755–761 CrossRef CAS.
- C. G. Hadjipanayis and E. G. V. Meir, Trends Mol. Med., 2009, 15, 519–530 CrossRef CAS.
- C. S. Riccardi, C. Kranz, J. Kowalik, H. Yamanaka, B. Mizaikoff and M. Josowicz, Anal. Chem., 2008, 80, 237–245 CrossRef.
- S. F. Wolf, L. Haines, J. Fisch, J. N. Kremsky, J. P. Dougherty and K. Jacobs, Nucleic Acids Res., 1987, 15, 2911–2926 CrossRef CAS.
- C. A. Miller, W. L. Patterson, P. K. Johnson, C. T. Swartzell, F. Wogoman, J. P. Albarella and R. J. Carrico, J. Clin. Microbiol., 1988, 26, 1271–1276 CAS.
- J. Hassmann, A. Misch, J. Schülein, J. Krause, B. Graßl, P. Müller and W. M. Bertling, Biosens. Bioelectron., 2001, 16, 857–863 CrossRef CAS.
- A. Granzhan and H. Ihmels, Org. Lett., 2005, 7, 5119–5122 CrossRef CAS.
- B. A. D. Neto, A. A. M. Lapis, F. S. Mancilha, I. B. Vasconcelos, C. Thum, L. A. Basso, D. S. Santos and J. Dupont, Org. Lett., 2007, 9, 4001–4004 CrossRef CAS.
- L. Wang, H. L. Li, Y. L. Luo, Y. W. Zhang, J. Q. Tian and X. P. Sun, RSC Adv., 2011, 1, 1318–1323 RSC.
- H. L. Li, J. F. Zhai and X. P. Sun, RSC Adv., 2011, 1, 725–730 RSC.
- Q. F. Luan, Y. Xue and X. Yao, Sens. Actuators, B, 2010, 147, 561–565 CrossRef.
- S. G. Wang, R. L. Wang, P. J. Sellina and Q. Zhang, Biochem. Biophys. Res. Commun., 2004, 325, 1433–1437 CrossRef CAS.
- J. Anglister and I. Z. Steinberg, J. Chem. Phys., 1983, 78, 5358–5368 CrossRef CAS.
- R. F. Pasternack, C. Bustamante, P. J. Collings, L. A. Giannetto and E. J. Gibbs, J. Am. Chem. Soc., 1993, 115, 5393–5399 CrossRef CAS.
- C. Z. Huang, K. A. Li and S. Y. Tong, Anal. Chem., 1996, 68, 2259–2263 CrossRef CAS.
- J. Ling, C. Z. Huang, Y. F. Li, L. Zhang, L. Q. Chen and S. J. Zhen, TrAC, Trends Anal. Chem., 2009, 28, 447–453 CrossRef CAS.
- S. K. Brar and M. Verma, TrAC, Trends Anal. Chem., 2011, 30, 4–17 CrossRef CAS.
- Z. G. Chen, W. F. Ding, F. L. Ren, J. B. Liu and Y. Z. Liang, Anal. Chim. Acta, 2005, 550, 204–209 CrossRef CAS.
- H. P. Zhou, X. Wu and J. H. Yang, Talanta, 2009, 78, 809–813 CrossRef CAS.
- C. Q. Cai, H. Gong and X. M. Chen, Microchim. Acta, 2007, 157, 165–171 CrossRef CAS.
- Z. G. Chen, G. L. Liu, M. H. Chen, Y. R. Peng and M. Y. Wu, Anal. Biochem., 2009, 384, 337–342 CrossRef CAS.
- Z. G. Chen, G. M. Zhang, X. Chen, J. H. Chen, S. H. Qian and Q. Li, Analyst, 2012, 137, 722–728 RSC.
- Z. G. Chen, T. H. Song, S. B. Wang, X. Chen, J. H. Chen and Y. Q. Li, Biosens. Bioelectron., 2010, 25, 1947–1952 CrossRef CAS.
- Z. G. Chen, Y. R. Peng, M. H. Chen, X. Chen and G. M. Zhang, Analyst, 2010, 135, 2653–2660 RSC.
- Z. G. Chen, T. H. Song, Y. R. Peng, X. Chen, J. H. Chen, G. M. Zhang and S. H. Qian, Analyst, 2011, 136, 3927–3933 RSC.
- P. Bao, A. G. Frutos, C. Greef, J. Lahiri, U. Muller, T. C. Peterson, L. Warden and X. Y. Xie, Anal. Chem., 2002, 74, 1792–1797 CrossRef CAS.
- L. Zhang, C. Z. Huang, Y. F. Li, S. J. Xiao and J. P. Xie, J. Phys. Chem. B, 2008, 112, 7120–7122 CrossRef CAS.
- P. G. He and M. Bayachou, Langmuir, 2005, 21, 6086–6092 CrossRef CAS.
- M. Saito, M. Kobayashi, S. I. Iwabuchi, Y. Morita, Y. Takamura and E. Tamiya, J. Biochem., 2004, 136, 813–823 CrossRef CAS.
- K. Hashimoto, K. Ito and Y. Ishlmori, Anal. Chem., 1994, 66, 3830–3833 CrossRef CAS.
- C. V. Kumar' and E. H. Asuncion, J. Am. Chem. Soc., 1993, 115, 8541–8553 Search PubMed.
- F. G. Loontiens, P. Regenfuss, A. Zechel, L. Dumortier and R. M. Clegg, Biochemistry, 1990, 29, 9029–9039 CrossRef CAS.
- A. Adhikary, V. Buschmann, C. Muller and M. Sauer, Nucleic Acids Res., 2003, 31, 2178–2186 CrossRef CAS.
- H. G. Hansma, Annu. Rev. Phys. Chem., 2001, 52, 71–92 CrossRef CAS.
- L. Qi, Z. Q. Han and Y. Chen, J. Chromatogr., A, 2006, 1110, 235–239 CrossRef CAS.
- Y. Li, L. J. Dong, W. P. Wang, Z. D. Hu and X. G. Chen, Anal. Biochem., 2006, 354, 64–69 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
