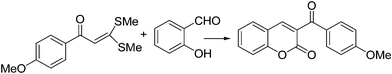
InCl3 catalyzed domino route to 2H-chromene-2-ones via [4 + 2] annulation of 2-hydroxyarylaldehydes with α-oxoketene dithioacetal under solvent-free conditions†
Rajiv Kumar
Verma
,
Girijesh Kumar
Verma
,
Gaurav
Shukla
and
Maya Shankar
Singh
*
Department of Chemistry (Centre of Advanced Study), Faculty of Science, Banaras Hindu University, Varanasi, 221005, India. E-mail: mssinghbhu@yahoo.co.in; Fax: +91-542-2368127; Tel: +91-542-6702502
First published on 1st February 2012
Abstract
A facile and efficient synthesis of 3-aroyl/heteroaroyl/ferrocenoyl/alkanoyl-2H-chromen-2-ones has been developed by the cyclocondensation of α-oxoketene dithioacetals and 2-hydroxyarylaldehydes catalyzed by InCl3 under solvent-free conditions. No co-catalyst or activator is needed and MeSH is the only by-product of this protocol. The methodology involves ring annulation of 2-hydroxyarylaldehydes with a variety of α-oxoketene dithioacetals offering rapid entry into differentially substituted chromen-2-ones. The condensation of ferrocene derived α-oxoketene dithioacetal and 2-hydroxyarylaldehyde furnished coumarin installed on a ferrocene platform.
Introduction
The coumarins (2H-chromen-2-ones, 2H-1-benzopyran-2-ones), an elite class of lactones, are important oxygen heterocycles that are widely present as a structural motif in numerous natural products.1 Among the scaffolds of natural products, coumarins exhibit a broad range of biological2 and therapeutic3 activities together with various applications in technological4 fields. Since the first isolation of coumarin in 1820, over 1400 natural coumarins have been isolated from natural sources, and their pharmacological and biochemical properties depend upon the pattern of substitutions. Several synthetic analogs of the coumarin such as novobiocin, chlorobiocin, coumermycin, simocyclinone, demiflin, and flavaxate have been developed into useful drugs.5Xanthotoxol (8-hydroxylpsoralen, Xan 1) is a natural psoralen with high capacity for the treatment of Alzheimer's disease6a and 4-(4-phenoxybutoxy)-7H-furo[3,2-g]chromene-7-thione, 2 is a blocker6b of the lymphocyte potassium channel Kv1.3. Scopoletin (7-hydroxy-6-methoxycoumarin) shows inhibitory activities on brain acetylcholinesterase.6cDibromo-7-hydroxy-4-methylchromen-2-one7a (DBC, 3), is the most promising inhibitor of Casein Kinase 2 (CK2). Other coumarin derivatives such as calanolide A 4, phenprocoumon 5, and warfarin 6 have been reported as potent anti-HIV agents.7bNovobiocin87 and its analogues have been shown to bind the C-terminal domain of heat-shock protein 90 (hsp90), an exciting new target in cancer drug discovery9 that lead to a decrease in hsp90 client proteins in various cancer cell lines10 (Chart 1).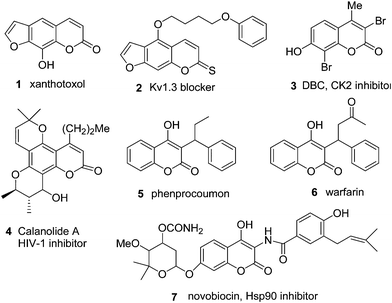 | ||
| Chart 1 Some biologically active coumarins. | ||
Coumarin, having an aldehyde group, was prepared as a doubly activated Michael acceptor and evaluated as a colorimetric and fluorescent probe for cyanide in water.11 Moreover, 3-aroylcoumarins are good Michael acceptors, amenable to further synthetic transformations,12 and demonstrated important roles in monoamine oxidase (MAO) enzymatic inhibition.13
Several elegant strategies14 for the syntheses of coumarins involving different types of catalysts have been developed.15 In recent years, versatile coumarin syntheses could be achieved through organopalladium intermediates.16 A few combinatorial library syntheses of derivatives of this important scaffold have also been developed, principally to test their utility as drug candidates.17 Among them, domino synthesis of coumarins has proved to be one of the most attractive methods, but only few reports18a,b are currently known. However, the reports on the synthesis of chromene-2-ones from α-oxoketene dithioacetals are limited,18a,b and suffer from drawbacks, such as long reaction times, harsh reaction conditions, and a lack of generality. Because of the great relevance to both synthetic and medicinal chemists, exploration of coumarins for drug discovery has been a vital subject of constant interest, and there has been a continuous effort to develop new, convenient, and versatile methods with defined structural features. Recently, indium trichloride has emerged as a powerful green and potential Lewis acid catalyst imparting high regio-and chemoselectivity in various chemical transformations,19 due to its low toxicity, air and water compatibility, operational simplicity, and remarkable ability to suppress side reactions in acid sensitive substrates.
In continuation of our efforts on the development of synthetic methodologies for heterocycles20 and our current interest in polarized ketene acetals,21 we report for the first time a two-component condensation of 2-hydroxyarylaldehydes and α-oxoketene dithioacetals in the presence of InCl3 under solvent-free conditions. Condensations occurred rapidly and were completed within two hours, consuming both the starting materials completely to give good yields. This procedure offers a selective and straightforward approach to various 3-substituted-2H-chromene-2-ones 10 and benzo[f]2H-chromene-2-ones 12. The generality of this protocol has been established with several examples.
Results and discussion
Our literature survey at this stage revealed that the α-oxoketene dithioacetals have been shown to be a important class of versatile intermediates, which have been exploited extensively in many synthetic transformations.22 The desired α-oxoketene dithioacetals 8 were prepared in good yields by treating the corresponding aryl/heteroaryl/aliphatic ketones with carbon disulphide in the presence of potassium tert-butoxide followed by alkylation with methyl iodide (Scheme 1).18 The β-alkylthio groups in this intermediate are activated by the presence of the polar carbonyl group at the α-position, and can therefore be displaced sequentially by various nucleophiles, creating further scope for introducing new functionalities at the β-position, which may find applications in many synthetic transformations.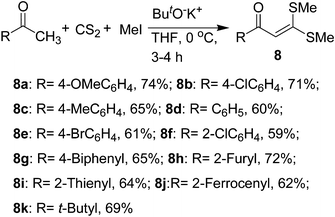 | ||
| Scheme 1 Synthesis of α-oxoketene dithioacetals 8a–k. | ||
Recently, we have succeeded on the straightforward syntheses of chromene-2-thiones via ring annulation of β-oxodithioesters20f under solvent-free conditions. The key strategy relies on the creation of a pyran ring through Lewis acid-catalyzed condensation. We envisioned that the similar construction of a pyran ring might be subsequently achieved via intermolecular cyclocondensation between α-oxoketene dithioacetal and 2-hydroxyarylaldehyde in the presence of Lewis acid catalyst under solvent-free conditions. It is worth noting that Rao et al.18a utilized α-aroylketene dithioacetals for the first time for the synthesis of 3-aroylcoumarins in the presence of piperidine in THF, which required a longer reaction time (12–15 h) and a high temperature (80 °C). In the present work 3-aroylcoumarins have been synthesized utilizing α-oxoketene dithioacetals in the presence of InCl3 at 65 °C under solvent-free conditions within 2 h.
The synthesis of 3-aroyl-2H-chromene-2-ones 10 was first undertaken. Indeed, our first attempt began with the condensation of 3,3-bis(methylthio)-1-(4-methoxyphenyl)-propen-1-one 8a (1 equiv) with salicylaldehyde 9a (1 equiv) as a model substrate. The reaction took place smoothly in the presence of InCl3 (10 mol%) under solvent-free conditions at 65 °C to give the desired product 3-(4-methoxybenzoyl)-2H-chromene-2-one (10a) in 94% yield (Table 1, entry 1). The above model reaction was investigated under a variety of conditions (catalyst, solvent, solvent-free, temperature, and time), as a test case to optimize the reaction conditions, and the results are gathered in Table 1.
| Entry | Catalyst (mol%) | Solvent | T/°C | Time | Yield (%)b |
|---|---|---|---|---|---|
| a Reaction of 3,3-bis(methylthio)-1-(4-methoxyphenyl)-propen-1-one (1 mmol) with salicylaldehyde (1 mmol). b Isolated yields. | |||||
| 1 | InCl3 (10) | None | 65 | 0.5 h | 94 |
| 2 | SbCl3 (10) | None | 65 | 10 h | 48 |
| 3 | CuBr2 (10) | None | 65 | 4 h | 78 |
| 4 | p-TSA (10) | None | 65 | 10 h | 45 |
| 5 | SiO2–H2SO4(10) | None | 65 | 8 h | 40 |
| 6 | SiO2–HClO4(10) | None | 65 | 6 h | 46 |
| 7 | InCl3 (5) | None | 65 | 1 h | 82 |
| 8 | InCl3 (20) | None | 65 | 25 min | 92 |
| 9 | InCl3 (10) | None | 40 | 1.5 h | 64 |
| 10 | InCl3 (10) | None | 55 | 1.0 h | 76 |
| 11 | InCl3 (10) | None | 80 | 0.5 h | 92 |
| 12 | InCl3 (10) | C6H6 | Reflux | 24 h | trace |
| 13 | InCl3 (10) | CHCl3 | Reflux | 24 h | No reaction |
| 14 | InCl3 (10) | THF | Reflux | 24 h | No reaction |
| 15 | InCl3 (10) | CH3CN | Reflux | 24 h | No reaction |
| 16 | InCl3 (10) | DMF | Reflux | 24 h | trace |
| 17 | InCl3 (10) | EtOH | Reflux | 24 h | No reaction |
| 18 | None | None | 65 | 24 h | No reaction |
A control experiment with the above model substrates in the absence of catalyst did not provide the desired products (Table 1, entry 18). Initially, a variety of catalysts such as InCl3, H2SO4–SiO2, SbCl3, p-TSA, CuBr2 and HClO4–SiO2 were screened to identify a suitable promoter for this transformation. Both InCl3 and CuBr2 facilitated chromene-2-one formation but InCl3 was found to be better than CuBr2 in terms of yield and time (Table 1, entries 1 and 3). In order to evaluate the most appropriate loading of InCl3, the above model reaction was performed with varying loading percentages, and it was found that 10 mol% of InCl3 was sufficient enough to promote the reaction. Further screening of non-polar, polar-aprotic, and polar-protic solvents such as C6H6, CHCl3, THF, DMF, CH3CN, and EtOH revealed that solvent-free conditions were found to be the best choice, which furnished the products not only in excellent yields but also with higher reaction rates. In addition, the effect of temperature was also studied, and the optimum temperature of the reaction was found to be 65 °C. Increasing the temperature above 65 °C neither improved the yield nor reduced the reaction time considerably; whereas lowering the temperature resulted in longer reaction times (Table 1, entry 9). Thus, the optimum condition was found to be 10 mol% of InCl3 at 65 °C under solvent-free conditions. Overall, the method works well and tolerates a variety of electron-rich and electron-deficient groups in the salicylaldehyde as well as on the aromatic ring of the α-aroylketene dithioacetal.
To test generality and scope of the condensation, and to generate a small combinatorial library of functionalized 3-substituted chromene-2-ones 10 using the optimized reaction condition, eleven α-oxoketene dithioacetals 8a–k were treated with six 2-hydroxyarylaldehydes 9a–f to furnish eighteen aroyl coumarins 10 in good yields (Scheme 2).
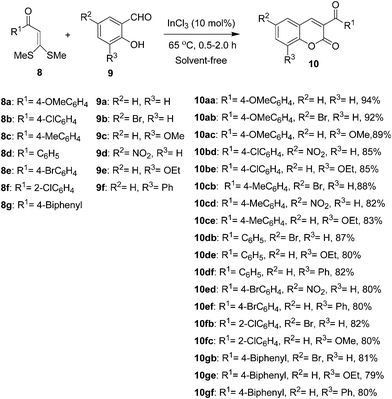 | ||
| Scheme 2 Synthesis of 3-aroyl-2H-chromene-2-ones 10. | ||
A number of functional groups, such as bromo, nitro, methoxy, and ethoxy in the salicylaldehyde component, and methoxy, methyl, phenyl, chloro and bromo groups at the o-and p-positions of the α-aroylketene dithioacetals 8a–g are well tolerated under the reaction conditions to provide structurally interesting chromene-2-ones 10 in good yields. All the reactions were monitored by TLC up to the total consumption of the starting materials, which required 0.5–2.0 h for their completion. All products were purified either by recrystallization or by column chromatography on silica gel.
To further add diversity to the chromene-2-one framework, the ketene dithioacetals 8h and 8i were subjected to cyclocondensation with 2-hydroxyarylaldehydes 9a–c and 9f to afford 3-heteroaroyl-2H-chromene-2-ones 10 in good yields (Scheme 3).
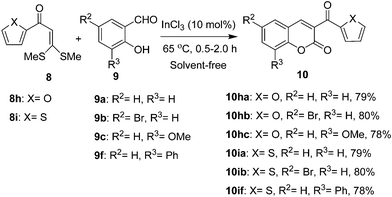 | ||
| Scheme 3 Synthesis of 3-heteroaroyl-2H-chromene-2-ones 10. | ||
As a next attempt, we have installed the coumarin motif on a ferrocene platform following the method presently developed. The ferrocene based α-oxoketene dithioacetal 8j was smoothly transformed into 3-η5-ferrocenoyl-2H-chromene-2-ones 10ja–je by condensation with 2-hydroxyarylaldehyde 9a–e under the above optimized conditions (Scheme 4). The α-oxoketene dithioacetal 8j was prepared from acetyl ferrocene by reaction with carbon disulfide and sodium tert-butoxide followed by alkylation with methyl iodide.
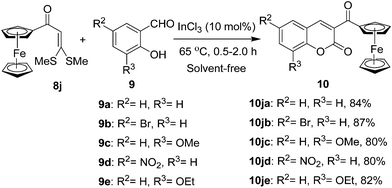 | ||
| Scheme 4 Synthesis of 3-ferrocenoyl-2H-chromene-2-ones 10. | ||
Similarly, 3-tert-butanoyl-2H-chromen-2-ones could be obtained by treatment of α-oxoketene dithioacetal 8k with 2-hydroxyarylaldehydes 9b, 9e, and 9f under identical conditions. As expected, all the reactions proceeded smoothly to afford coumarins10kb, 10ke, and 10kf in 80–82% yields (Scheme 5). The α-oxoketene dithioacetal 8k was prepared from tert-butyl methyl ketone by reaction with carbon disulfide and sodium tert-butoxide followed by alkylation.
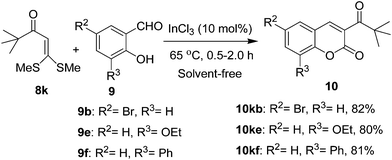 | ||
| Scheme 5 Synthesis of 3-tert-butanoyl-2H-chromene-2-ones 10. | ||
In a further elaboration to construct novel benzo[f]2H-chromene-2-ones, and to explore the limitations and generality of the reaction, 2-hydroxy-1-naphthaldehyde 11 as a cyclocondensation partner was treated with various α-oxoketene dithioacetals 8a–k under optimized reaction conditions to afford benzo[f]2H-chromene-2-ones 12a–k in 78–90% yields (Scheme 6). Such coumarins exhibit characteristics of triplet sensitizers, particularly when there is extended conjugation to aromatic rings.
![Synthesis of 3-substituted benzo[f]2H-chromene-2-ones 12.](/image/article/2012/RA/c2ra00987k/c2ra00987k-s6.gif) | ||
| Scheme 6 Synthesis of 3-substituted benzo[f]2H-chromene-2-ones 12. | ||
The structural determination of all the products 10 and 12 was achieved by IR, 1H and 13C NMR, and MS spectral analyses, and unequivocally established by X-ray single crystal diffraction analysis of two representative compounds 3-(ferrocene-2-carbonyl)-chromen-2-one10ja and 3-(2-chlorobenzoyl) benzo[f]chromen-2-one 12f. The ORTEP drawing of 10ja and 12f are presented in Fig. 1, and their CIF data are given in the ESI†.
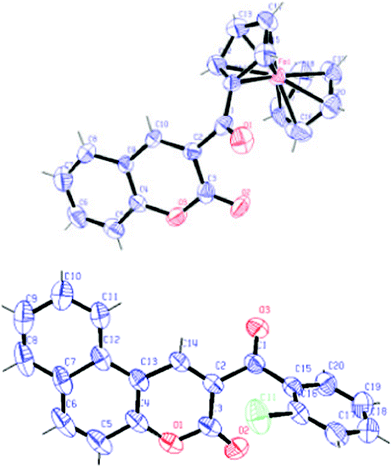 | ||
| Fig. 1 ORTEP diagram of 10ja and 12f respectively. | ||
On the basis of the above experimental results together with the related reports, a plausible reaction scenario for this two-component cyclocondensation reaction is proposed and outlined in Scheme 7. The α-oxoketene dithioacetals 8 are polarized alkenes (polarization flows from two electron-donating alkylthio groups on C-1 to the electron-withdrawing carbonyl group on C-2), showing C-1 electrophilic and C-2 nucleophilic characteristics. First, an oxa-Michael type reaction of 2-hydroxyarylaldehyde 9 or 11 (molecule with nucleophilic oxygen and electrophilic carbonyl carbon) on the C-1 of the α-oxoketene dithioacetal 8 occurs to generate enolate A, which undergoes intramolecular cyclization to produce Bvia aldol type condensation. The intermediate B immediately undergoes dehydration to give C, which upon subsequent hydrolysis of methylthio groups (by the H2O released during dehydration) with the extrusion of MeSH leads to 3-substituted coumarins 10 and 12. This operationally simple and two-component domino process concomitantly created two chemical bonds (one C–O bond and one C–C bond) leading to a new pyran ring.
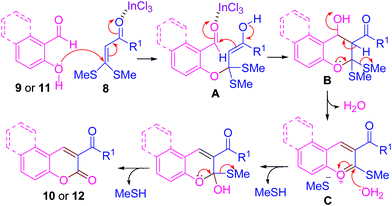 | ||
| Scheme 7 Plausible mechanism for the synthesis of chromen-2-ones. | ||
In summary, we have developed a highly efficient two-component synthesis of 3-substituted-2H-chromene-2-ones 10 and benzo[f]2H-chromene-2-ones 12 from α-oxoketene dithioacetals and 2-hydroxyarylaldehydes in high yields under solvent-free conditions in the presence of InCl3. In this experimentally simple process, one C–O bond, one C–C bond, and one new pyran ring are constructed with both reactants efficiently utilized. The scaffold could be further decorated to introduce more diversity through subsequent coupling reaction. The facile assembly of a 2H-chromene-2-one library could be expected because of the high efficiency, good substrate generality, mild conditions, and the ready availability of the starting materials.
Experimental
All starting materials were commercially available and used as received without further purification. α-Oxoketene dithioacetals (8a–k) were prepared according to the protocol described in literature18 and displayed in Scheme 1. Thin layer chromatography (TLC) was performed using silica gel 60 F254 precoated plates. Infrared (IR) spectra are measured in KBr and wavelengths are reported in cm−1. 1H and 13C NMR spectra were recorded on NMR spectrometer operating at 300 and 75 MHz, respectively. Chemical shifts (δ) are given in parts per million (ppm) using the residue solvent peaks as reference relative to TMS. J values are given in Hz. Mass spectra (MS) were recorded using Electron-spray ionization (ESI) mass spectrometry. The C, H, and N analyses were performed from microanalytical laboratory. The melting points are uncorrected.General procedure for synthesis of α-oxoketene dithioacetals18 (8a–k)
To a stirred suspension of Potassium tert-butoxide (4.48 g, 40 mmol) in dry THF (10 mL) at 0 °C, a solution of ketone (10 mmol) and carbon disulfide (12 mmol, 0.913 g, 0.73 mL) in dry THF (10 mL) was added dropwise. The reaction mixture was vigorously stirred at 0 °C for 90 min. To this suspension, a solution of methyl iodide (22 mmol, 1.37 mL) in dry THF (5 mL) was added dropwise during 10 min at 0 °C. The reaction mixture was allowed to stir for 3 h. After completion of the reaction (monitored by TLC), the mixture was poured over crushed ice/saturated ammonium chloride solution, and extracted using ethyl acetate (3 × 25 mL). The combined organic extract was washed with brine (1 × 25 mL), dried, and concentrated under vacuum to obtain the crude product, which was finally purified by silica gel column chromatography using ethyl acetate-n-hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as eluent. Spectral and analytical data of representative α-oxoketene dithioacetals are given below.
10) as eluent. Spectral and analytical data of representative α-oxoketene dithioacetals are given below.
General procedure for the synthesis of 3-substituted coumarins (10 and 12)
A mixture of α-oxoketene dithioacetal 8 (1.0 mmol) and salicylaldehyde 9 or 2-hydroxy-1-naphthaldehyde 11 (1.0 mmol) were treated in the presence of InCl3 (10 mol%, 0.1 mmol, 0.022 g) with constant stirring at 65 °C. The heating was continued for the stipulated period of time (0.5–2.0 h) till the completion of the reaction (monitored by TLC). The reaction mixture was treated with water (20 mL) and extracted with ethyl acetate (2 × 20 mL). The organic layer was washed with brine (1 × 20 mL) and dried over anhyd. Na2SO4. The solvent was evaporated under vacuum to give the product, which was purified either by recrystallization from ethanol or by column chromatography over silica gel using ethyl acetate-n-hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) as eluent. Spectral data of all new compounds are given below.
8) as eluent. Spectral data of all new compounds are given below.
Acknowledgements
This work was carried out under financial support from Council of Scientific and Industrial Research (Grant 01(2260)/08/EMR-II) and Department of Science and Technology (Grant SR/S1/OC-66/2009), New Delhi. R.K.V. and G.S. are grateful to CSIR, New Delhi for SRF and JRF, respectively and G.K.V. is thankful to University Grants Commission (UGC), New Delhi for junior research fellowship.References
- (a) R. D. H. Murray, J. Mendez and S. A. Brown, In The Natural Coumarins: Occurrences, Chemistry and Biochemistry; John Wiley and Sons: New York, USA, 1982 Search PubMed; (b) J. D. Hepworth, In Comprehensive Heterocyclic Chemistry; A. R. Katritzky, C. W. Rees, ed.; Pergamon: New York, 1984; Vol. 3, pp 799 Search PubMed; (c) J. D. Hepworth, C. D. Gabbutt and B. M. Heron, In Comprehensive Heterocyclic Chemistry-II; A. R. Katritzky, C. W. Rees and E. F. V. Scriven, ed.; Pergamon: New York, 1996; Vol. 5, pp 417 Search PubMed; (d) F. M. Deans, Naturally Occurring Oxygen Ring Compounds, London, Butterworths, 1963 Search PubMed.
- (a) H.-X. Xu and S. F. Lee, Phytother. Res., 2001, 15, 39 CrossRef CAS; (b) J. M. Hamilton miller, Antimicrob. Agents Chemother., 1995, 39, 2375 CAS; (c) K. C. Fylaktakidou, D. J. Hadjipavlou-Litina, K. E. Litinas and D. N. Nicolaides, Curr. Pharm. Des., 2004, 10, 3813 CrossRef CAS; (d) J. R. Hwu, R. Singh, S. C. Hong, Y. H. Chang, A. R. Das, I. Vliegen, E. De Clercq and J. Neyts, Antiviral Res., 2008, 77, 157 CrossRef CAS; (e) S. Sardari, Y. Mori, K. Horita, R. G. Micetich, S. Nishibe and M. Daneshtalab, Bioorg. Med. Chem., 1999, 7, 1933 CrossRef CAS; (f) D. Egan, P. James, D. Cooke and R. O'Kennedy, Cancer Lett., 1997, 118, 201 CrossRef CAS; (g) P. Valenti, A. Rampa, M. Recanatini, A. Bisi, F. Belluti, P. Da Re, M. Carrara and L. Cima, Anti-cancer Drug Des., 1997, 12, 443 CAS; (h) C. Spino, M. Dodier and S. Sotheeswaran, Bioorg. Med. Chem. Lett., 1998, 8, 3475 CrossRef CAS.
- (a) R. D. Thornes, In Coumarins: Biology, Application, and Mode of Action; R. O'Kennedy and R. D. Thornes, ed.; Wiley: Chichester, UK, 1997; pp 348 Search PubMed; (b) H. Madari, D. Panda, L. Wilson and R. S. Jacobs, Cancer Res., 2003, 63, 1214 CAS; (c) G. Finn, B. Creaven and D. Egan, Eur. J. Pharmacol., 2003, 481, 159 CrossRef CAS; (d) G. J. Finn, B. S. Creaven and D. A. Egan, Cancer Lett., 2004, 214, 43 CrossRef CAS; (e) G. J. Finn, B. S. Creaven and D. A. Egan, Eur. J. Pharm. Sci., 2005, 26, 16 CrossRef CAS; (f) M. Basanagouda, K. Shivashankar, M. V. Kulkarni, V. P. Rasal, H. Patel, S. S. Mutha and A. A. Mohite, Eur. J. Med. Chem., 2010, 45, 1151 CrossRef CAS.
- (a) Perfumery: G. S. Clark, Perfum. Flavor., 1995, 20, 23 CAS; (b) Fluorescent chemo-sensors: C.-T. Chen and W.-P. Huang, J. Am. Chem. Soc., 2002, 124, 6246 CrossRef CAS; (c) Laser technology: N. Sekar, Colourage, 2003, 50, 55 Search PubMed; (d) Fluorescent indicators: M.-P. Brun, L. Bischoff and C. Garbay, Angew. Chem., Int. Ed., 2004, 43, 3432 CrossRef CAS; (e) L. Shastri, S. Kalegowda and M. Kulkarni, Tetrahedron Lett., 2007, 48, 7215 CrossRef CAS; (f) M. Maeda, Laser Dyes: Properties of Organic Compounds for Laser Dyes; Academic Press, Tokyo, 1984; pp 335 Search PubMed; (g) M. Zabradnik, The Production and Application of Fluorescent Brightening Agent, John Wiley and Sons, New York, 1992 Search PubMed; (h) D. P. Specht, P. A. Martic and S. Farid, Tetrahedron, 1982, 38, 1203 CrossRef CAS.
- K. S. Atwal, G. J. Grover, S. Z. Ahmed, F. N. Ferrara, T. W. Harper, K. S. Kim, P. G. Sleph, S. Dzwonczyk and A. D. Russell, J. Med. Chem., 1993, 36, 3971 CrossRef CAS.
- (a) N. H. Zawia, D. K. Lahiri and F. Cardozo-Pelaez, Free Radical Biol. Med., 2009, 46, 1241 CrossRef CAS; (b) S. B. Bodendiek, C. Mahieux, W. Hansel and H. Wulff, Eur. J. Med. Chem., 2009, 44, 1838 CrossRef CAS; (c) J. M. Rollinger, A. Hornick, T. Langer, H. Stuppner and H. Prast, J. Med. Chem., 2004, 47, 6248 CrossRef CAS.
- (a) F. Meggio, M. A. Pagano, S. Moro, G. Zagotto, M. Ruzzene, S. Sarno, G. Cozza, J. Bain, M. Elliott, A. D. Deana, A. M. Brunati and L. A. Pinna, Biochemistry, 2004, 43, 12931 CrossRef CAS; (b) D. Yu, M. Suzuki, L. Xie, S. L. Morris-Natschke and K.-H. Lee, Med. Res. Rev., 2003, 23, 322 CrossRef CAS.
- M. G. Marcu, A. Chadli, I. Bouhouche, M. Catelli and L. M. Neckers, J. Biol. Chem., 2000, 275, 37181 CrossRef CAS.
- (a) Y. L. Janin, J. Med. Chem., 2005, 48, 7503 CrossRef CAS; (b) J. A. Burlison, L. Neckers, A. B. Smith, A. Maxwell and B. S. J. Blagg, J. Am. Chem. Soc., 2006, 128, 15529 CrossRef CAS; (c) M. G. Marcu, T. W. Schulte and L. Neckers, J. Natl. Cancer Inst., 2000, 92, 242 CrossRef CAS; (d) S. Messaoudi, J. F. Peyrat, J. D. Brion and M. Alami, Anti-Cancer Agents Med. Chem., 2008, 8, 761 CAS.
- (a) G. Le Bras, C. Radanyi, J. F. Peyrat, J. D. Brion, M. Alami, V. Marsaud, B. Stella and J. M. Renoir, J. Med. Chem., 2007, 50, 6189 CrossRef CAS; (b) C. Radanyi, G. Le Bras, S. Messaoudi, C. Bouclier, J. -F. Peyrat, J. -D. Brion, V. Marsaud, J. -M. Renoir and M. Alami, Bioorg. Med. Chem. Lett., 2008, 18, 2495 CrossRef CAS; (c) C. Radanyi, G. Le Bras, V. Marsaud, J. -F. Peyrat, S. Messaoudi, M. -G. Catelli, J. -D. Brion, M. Alami and J. -M. Renoir, Cancer Lett., 2009, 274, 88 CrossRef CAS; (d) C. Radanyi, G. Le Bras, C. Bouclier, S. Messaoudi, J. -F. Peyrat, J. -D. Brion, M. Alami and J. -M. Renoir, Biochem. Biophys. Res. Commun., 2009, 379, 514 CrossRef CAS.
- G. -J. Kim and H. -J. Kim, Tetrahedron Lett., 2010, 51, 2914 CrossRef CAS.
- (a) V. D. N. Sastry and T. R. Seshadri, Proc. Indian Acad. Sci., 1942, 16A, 29 CAS ; Chem. Abstr., 1943, 37, 880; (b) M. Yamashita, K. Okuyama, T. Kawajiri, A. Takada, Y. Inagaki, H. Nakano, M. Tomiyama, A. Ohnaka, I. Teryama, I. Kawasaki and S. Ohta, Tetrahedron, 2002, 58, 1497 CrossRef CAS; (c) M. P. Nemeryuk, V. D. Dimitrova, O. S. Anisimova, A. L. Sedov, N. P. Soloveva and V. F. Traven, Chem. Heterocycl. Compd., 2003, 39, 1454 CrossRef CAS; (d) J. M. Rollinger, A. Hornick, T. Langer, H. Stuppner and H. Prast, J. Med. Chem., 2004, 47, 6248 CrossRef CAS.
- (a) M. J. Matos, D. Vina, E. Quezada, C. Picciau, G. Delogu, F. Orallo, L. Santana and E. Uriarte, Bioorg. Med. Chem. Lett., 2009, 19, 3268 CrossRef CAS; (b) F. Chimenti, D. Secci, A. Bolasco, P. Chimenti, A. Granese, O. Befani, P. Turini, S. Alcaro and F. Ortuso, Bioorg. Med. Chem. Lett., 2004, 14, 3697 CrossRef CAS.
- (a) D. S. Bose, A. P. Rudradas and M. Hari Babu, Tetrahedron Lett., 2002, 43, 9195 CrossRef; (b) N. N. Karade, S. V. Gampawar, S. V. Shinde and W. N. Jadhav, Chin. J. Chem., 2007, 25, 1686 CrossRef CAS; (c) Y.-L. Chen, T.-C. Wang, H.-H. Lee, C.-C. Tzeng, Y.-L. Chang and C. M. Teng, Helv. Chim. Acta, 1996, 79, 651 CrossRef CAS; (d) P. K. Upadhyay and P. Kumar, Tetrahedron Lett., 2009, 50, 236 CrossRef CAS; (e) A. Ramazani and A. Souldozi, Phosphorus, Sulfur Silicon Relat. Elem., 2004, 179, 529 CrossRef CAS; (f) G. A. Cartwright and H. McNab, J. Chem. Res. (S), 1997, 296 RSC; (g) G. Brufola, F. Fringuelli, O. Piermatti and F. Pizzo, Heterocycles, 1996, 43, 1257 CrossRef CAS; (h) T. Sugino and K. Tanaka, Chem. Lett., 2001, 110 CrossRef CAS; (i) N. S. Narasimhan, R. S. Mali and M. V. Barve, Synthesis, 1979, 906 CrossRef CAS; (j) D. C. Dittmer, Q. Li and D. V. Avilov, J. Org. Chem., 2005, 70, 4682 CrossRef CAS; (k) V. M. Alexander, R. P. Bhat and S. D. Samant, Tetrahedron Lett., 2005, 46, 6957 CrossRef CAS; (l) E. Altieri, M. Cordaro, G. Grassi, F. Risitano and A. Scala, Tetrahedron, 2010, 66, 9493 CrossRef CAS; (m) M. J. Matos, G. Delogu, G. Podda, L. Santana and E. Uriarte, Synthesis, 2010, 2763 CrossRef CAS.
- (a) For recent publications on coumarin synthesis see: P. Konigs, O. Neumann, K. Hackeloeer, O. Kataeva and S. R. Waldvogel, Eur. J. Org. Chem., 2008, 343 CrossRef CAS , and references therein; (b) E. Ramesh and R. Raghunathan, Tetrahedron Lett., 2008, 49, 1812 CrossRef CAS; (c) F. -F. Ye, J. -R. Gao, W. -J. Sheng and J. -H. Jia, Dyes Pigm., 2008, 77, 556 CrossRef CAS , and references therein; (d) J. Oyamada, C. Jia, Y. Fujiwara and T. Kitamura, Chem. Lett., 2002, 380 CrossRef CAS; (e) G. Athanasellis, G. Melagraki, H. Chatzidakis, A. Afantitis, A. Detsi, O. Igglessi-Markopoulou and J. Markopoulos, Synthesis, 2004, 1775 CAS; (f) D. Audisio, S. Messaoudi, J.-D. Brion and M. Alami, Eur. J. Org. Chem., 2010, 1046 CAS; (g) M. M. Heravi, S. Sadjadi, H. A. Oskooie, R. H. Shoar and F. F. Bamoharram, Catal. Commun., 2007, 9, 470 CrossRef; (h) J. Oyamada and T. Kitamura, Tetrahedron, 2006, 62, 6918 CrossRef CAS; (i) K. Taksande, D. S. Borse and P. Lokhande, Synth. Commun., 2010, 40, 2284 CrossRef CAS.
- (a) C. Jia, D. Piao, J. Oyamada, W. Lu, T. Kitamura and Y. Fujiwara, Science, 2000, 287, 1992 CrossRef CAS; (b) C. Jia, W. Lu, J. Oyamada, T. Kitamura, K. Matsuda, M. Irie and Y. Fujiwara, J. Am. Chem. Soc., 2000, 122, 7252 CrossRef CAS; (c) C. Jia, D. Piao, T. Kitamura and Y. Fujiwara, J. Org. Chem., 2000, 65, 7516 CrossRef CAS; (d) P. Selles and U. Mueller, Org. Lett., 2004, 6, 277 CrossRef CAS; (e) K. Li, Y. Zeng, B. Neuenswander and J. A. Tunge, J. Org. Chem., 2005, 70, 6515 CrossRef CAS; (f) S. Aoki, C. Amamoto, J. Oyamada and T. Kitamura, Tetrahedron, 2005, 61, 9291 CrossRef CAS; (g) B. M. Trost, F. D. Toste and K. Greenman, J. Am. Chem. Soc., 2003, 125, 4518 CrossRef CAS.
- (a) M. S. Schiedel, C. A. Briehn and P. Bauerle, J. Organomet. Chem., 2002, 653, 200 CrossRef CAS; (b) A. Song, J. Zhang and K. S. Lam, J. Comb. Chem., 2004, 6, 112 CrossRef CAS.
- (a) H. S. P. Rao and S. Sivakumar, J. Org. Chem., 2006, 71, 8715 CrossRef CAS; (b) H.-J. Yuan, M. Wang, Y.-J. Liu and Q. Liu, Adv. Synth. Catal., 2009, 351, 112 CrossRef CAS; (c) K. T. Potts and P. A. Winslow, Synthesis, 1987, 839 CrossRef CAS; (d) K. T. Potts, M. J. Cipullo, P. Ralli and G. Theodoridis, J. Org. Chem., 1982, 47, 3027 CrossRef CAS; (e) I. Shahak and Y. Sasson, Tetrahedron Lett., 1973, 14, 4207 CrossRef; (f) A. Thuillier and J. Vialle, Bull. Soc. Chim. France, 1959, 1398 CAS; (g) J. Maignan and J. Vialle, Bull. Soc. Chim. France, 1973, 2388 CAS.
- (a) J. S. Yadav, Eur. J. Org. Chem., 2010, 591 CrossRef CAS; (b) C. G. Frost and K. K. Chauhan, Perkin 1, 2000, 3015 Search PubMed; (c) F. Fringuelli, O. Piermatti, F. Pizzo and L. Vaccaro, Curr. Org. Chem., 2003, 7, 1661 CrossRef CAS; (d) C. G. Frost and J. P. Hartley, Mini-Rev. Org. Chem., 2004, 1, 1 CrossRef CAS; (e) Z.-H. Zhang, Synlett, 2005, 711 CrossRef; (f) Y. Zhang, P. Li, M. Wang and L. Wang, J. Org. Chem., 2009, 74, 4364 CrossRef CAS; (g) B. V. S. Reddy, Ch. Divyavani, Z. Begam and J. S. Yadav, Synthesis, 2010, 1719 CrossRef CAS.
- (a) G. C. Nandi, S. Samai, R. Kumar and M. S. Singh, Tetrahedron, 2009, 65, 7129 CrossRef CAS; (b) R. Kumar, G. C. Nandi, R. K. Verma and M. S. Singh, Tetrahedron Lett., 2010, 51, 442 CrossRef CAS; (c) G. C. Nandi, S. Samai and M. S. Singh, Synlett, 2010, 1133 CAS; (d) R. Kumar, K. Raghuvanshi, R. K. Verma and M. S. Singh, Tetrahedron Lett., 2010, 51, 5933 CrossRef CAS; (e) G. K. Verma, K. Raghuvanshi, R. K. Verma, P. Dwivedi and M. S. Singh, Tetrahedron, 2011, 67, 3698 CrossRef CAS; (f) R. K. Verma, G. K. Verma, K. Raghuvanshi and M. S. Singh, Tetrahedron, 2011, 67, 584 CrossRef CAS; (g) G. C. Nandi, S. Samai, R. Kumar and M. S. Singh, Tetrahedron Lett., 2009, 50, 7220 CrossRef CAS; (h) S. Samai, G. C. Nandi, R. Kumar and M. S. Singh, Tetrahedron Lett., 2009, 50, 7096 CrossRef CAS; (i) G. C. Nandi, S. Samai and M. S. Singh, J. Org. Chem., 2010, 75, 7785 CrossRef CAS; (j) G. C. Nandi, S. Samai, R. Kumar and M. S. Singh, Synth. Commun., 2011, 41, 417 CrossRef CAS; (k) S. Samai, G. C. Nandi, P. Singh and M. S. Singh, Tetrahedron, 2009, 65, 10155 CrossRef CAS; (l) S. Samai, G. C. Nandi and M. S. Singh, Tetrahedron Lett., 2010, 51, 5555 CrossRef CAS.
- R. K. Verma, H. Ila and M. S. Singh, Tetrahedron, 2010, 66, 7389 CrossRef CAS.
- (a) For reviews, see: R. K. Dieter, Tetrahedron, 1986, 42, 3029 CrossRef CAS; (b) H. Junjappa, H. Ila and C. V. Asokan, Tetrahedron, 1990, 46, 5423 CrossRef CAS; (c) For selected recent reports, see: B. K. Joseph, B. Verghese, C. Sudarsanakumar, S. Deepa, D. Viswam, P. Chandran and C. V. Asokan, Chem. Commun., 2002, 736 RSC; (d) K. Panda, J. R. Suresh, H. Ila and H. Junjappa, J. Org. Chem., 2003, 68, 3498 CrossRef CAS; (e) S. Peruncheralathan, T. A. Khan, H. Junjappa and H. Ila, J. Org. Chem., 2005, 70, 10030 CrossRef CAS; (f) H. Yu and Z. Yu, Angew. Chem., Int. Ed., 2009, 48, 2929 CrossRef CAS; (g) Y. Yin, M. Wang, Q. Liu, J. -L. Hu, S. -G. Sun and J. Kang, Tetrahedron Lett., 2005, 46, 4399 CrossRef CAS; (h) Z. Fu, M. Wang, Y. Ma, Q. Liu and J. Liu, J. Org. Chem., 2008, 73, 7625 CrossRef CAS; (i) X. Bi, D. Dong, Q. Liu, W. Pan, L. Zhao and B. Li, J. Am. Chem. Soc., 2005, 127, 4578 CrossRef CAS; (j) D. Dong, X. Bi, Q. Liu and F. Cong, Chem. Commun., 2005, 3580 RSC; (k) X. Bi, D. Dong, Y. Li, Q. Liu and Q. Zhang, J. Org. Chem., 2005, 70, 10886 CrossRef CAS; (l) J. Hu, Q. Zhang, H. Yuan and Q. Liu, J. Org. Chem., 2008, 73, 2442 CrossRef CAS; (m) J. Tan, X. Xu, L. Zhang, Y. Li and Q. Liu, Angew. Chem., Int. Ed., 2009, 48, 2868 CrossRef CAS; (n) L. Zhang, F. Liang, X. Cheng and Q. Liu, J. Org. Chem., 2009, 74, 899 CrossRef CAS; (o) Y. Ma, M. Wang, D. Li, B. Bekturhun, J. Liu and Q. Liu, J. Org. Chem., 2009, 74, 3116 CrossRef CAS; (p) N. C. Misra, K. Panda, H. Ila and H. Junjappa, J. Org. Chem., 2007, 72, 1246 CrossRef CAS; (q) N. C. Misra and H. Ila, J. Org. Chem., 2010, 75, 5195 CrossRef CAS; (r) A. K. Yadav, S. Peruncheralathan, H. Ila and H. Junjappa, J. Org. Chem., 2007, 72, 1388 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: detailed experimental and spectral data, crystallographic data, and original 1H and 13C NMR spectrum of all compounds. CCDC reference numbers 805566 and 805567. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra00987k |
| This journal is © The Royal Society of Chemistry 2012 |