pH-induced formation of various hierarchical structures from amphiphilic core–shell nanotubes†
Cheng Hao
Lee
and
Pei
Li
*
Department of Applied Biology and Chemical Technology, The Hong Kong Polytechnic University, Hung Hom, Kowloon, Hong Kong, China. E-mail: bcpeili@polyu.edu.hk; Fax: +(852) 2364 9932; Tel: +(852) 3400 8721
First published on 3rd January 2012
Abstract
Intriguing hierarchical structures, including nanotubular bundle, columnar, reticular plate-like and highly packed interwoven plate-like materials, have been created viaself-assembly of amphiphilic core–shell nanotubes in water through varying the solution pHs between 3 and 13.
Hierarchical structures are assemblies of molecules or nanostructured units into organized patterns in multiple length scales. The hierarchical self-assembly of a wide range of biomolecules such as proteins, peptides, DNA and lipids is a well-known phenomenon in nature.1–7 Thus there is much interest in both fundamental research and practical applications to develop new approaches to create microscopic or macroscopic higher-ordered superstructures through the self-organization of preformed discrete nanomaterials that serve as building blocks. In principle, creation of well-defined hierarchical structures through the self-assembly of preformed building blocks in water strongly depends on their molecular interactions such as hydrophobic interactions, hydrogen bonding, and electrostatic interactions under experimental conditions, as well as their size distribution, shape and surface properties.8–12 Various template-free self-assembly approaches to prepare hierarchical structures have been reported, including droplet evaporation based on capillary flow kinetics,13–15 the Langmuir–Blodgett technique,16–18nanocrystal-micelle,19 2D self-assembly of rod amphiphiles,20 tubular stacking of grafted amphiphiles,21polymer mediator-induced assembly22 and self-assembly of polymeric Janus particles.23 In addition to these approaches, surface template-assisted self-assembly based on a lithographically defined polyelectrolyte template24 or self-organized chemical template25 has been widely investigated because it can directly achieve desired patterns on substrates. Here, we report a new and simple template-free self-assembly approach to produce various intriguing hierarchical nanostructures such as nanotubular bundles, columnar, reticular plate-like and highly packed interwoven plate assemblies through pH-induced self-assembly of amphiphilic core–shell nanotubes in water.
The nanotubes used in this study are comprised of a hydrophilic branched polyethyleneimine (PEI) shell (Mn ∼60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) and hydrophobic poly(methyl methacrylate) (PMMA) grafts in the interior. The estimated average molecular weight of PMMA grafts is three times higher than that of PEI.26 The degree of PMMA grafting onto the PEI backbone is approximately 200% (weight of PMMA grafts divided by weight of branched PEI) (see ESI†). The nanotube diameters are less than 150 nm and their length scales range from 1 to 5 μm. The amphiphilic nanotubes are very stable in water due to the presence of the water-soluble PEI shell. The nanotubes were prepared according to our previously established method, which involves the self-assembly of PEI-g-PMMA hollow particles via the controlled aggregation and coalescence of particles in a dichloromethane–water mixture.27 The mechanism of the nanotube formation involves three key steps:28 (1) the spherical hollow particles are first elongated to an ellipsoidal shape via shear-induced stretching; (2) aggregation of the elongated particles via a tip-to-tip connection; (3) coalescence and fusion of the aggregates under appropriate stirring and continuous evaporation of DCM.
000 g mol−1) and hydrophobic poly(methyl methacrylate) (PMMA) grafts in the interior. The estimated average molecular weight of PMMA grafts is three times higher than that of PEI.26 The degree of PMMA grafting onto the PEI backbone is approximately 200% (weight of PMMA grafts divided by weight of branched PEI) (see ESI†). The nanotube diameters are less than 150 nm and their length scales range from 1 to 5 μm. The amphiphilic nanotubes are very stable in water due to the presence of the water-soluble PEI shell. The nanotubes were prepared according to our previously established method, which involves the self-assembly of PEI-g-PMMA hollow particles via the controlled aggregation and coalescence of particles in a dichloromethane–water mixture.27 The mechanism of the nanotube formation involves three key steps:28 (1) the spherical hollow particles are first elongated to an ellipsoidal shape via shear-induced stretching; (2) aggregation of the elongated particles via a tip-to-tip connection; (3) coalescence and fusion of the aggregates under appropriate stirring and continuous evaporation of DCM.
In this study, we first prepared the PEI-g-PMMA hollow particles, followed by the generation of PEI-g-PMMA nanotubes through stirring (350 rpm) the hollow particles in a mixture of DCM–water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v) at 25 °C for 24 h (the detailed procedure is described in the ESI†). Unlike in previous studies, we purposely carried out the assembling process at 25 °C instead of 15 °C in order to obtain shorter nanotubes (<5 μm). The resulting aqueous solution containing PEI-g-PMMA nanotubes was clear and had a pH of 6.5. The sample was then stored at room temperature for subsequent studies. Fig. 1a shows the morphology of as-prepared nanotubes at pH 6.5. The nanotubes seem to have aggregated into aligned bundles with a quite uniform distribution. Observation by transmission electron microscopy further confirmed the formation of nanotube bundles (Fig. 1b). These as-prepared nanotubes were then used to study the formation of hierarchical structures in various solution pHs (from 3 to 13). The assembling process was simple; it was carried out by gently stirring (50 rpm) the nanotube dispersion (5 mg mL−1) at 25 °C for 24 h at a specific solution pH. Morphologies of the resulting nanostructures were then observed by field emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM) and atomic force microscopy (AFM) in fluid mode.
7 v/v) at 25 °C for 24 h (the detailed procedure is described in the ESI†). Unlike in previous studies, we purposely carried out the assembling process at 25 °C instead of 15 °C in order to obtain shorter nanotubes (<5 μm). The resulting aqueous solution containing PEI-g-PMMA nanotubes was clear and had a pH of 6.5. The sample was then stored at room temperature for subsequent studies. Fig. 1a shows the morphology of as-prepared nanotubes at pH 6.5. The nanotubes seem to have aggregated into aligned bundles with a quite uniform distribution. Observation by transmission electron microscopy further confirmed the formation of nanotube bundles (Fig. 1b). These as-prepared nanotubes were then used to study the formation of hierarchical structures in various solution pHs (from 3 to 13). The assembling process was simple; it was carried out by gently stirring (50 rpm) the nanotube dispersion (5 mg mL−1) at 25 °C for 24 h at a specific solution pH. Morphologies of the resulting nanostructures were then observed by field emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM) and atomic force microscopy (AFM) in fluid mode.
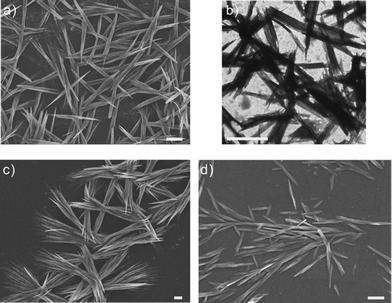 | ||
| Fig. 1 (a) FE-SEM image of the tightly packed nanotube bundles as prepared at pH 6.5; (b) TEM image of the nanotube bundles at pH 6.5; (c) FE-SEM image of the loosely packed nanotube bundles obtained at pH 5; (d) FE-SEM image of the well-separated nanotubes obtained at pH 3. All scale bars are 1 μm. | ||
On lowering the original solution pH of the as-prepared nanotube dispersion from 6.5 to 5.0, the nanotubes formed loose straw-like bundles, as illustrated in Fig. 1c. On further reducing the solution pH of the nanotube dispersion from 6.5 to 3.0, almost all the tightly packed nanotube bundles were freed, and dispersed as well-separated individual nanotubes with diameters ranging from 80 to 150 nm (Fig. 1d). In addition, the distribution of nanotube lengths became broader, and many short nanotubes (less than 1 μm) were found.
The above assemblies at pH 3, 5, and 6.5 are considered to be a result of a balance between three key molecular interactions of the PEI molecule located at the outer shell of the nanotube: (i) electrostatic repulsion; (ii) attractive binding (hydrogen bonding, dipole–dipole interactions, van der Waals forces); and (iii) polymer chain diffusion and entanglement. Under acidic conditions, electrostatic repulsion is expected to play a key role. The protonation degree of the branched PEI at pH 5 is approximately 50%, a percentage that is higher than that at pH 6.5.29 A higher charge density of the PEI molecule increases the electrostatic repulsion between the nanotubes, thus reducing the packing density of the nanotube bundles. At pH 3, the protonation degree of the PEI molecule reaches nearly 75%.29 The highly charged nanotubes encounter strong electrostatic repulsion, thus resulting in a dissociation of the bundles into individual nanotubes. Moreover, the strong electrostatic repulsion could even break the nanotubes into shorter segments since some PEI junctions of the nanotubes were still present within the nanotubes. This phenomenon has been described in our previous work.28 We have also investigated the self-assembly of nanotubes under neutral and alkaline conditions.
After treating the initial fused nanotube bundles at pH 7, the morphologies of the resulting materials were observed by SEM. Fig. 2a illustrates a fractal pattern with a straw sheaf-like centre formed on the substrate surface. A close look at the centre part reveals fused nanotube bundles (Fig. 2b). At pH 7, the protonation degree of the PEI molecule dropped to approximately 25%,29 thus the electrostatic repulsion among the nanotubes was much reduced. The attractive binding, chain diffusion and entanglement of the PEI molecules might become dominant forces, leading to the formation of tightly packed bundles. Furthermore, solubility of the PEI at pH 7 was also lowered due to the decrease in amine protonation. Therefore, the tight bundle unit would undergo secondary self-assembly into fractal structure during drying in order to reduce the surface energy through minimizing its surface areas. Chau and Wang have reported a similar fractal formation using peptide nanorods as building blocks.30 They have pointed out that the diffusion units should be short (a few micrometres in length) and sufficiently rigid in order to form fractal patterns. In our system, the tight nanotube bundles have the appropriate length scale (<5 μm) and contain rigid PMMA graft segments. Thus we believe that they may undergo a similar fractal assembly mechanism: once the nucleation site is formed from the side-by-side aggregation of the nanotube bundles, the free bundles could undergo random diffusion to attach to each other and become a part of the assembly based on a diffusion-limited aggregation mechanism.31
 | ||
| Fig. 2 FE-SEM micrographs of aggregated nanotube bundles at pH 7: (a) formation of a fractal pattern. The scale bar is 5 μm. (b) Aggregation of tightly packed nanotube units. The scale bar is 1 μm. | ||
When the preformed nanotube bundles were treated at pH 9, three-dimensional single columnar assemblies were found to be the major product as shown in Fig. 3a and 3b. The columnar diameters ranged from 5 to 8 μm, consisting of many fused nanotube bundles (Fig. 3c). The presence of individual nanotubes was identified with AFM images (Fig. S1 in ESI†). The SEM image in Fig. 3d shows that the columnar assembly may form through aggregation of a higher number of nanotube bundles.
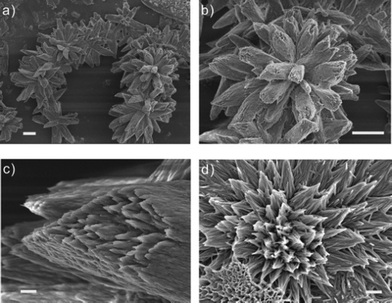 | ||
| Fig. 3 FE-SEM micrographs of a three-dimensional single columnar unit at pH 9: (a) assembled into a flower-like chain pattern. (b) Highly uniform columnar unit. (c) Enlarged image of the tail part of the columnar unit. (d) Aggregation of nanotubular bundles to form a columnar structure. The scale bars of (a–b) and (c–d) are 10 μm and 1 μm, respectively. | ||
At pH 9, the protonation degree of PEI is only 8%,29 thus the electrostatic repulsion between the nanotubes is at its minimum. At this pH, PEI solubility is also lower, thus the bundles would undergo further aggregation to form a larger columnar structure in order to reduce their surface energy through minimization of the surface area. The above results suggest that morphologies of hierarchical structures could be easily manipulated through varying solution pHs.
When treating the nanotube bundles at pH 11, we were surprised to find that a reticular plate-like material was formed instead of nanotube bundles (Fig. 4a). Observation of the detailed architecture reveals that the hierarchical plate-like structure is assembled from individual nanotubes perpendicular to one another (Fig. 4b). A TEM image confirms this kind of arrangement, as shown in the inset of Fig. 4b. These images indicate that the nanotubes are dispersed as individual units instead of in bundle units under strong alkaline conditions. Unlike under acidic conditions in which the PEI chains are highly extended in water, the PEI chains in strong alkaline solution tend to compact onto the nanotube surface due to a lack of amine protonation and reduced water solubility. As a result, chain diffusion and entanglement of the PEI molecule between the nanotube shells are much reduced. Furthermore, the hydroxyl ions (OH−), which were added to adjust the solution pH to strongly alkaline, tend to adsorb onto the nanotube surface, providing a negative surface charge (zeta-potential of nanotubes at pH 11 was approximately −40 mV (Fig. S2 in ESI†). Thus, the strong negative electrostatic repulsion causes the nanotubes to arrange themselves in a perpendicular fashion in order to minimize their charge repulsion. When treating the nanotube dispersion at pH 13, interwoven plate-like materials with a similar shape were obtained, as illustrated in Fig. 4c. But the nanotubes interweave with one another at an angle of approximately 45° to form a much tighter structure (Fig. 4d). This transformation may be induced by the “salting out” effect due to the increased electrolyte concentration.
 | ||
| Fig. 4 FE-SEM images of self-assembled plate-like assemblies at pH 11 and 13: (a) reticular plate-like structure formed at pH 11. (b) The nanotubes are perpendicularly interwoven together at pH 11. Scale bar: 200 nm. (TEM inset scale bar: 500 nm). (c) Tightly packed interwoven plate-like structure at pH 13. (d) The nanotubes are interwoven with one another at an angle of approximately 45°. The scale bars are 1 μm (a, c and d). | ||
In summary, we have discovered a simple pH-induced hierarchical assembly using amphiphilic PEI-g-PMMA core–shell nanotubes as building blocks. Since the PEI molecule is highly sensitive to solution pH, variation of the pH could significantly alter the surface charge density of the nanotubes, PEI chain diffusion and entanglement as well as water solubility. Our results indicate that the preformed nanotubes can be arranged in parallel, fantail-shape or perpendicular to one another, depending on the solution pH. Thus various intriguing hierarchical assemblies have been generated at different pH values. This study demonstrates the bottom-up assembly of synthetic amphiphilic core–shell nanotubes under mild conditions. This versatile approach enables us to create various microscopic or macroscopic higher-ordered superstructures which may serve as novel templates for other complex organic–inorganic hybrid nanostructures.32,33
Acknowledgements
We gratefully acknowledge the Hong Kong Polytechnic University and the Research Grant Council of Hong Kong SAR (Poly5016/09P) for their financial support of this research.References
- G. M. Whitesides and M. Boncheva, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4769–4774 CrossRef CAS.
- S. Zhang, T. Holmes, C. Lockshin and A. Rich, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 3334–3338 CrossRef CAS.
- R. P. Goodman, I. A. T. Schaap, C. F. Tardin, C. M. Erben, R. M. Berry, C. F. Schmidt and A. J. Turberfield, Science, 2005, 310, 1661–1665 CrossRef CAS.
- A. M. Hung and S. I. Stupp, Nano Lett., 2007, 7, 1165–1171 CrossRef CAS.
- P. Ringler and G. E. Schulz, Science, 2003, 302, 106–109 CrossRef CAS.
- Y. He, T. Ye, C. Zhang, A. E. Ribbe, W. Jiang and C. Mao, Nature, 2008, 452, 198–201 CrossRef CAS.
- S. M. Douglas, H. Dietz, T. Liedl, B. Hogberg, F. Graf and W. M. Shih, Nature, 2009, 459, 414–418 CrossRef CAS.
- M. P. Pileni, J. Phys. Chem. B, 2001, 105, 3358–3371 CrossRef CAS.
- V. Percec, G. Ungar and M. Peterca, Science, 2006, 313, 55–56 CrossRef.
- N. Kol, L. Adler-Abramovich, D. Barlam, R. Z. Shneck, E. Gazit and I. Rousso, Nano Lett., 2005, 5, 1343–1346 CrossRef CAS.
- S. Zhang, Nat. Biotechnol., 2003, 21, 1171–1178 CrossRef CAS.
- P. W. K. Rothemund, Nature, 2006, 440, 297–302 CrossRef CAS.
- J. Xu, J. F. Xia and Z. Q. Lin, Angew. Chem., Int. Ed., 2007, 46, 1860–1863 CrossRef CAS.
- C. Querner, M. D. Fischbein, P. A. Heiney and M. Drndió, Adv. Mater., 2008, 20, 2308–2314 CrossRef CAS.
- T. Ming, X. S. Kou, H. J. Chen, T. Wang, H. L. Tam, K. W. Cheah, J. Y. Chen and J. F. Wang, Angew. Chem., Int. Ed., 2008, 47, 9685–9690 CrossRef CAS.
- J. X. Huang, F. Kim, A. R. Tao, S. Connor and P. D. Yang, Nat. Mater., 2005, 4, 896–900 CrossRef CAS.
- A. R. Tao, P. Sinsermsuksakul and P. D. Yang, Nat. Nanotechnol., 2007, 2, 435–440 CrossRef CAS.
- A. R. Tao, J. X. Huang and P. D. Yang, Acc. Chem. Res., 2008, 41, 1662–1673 CrossRef CAS.
- H. Y. Fan, E. Leve, J. Gabaldon, A. Wright, R. E. Haddad and C. J. Brinker, Adv. Mater., 2005, 17, 2587–2590 CrossRef CAS.
- E. Lee, J. K. Kim and M. Lee, Angew. Chem., Int. Ed., 2009, 48, 3657–3660 CrossRef CAS.
- E. Lee, J. K. Kim and M. Lee, J. Am. Chem. Soc., 2009, 131, 18242–18243 CrossRef CAS.
- K. P. Pernstich, R. Ginés and W. R. Caseri, Small, 2011, 7, 788–795 CrossRef CAS.
- L. Cheng, G. Zhang, L. Zhu, D. Chen and M. Jiang, Angew. Chem., Int. Ed., 2008, 47, 10171–10174 CrossRef CAS.
- I. Lee, H. P. Zheng, M. F. Rubner and P. T. Hammond, Adv. Mater., 2002, 14, 572–577 CrossRef CAS.
- J. H. Choi, S. M. Adams and R. Ragan, Nanotechnology, 2009, 20, 065301 CrossRef CAS.
- P. Li, J. Zhu, P. Sunintaboon and F. W. Harris, Langmuir, 2002, 18, 8641–8646 CrossRef.
- P. Sunintaboon, K. M. Ho, P. Li, S. Z. D. Cheng and F. W. Harris, J. Am. Chem. Soc., 2006, 128, 2168–2169 CrossRef CAS.
- C. H. Lee, K. M. Ho, F. W. Harris, S. Z. D. Cheng and P. Li, Soft Matter, 2009, 5, 4914–4921 RSC.
- J. H. Suh, S. H. Lee, S. M. Kim and S. S. Hah, Bioorg. Chem., 1997, 25, 221–231 CrossRef CAS.
- W. P. Wang and Y. Chau, Soft Matter, 2009, 5, 4893–4898 RSC.
- K. Giri, N. P. Bhattacharyya and S. Basak, Biophys. J., 2007, 92, 293–302 CrossRef CAS.
- Y. Y. Lin, Y. Qiao, C. Gao, P. F. Tang, Y. Liu, Z. B. Li, Y. Yan and J. B. Huang, Chem. Mater., 2010, 22, 6711–6717 CrossRef CAS.
- V. M. Yuwono and J. D. Hartgerink, Langmuir, 2007, 23, 5033–5038 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details of the preparation of PEI-g-PMMA nanotubes, AFM images of nanotubular bundles formed at solution pH 9, zeta-potential of nanotubesversus solution pH. See DOI: 10.1039/c2ra00959e |
| This journal is © The Royal Society of Chemistry 2012 |
